ABSTRACT
Background
Hypertension (HTN) is common following renal transplantation and it is associated with adverse effects on cardiovascular (CV) and graft health. Ambulatory blood pressure monitoring (ABPM) is the preferred method to characterize blood pressure (BP) status, since HTN misclassification by office BP (OBP) is quite common in this population. We performed a systematic review and meta-analysis aimed at determining the clinical utility of 24-h ABPM and its potential implications for the management of HTN in this population.
Methods
Ovid-MEDLINE and PubMed databases were searched for interventional or observational studies enrolling adult kidney transplant recipients (KTRs) undergoing 24-h ABP readings compared with OBP or home BP. The main outcome was the proportion of KTRs diagnosed with HTN by ABPM, home or OBP recordings. Additionally, day–night BP variability and dipper/non-dipper status were assessed.
Results
Forty-two eligible studies (4115 participants) were reviewed. A cumulative analysis including 27 studies (3481 participants) revealed a prevalence of uncontrolled HTN detected by ABPM of 56% [95% confidence interval (CI) 46–65%]. The pooled prevalence of uncontrolled HTN according to OBP was 47% (95% CI 36–58%) in 25 studies (3261 participants). Very few studies reported on home BP recordings. The average concordance rate between OBP and ABPM measurements in classifying patients as controlled or uncontrolled hypertensive was 66% (95% CI 59–73%). ABPM revealed HTN phenotypes among KTRs. Two pooled analyses of 11 and 10 studies, respectively, revealed an average prevalence of 26% (95% CI 19–33%) for masked HTN (MHT) and 10% (95% CI 6–17%) for white-coat HTN (WCH). The proportion of non-dippers was variable across the 28 studies that analysed dipping status, with an average prevalence of 54% (95% CI 45–63%).
Conclusions
In our systematic review, comparison of OBP versus ABP measurements disclosed a high proportion of MHT, uncontrolled HTN and, to a lesser extent, WCH in KTRs. These results suggest that HTN is not adequately diagnosed and controlled by OBP recordings in this population. Furthermore, the high prevalence of non-dippers confirmed that circadian rhythm is commonly disturbed in KTRs.
Keywords: ambulatory blood pressure monitoring, hypertension, kidney transplantation, meta-analysis, systematic review
INTRODUCTION
Arterial hypertension (HTN) is a highly prevalent complication among kidney transplant recipients (KTRs) [1] and is a major contributing factor to graft failure and cardiovascular (CV) morbidity and mortality [2, 3] in this population. HTN is a modifiable risk factor and well-controlled blood pressure (BP) associates with longer transplant and patient survival [4–6].
The diagnosis and clinical decisions about the treatment of HTN in the transplant population have traditionally been based on measurements of BP in the clinic setting. However, office BP (OBP) has important limitations in diagnosing HTN because of its intra- and inter-individual variability [7], thus misclassifying a proportion of KTRs [8, 9]. Furthermore, it has been shown that nighttime BP, rather than isolated OBP readings, correlates better with markers of vascular damage [i.e. carotid intima-media thickness (IMT)] [9] and morbid CV events [10, 11] in these individuals. Nocturnal [9], masked (MHT) [12] and white-coat HTN (WCH) [13] are all common in this population.
Current recommendations by the National Institute for Health and Care Excellence (NICE) [14] and the American US Preventive Services Task Force [15] recommend that in individuals with high-normal OBP at high risk of developing CV disease, the diagnosis of HTN be confirmed with ambulatory BP monitoring (ABPM). However, the use of this technique remains scarcely applied in KTRs, a population notoriously at high risk for CV disease [1–3].
Since diagnostic biomarkers should be specifically tested in the population where they are applied in clinical practice, we conducted a systematic review and meta-analysis aimed at comparing the prevalence of KTRs diagnosed with uncontrolled HTN by 24-h, daytime and/or nighttime ABPM, home BP and OBP.
Additionally, we collected data from studies reporting day–night BP variability and assessment of dipper/non-dipper status in the same population.
MATERIALS AND METHODS
We performed this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16] and published the protocol of the meta-analysis [17]. Due to the length of the originally planned systematic review and meta-analysis, we decided to split it into two different systematic reviews. Thus, some pre-specified outcomes reported in the aforementioned protocol (i.e. association between BP recordings and renal and CV outcomes) will be mentioned in a second manuscript.
Data source and search strategy
We searched Ovid-MEDLINE and PubMed databases for articles without time or language restriction through 16 November 2020 using focused, high-sensitive search strategies (Supplementary data, Table S1). Bibliographies of relevant studies and reviews were screened for additional articles. The search was designed and performed by two authors (A.Pisano and D.B.).
Study selection and data extraction
Any interventional [randomized and non-randomized controlled trials (RCTs) or uncontrolled trials] or observational study (prospective or retrospective study) dealing with the reference population undergoing 24-h ABP readings compared with traditional clinic or home BP measurements was included. Studies enrolling adult KTRs from either live or deceased organ donors were included. Studies where at least part of the population fulfilled the above criteria were included in the review. Studies were excluded if they: (i) did not include KTRs; (ii) did not compare 24-h ABPM with at least one of the traditional OBP or home BP measurements; (iii) did not provide data on the outcomes of interest. We aimed at comparing the agreement of different BP measurements in diagnosing HTN and assessing the relation between BP recordings by different methods in KTRs.
The main outcome of interest was assessment of proportion of KTRs diagnosed with uncontrolled HTN by 24-h, daytime and/or nighttime ABPM, home or OBP recordings. Pre-specified additional outcomes were day–night BP variability and assessment of dipper/non-dipper status.
Two investigators (A.Pisano and D.B.) independently screened titles and abstracts, excluding studies not pertinent to the topic, and assessed the retrieved full texts to determine eligibility according to the pre-specified inclusion/exclusion criteria. A third reviewer (C.Z.) solved possible discrepancies on study judgements. Reviews, editorials, letters, case reports and studies performed on children or adolescents (age <18 years) were excluded, but screened for additional references. If more than one article from a single study was identified, eligible data from all reports were considered, but each study was included only once. Data extraction was performed by one Author (A.Pisano), using a customized table (see Supplementary data, Table S2). The following properties were extracted from each study: study characteristics (first author, year of publication, country, design and inclusion/exclusion criteria), population characteristics (number of patients, age, sex, body mass index, baseline renal function, transplantation vintage, baseline OBP, ABPM and home BP), comorbidities [diabetes, HTN, left ventricular hypertrophy (LVH), coronary heart disease and heart failure], and BP thresholds according to OBP, ABPM and home BP recordings.
Data analysis
The pooled HTN prevalence with 95% confidence intervals (CIs), by, respectively, ABPM and home BP/OBP methods, was obtained by aggregating single-study prevalence.
To avoid selection bias, studies not involving a random population sample (i.e. studies that selected fully uncontrolled and/or controlled hypertensive subjects by OBP) were not included in the pooled analysis. Nevertheless, in order to maximize information, prevalence data produced by the above studies were reported narratively.
Data were pooled using the random-effects model and, to guarantee robustness of the model, we also analysed data with the fixed-effects method. The χ2 test on N − 1 degrees of freedom, with an alpha of 0.05 considered for statistical significance, and the Cochrane-I2 [18] were used to assess the presence of heterogeneity. I2 values of ≤25, <50 and >50% were assumed to correspond to low, medium and high levels of heterogeneity, respectively. Values ≥75% correspond to a highly significant heterogeneity across studies with a strong effect in the pooled estimate of the outcomes. Possible sources of heterogeneity were explored performing sensitivity and subgroup analyses according to different BP metrics and/or BP thresholds. Meta-regression analyses were performed for identifying possible effect modifiers in meta-analyses including at least 10 studies.
Publication bias was investigated by Egger’s regression test and by visual inspection of funnel plots.
Data analyses were performed by two authors (A.Pisano and G.D.) using Stata/IC (version 13.1, StataCorp LP, College Station, TX, USA), and independently verified by a third author (C.Z.).
RESULTS
Search results
Figure 1 shows the flow diagram of the study selection process. Nine hundred and seven potentially relevant references were initially found. Four additional citations were added by personal search. By screening titles and abstracts, 765 citations were excluded for various reasons (search overlap, study population/clinical problem not pertinent and review articles). Among the 146 studies selected for full text examination, 95 were excluded due to studying other populations or not reporting outcomes of interest (n = 20), lack of a comparator group (n = 60), being review articles (n = 4) or any other reason (n = 11).
FIGURE 1:
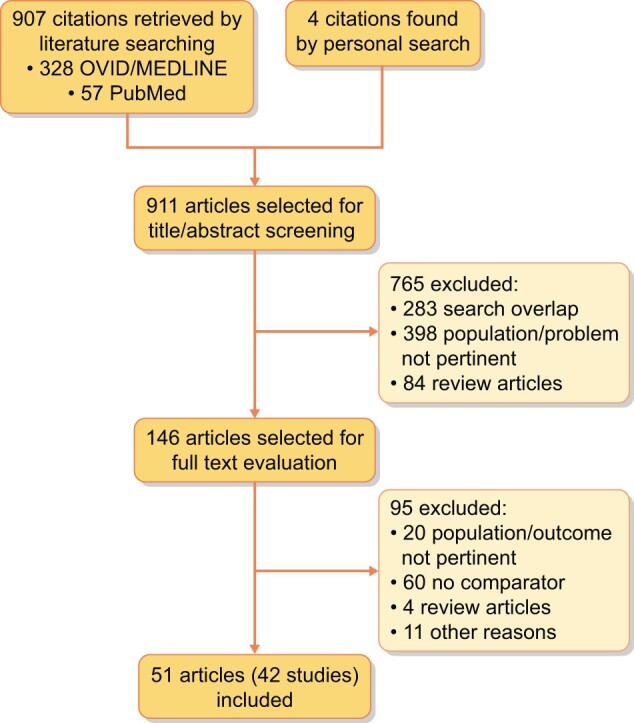
Study selection flow.
A total of 51 articles referring to 42 studies (4115 participants) were finally included in the review.
Study characteristics
Study design
The vast majority of the reviewed studies had an overall observational design, including three retrospective studies [8, 19, 20], 20 prospective studies [21–40], 14 studies with a cross-sectional design [7, 12, 13, 41–51] and one survey [9]. Four studies had an interventional design [52–55], of which three were RCTs [52, 53, 55]. Only 4 [7, 29, 34, 48] out of 42 studies compared ABPM with both the traditional OBP and home BP measurements.
BP categories considered in the various studies
The overwhelming majority of studies enrolled uncontrolled hypertensive KTRs (being treated with antihypertensive medication), except two studies [12, 40] that involved apparently controlled hypertensive individuals, and one additional study [21] that included normotensive stable KTRs not treated with antihypertensive drugs. The final population analysed in this review included 4115 patients and the range of patients enrolled in these studies was extremely variable, spanning from 10 [52] to 868 [13] individuals.
Criteria adopted for the definition of HTN according to OBP and 24-h ABPM
Overall, the classification of patients as hypertensive by OBP readings in these studies was based on contemporary documents issued by the 1996 World Health Organization (WHO) [56] and/or Reports of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High BP (JNC 6–7) [57, 58]. Definition of arterial HTN for the general population by these documents was office systolic BP (SBP) exceeding 140 mmHg or diastolic BP (DBP) exceeding 90 mmHg, and/or use of antihypertensive drugs. The European Society of HTN/European Society of Cardiology (ESH/ESC) 2013 guidelines (SBP ≥140 mmHg and/or DBP ≥90 mmHg or treatment with hypotensive drugs) [59] were adopted by six studies [9, 33, 36, 39, 47, 51]. Five studies [8, 13, 38, 43, 49] set office HTN diagnosis threshold at 130/80 mmHg, in accordance with Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [60].
In one study [50], office HTN was defined as mean office SBP ≥140 mmHg and/or mean office DBP ≥90 mmHg, according to the JNC 8 report [61].
Standardized OBP readings (measured by the same nurse/technician, a mean of 3 times after at least 10 min of quiet resting in a semi-recumbent position), adhering to WHO [56] and/or ESH/ESC recommendations (3–5 min of rest in a sitting position) [59], were carried out with a mercury sphygmomanometer in 21 studies [12, 19, 22–25, 27–29, 31, 34, 36, 37, 40, 42, 48, 51–55], whereas an automated oscillometric device was used in 10 studies [7, 9, 13, 30, 32, 33, 35, 39, 43, 47]. Not standardized (taken manually by different people or in a single reading) OBP measurements were performed by five studies [8, 21, 38, 45, 50]. Six studies [20, 26, 41, 44, 46, 49] reported no information on clinic BP methodology.
In four studies [7, 29, 34, 48] focusing on home BP, patients (354 participants) were considered hypertensive when the mean reading of self-measured BP taken several times during the day for 5–7 days (12–28 valid measurements) [7, 34, 48] exceeded 135/85 mmHg, i.e. the threshold recommended by the ESH/ESC 2003 guidelines [62, 63]. In one study, home BP was measured on a single day only with four measurements per patient [29].
Classification of patients as hypertensive by ABPM, following the ESH/ESC 2003 recommendations (24-h ABP >125/80 mmHg), was adopted by eight studies [7, 26, 27, 29, 30, 32, 43, 54]. In the majority of the studies, arterial HTN was recognized in accordance with ESH/ESC 2013 guidelines if 24-h ABPM exceeded the target set at 130/80 mmHg. Target diurnal and nocturnal BP in these studies were set at 135/85 and 120/70 mmHg, respectively [8, 9, 33, 35, 36, 38–40, 47, 49, 50, 55]. Five studies [19, 24, 41, 45, 51] defined HTN as 24-h ABPM >135/85 mmHg, daytime BP >140/90 mmHg or nighttime BP >120/80 mmHg [64]. Paoletti et al. [31] and Marcondes et al. [25] defined HTN as an average BP ≥130/80 mmHg according to the criteria issued by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) Clinical Practice Guidelines on HTN and Antihypertensive agents in CKD [65]. Two studies [12, 34] defined HTN according to the JNC 7 criteria (mean ABP >140/90 mmHg).
Mostly, ABPM technology used an automated cuff with an oscillometric device conforming to the advancement of medical instrumentation recommendations (i.e. Spacelabs 90207) with BP readings taken at 15- and 30-min intervals throughout the day and night, respectively [7, 9, 13, 19, 22, 23, 25, 26, 28–31, 33, 39, 40, 42, 45, 47–49, 52–54]. Twenty-four-hour recordings were carried out every 30 min during the day and every 60 min during the night in six studies [21, 37, 43, 49, 51, 55]. Other devices and customized analytical software were used in other studies [12, 24, 27, 32, 34–38, 41, 50, 51]. No information on ABPM methodology was provided by three studies [8, 44, 46].
Nocturnal BP dipping was calculated as the difference between mean daytime and mean nighttime BPs. Patients were classified as dippers if mean BP decreased by 10% or more during the nighttime period.
The main characteristics of the studies reviewed are described in Supplementary data, Table S2.
In-study and pooled prevalence of HTN by ABPM, home and OBP
Overall, the single-study prevalence of uncontrolled HTN by ABPM and OBP ranged from 19% [35] to 95% [31] and from 3% [36] to 95% [31], respectively. Studies that selected fully uncontrolled [43, 48, 52, 54, 55] or controlled hypertensive subjects [12, 40] by OBP were excluded from the cumulative analysis of uncontrolled HTN prevalence detected by OBP recordings.
Prevalence of HTN according to daytime and nighttime ABP was variable, spanning from 26% [9] to 87% [49] and 21.6% [54] to 84% [50], respectively.
Only two [7, 29] out of four studies [7, 29, 34, 48] reported information about the prevalence of HTN according to home BP. Among 183 KTRs, Agena et al. [7] observed a prevalence of uncontrolled HTN of 56.3, 36.1 and 44.8%, respectively, by OBP, ABPM and home BP. In the study by Stenehjem et al. [29], OBP, ABPM and home recordings revealed uncontrolled HTN in 47, 84 and 71% of patients, respectively.
Overall, 29 studies [7–9, 12, 13, 19, 22, 24, 26–33, 35, 36, 38–40, 42, 44, 45, 47, 49–51, 54] with randomly recruited subjects provided suitable data on the in-study prevalence of uncontrolled HTN by ABPM and/or OBP and were included in pooled meta-analyses.
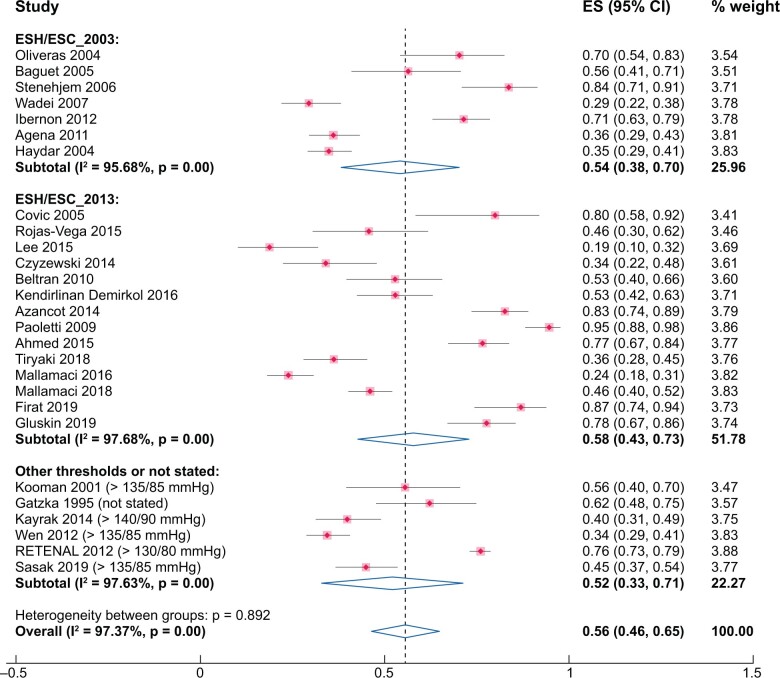
A cumulative analysis of 27 studies (3481 participants) [7–9, 12, 13, 19, 22, 26–33, 35, 36, 38–40, 44, 45, 47, 49–51, 54] based on ABPM exposed a high heterogeneity (97.4%). The cumulative prevalence of uncontrolled HTN by this technique was 56% (95% CI 46–65%, χ2 = 988.41, P = 0.00, I2 = 97.4%) (Figure 2). Sensitivity analyses, stratifying by different ABPM metrics (24 h or daytime; Supplementary data, Figure S1, χ2 = 816.71, P = 0.00, I2 = 97.5% and χ2 = 146.66, P = 0.00, I2 = 96.6%, respectively), different BP thresholds (ESH/ESC 2003, χ2 = 138.83, P = 0.00, I2 = 95.7% or ESH/ESC 2013 guidelines, χ2 = 560.68, P = 0.00, I2 = 97.7% or other thresholds, χ2 = 211.03, P = 0.00, I2 = 97.6%) (Figure 2) or type of devices used (Spacelab or other devices; Supplementary data, Figure S2, χ2 = 858.58, P = 0.00, I2 = 98.1% and χ2 = 96.97, P = 0.00, I2 = 92.8%, respectively) did not reduce the high heterogeneity observed in the cumulative analysis.
FIGURE 2:
Pooled prevalence of uncontrolled HTN by ABPM. Sensitivity analysis stratifying by different BP thresholds (ESH/ESC 2003 or ESH/ESC 2013 or other guidelines).
Visual inspection of the funnel plot and Egger’s regression test (P = 0.12) indicate that the presence of publication bias was unlikely (Supplementary data, Figure S3a).
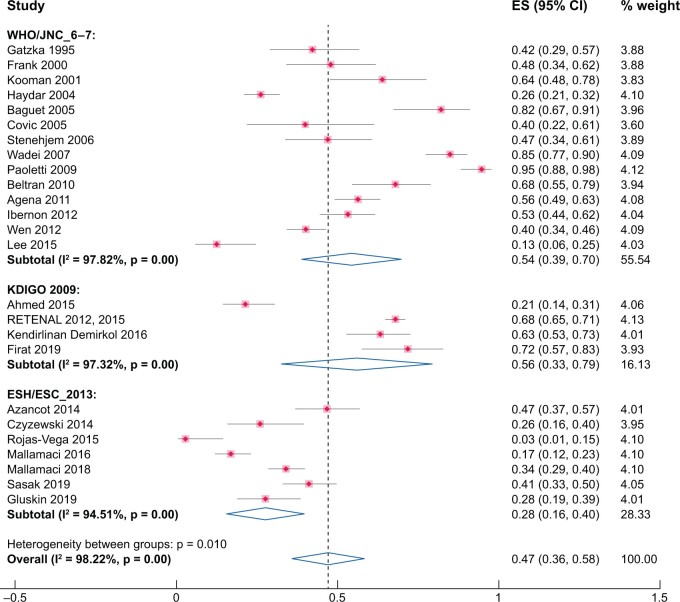
As for OBP, the cumulative prevalence (25 studies, 3261 participants) of uncontrolled HTN was 47% (95% CI 36–58%) [7–9, 13, 19, 26–33, 35, 36, 38, 39, 42, 44, 45, 47, 49–51]. Again there was considerable heterogeneity among the various studies (χ2 = 1349.18, P = 0.00, I2 = 98.2%). Heterogeneity remained high in sub-analyses carried out according to different BP thresholds (KDIGO or ESH/ESC 2013 guidelines or others) (Figure 3) or type of devices (Supplementary data, Figure S4; mercury sphygmomanometer, χ2 = 674.61, P = 0.00, I2 = 98.8%; automatic oscillometric devices, χ2 = 479.76, P = 0.00, I2 = 98.3%; not standardized or unreported methods, χ2 = 44.18, P = 0.00, I2 = 94.5%).
FIGURE 3:
Pooled prevalence of uncontrolled HTN by OBP. Sensitivity analysis stratifying by different BP thresholds (WHO/JNC 6–7 or KDIGO 2009 or ESH/ESC 2013).
Visual inspection of the funnel plot and the Egger’s regression test (P = 0.36) show absence of publication bias (Supplementary data, Figure S3b).
BP profile by different methods
Four studies compared BP profiles by ABPM, home BP and OBP recordings. In general, OBP and home BP were higher than ABPM but the results were not homogeneous [7, 29, 34, 48].
In 183 KTRs, Agena et al. [7] found both office and home SBP higher than ABPM values, whereas DBP was lower by home and higher by OBP when compared with ABPM. In 49 KTRs with early deterioration in graft function, Stenehjem et al. [29] observed morning and evening home BP significantly higher than 24 h, daytime ABP and OBP (P < 0.001 for all), and no difference between OBP and ABPM values.
In 49 KTRs enrolled by David et al., no significant difference between awake and, respectively, home and office SBP was observed, whereas daytime DBP resulted lower (P < 0.05) than the corresponding OBP and home values [34].
The vast majority of the studies comparing OBP versus ABPM, reported overall higher values by OBP than ABPM [13, 23, 25–27, 30, 32, 36–38, 40, 41, 45, 52, 53, 55]. Only six studies (349 participants) [8, 20, 21, 28, 49, 50] recorded lower OBP than 24-h ABPM. No significant difference between OBP and ABPM recordings (24-h, daytime and nighttime BP) was found in 11 studies (965 participants) [9, 19, 22, 24, 31, 33, 35, 39, 42, 46, 47].
Steigerwalt et al. [43] reported that patients receiving tacrolimus (n = 20) had higher OBP than ABPM values while those receiving sirolimus (n = 18) had higher ABPM than OBP values.
Concordance rate among methods
Agreement in the diagnosis of controlled and uncontrolled HTN
Agreement amongst different BP measurements in classifying patients as controlled or uncontrolled hypertensive were reported in 17 studies [7, 8, 13, 19, 23, 24, 26, 29, 32–35, 38, 41, 45, 47, 51], with variable concordance rate among studies, spanning from 39% [8] to 90% [35]. A pooled analysis including data from 10 studies [7, 8, 13, 26, 32, 33, 35, 38, 47, 51] revealed an average prevalence of the agreement of 66% (95% CI 59–73%) (Figure 4).
FIGURE 4:
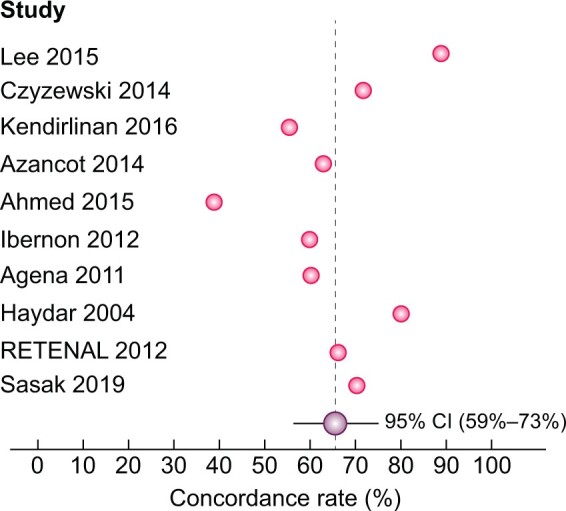
Concordance rate between ABPM and OBP in diagnosing controlled and uncontrolled HTN.
Nominally significant correlations between OBP and 24-h ABPM were also reported by five studies (all comparisons, r = 0.46–0.69, P < 0.05 to P < 0.001) [19, 24, 29, 41, 45]. No correlation between methods was found by Jacobi et al. [23].
Using receiver operating characteristics curve analyses, Agena et al. [7] reported a significant but unsatisfactory diagnostic concordance of OBP (61.2%) with ABPM (the gold standard) and a higher diagnostic concordance with home BP (72.7%). David et al. [34] found OBP more specific than home BP in diagnosing HTN (98% versus 89% specificity) as defined by ABPM. In contrast, home BP was superior to OBP in identifying patients achieving the BP goal (83% versus 50% specificity) defined on the basis of ABPM.
OBP–ABP discordance in diagnosing uncontrolled HTN
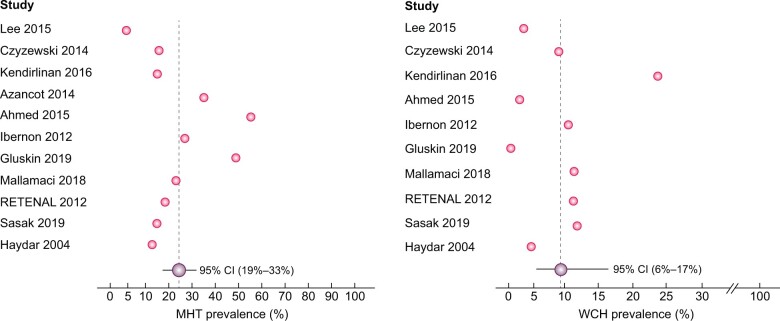
ABPM gave information on HTN phenotypes among KTRs, revealing a considerable disagreement with OBP. Thirteen studies [8, 12, 13, 26, 32, 33, 35, 38–40, 47, 50, 51] addressed the OBP-24-h ABP discordance, finding a variable proportion of MHT, spanning from 6% [35] to 58% [8], and WCH ranging from 0% [47] to 24.7% [38].
Two cumulative analyses of 11 and 10 studies, respectively, revealed an average prevalence of 26% (95% CI 19–33%) for MHT and 10% (95% CI 6–17%) for WCH (Figure 5). Meta-regression analyses did not reveal any association between the prevalence of MHT (P = 0.12) and WCH (P = 0.065), respectively, and the severity of renal impairment [mean estimated glomerular filtration rate (eGFR)].
FIGURE 5:
Average prevalence of MHT and WCH.
Nominally, MHT was common among patients with apparently controlled HTN, according to OBP, with a prevalence of 36 and 40% in two surveys by Tiryaki et al. [40] and Kayrak et al. [12], respectively. Similarly, in a cross-sectional study involving 92 stable KTRs, 36% of patients had MHT and no patient had WCH [47]. A high discordance (61%) between OBP and ABPM emerged also in a study by Ahmed et al. [8]. In this study, 58% of patients had MHT and more than a half of this proportion (33%) resulted from isolated nocturnal HTN. Conversely, an overall lower disagreement (20%) was observed in the study by Haydar et al. [26].
In a longitudinal study by Mallamaci et al., with an average follow-up of 3.9 years, in ∼37% of outpatient visits, OBP measurements provided indications for changes in antihypertensive therapy discordant from those by 24-h ABPM [66]. In detail, in 12% of all visits, OBP provided a wrong indication to HTN treatment (OBP >140/90 versus 24-h ABPM <130/80 mmHg), whereas in 25% of all visits, it failed to correctly indicate the need of starting or intensifying HTN treatment (OBP <140/90 versus 24-h ABPM >130/80 mmHg).
Circadian BP pattern
Prevalence of abnormal dipping status
Overall, 28 studies [9, 12, 13, 19, 22–33, 35, 37, 38, 41, 43–47, 50, 51, 54] assessed the percentage of nocturnal dipping. Among these, 10 studies [13, 19, 28–32, 35, 37, 50] reported variable proportion of patients with a reverse dipping pattern (i.e. an actual BP rise during nighttime), spanning from 14% [13] to 42% [19]. The proportion of non-dippers was variable across studies, ranging from 8.1% (non-diabetic KTRs without LVH) [46] to 85% (hypertensive KTRs receiving tacrolimus) [43].
A cumulative analysis including 28 studies revealed an average prevalence of non-dipping of 54% (95% CI 45–63%), thus confirming that circadian rhythm is commonly disturbed in KTRs. Meta-regression analysis did not reveal any association between prevalence of non-dipping status and the mean eGFR (P = 0.25).
Kooman et al. [19] reported a nocturnal decrease of 0.42 ± 11.7 (range –35 to 22) mmHg for SBP and 2.3 ± 6.2 (range –10–10) mmHg for DBP. Using the criterion of a ≥10% SBP decrease during sleep defining normal day-to-night BP variability (dipping), a high proportion of non-dippers (94.5%) was observed; among these, 41.6% were reverse dippers.
Gatzka et al. [22] reported a mean nighttime fall of 9 ± 8 mmHg with respect to daytime values, classifying 51% of patients as non-dippers. Stratifying patients according to transplantation vintage in early (130–210 days, n = 15), intermediate (211–348 days, n = 15) and late (356–598 days, n = 15) transplant patients, the dippers percentage increased with the time after transplantation (27% versus 47% versus 73%, respectively), thus reducing the proportion of non-dippers. The more time had passed since KT, the higher was the fall of BP during sleep (r = 0.38, P < 0.01).
In line with this finding was a study by Covic et al. [28]. Defining normal circadian rhythm (dipping status) as a sleep-to-awake ratio >0.92 for SBP and >0.90 for DBP, at 1-month post-transplantation 100% of patients were complete non-dippers (including 80% reverse dippers), whereas, after >1 year, the proportion of non-dippers decreased to 60%, among which 20% were reverse dippers.
Wadei et al. [30] observed 24% of dippers (ΔSBP 13.7 ± 3.8%), 42% non-dippers (ΔSBP 5.2 ± 2.4%) and 34% reverse dippers (ΔSBP −9.1 ± 8.4%). A high proportion of non-dippers, 67.8% [38] and 73% [25], respectively, was observed in two different studies.
A study by Sasak and Ecder [51] recorded different proportions of non-dippers among sustained normotensive (24.1%), WCH (29.4%), MHT hypertensive (31.8%) and sustained-hypertensive patients (25%). Gluskin et al. [50] observed a non-dipping BP pattern in 73% of patients and such an alteration was associated with tacrolimus use (P = 0.020). Among 76 KTRs, the rates of BP dipping, non-dipping and reverse dipping patterns were 26.7, 53.3 and 20%, respectively. Stenehjem et al. [29] observed 82% of non-dippers, according to SBP; among these patients, 39% were reverse dippers. In the RETENAL study [13], there was a high proportion of non-dippers (48%), including 34% of reverse dipper patients.
In a survey by Mallamaci et al. [9], including 172 stable KTRs, 36% of patients had a night–day ratio ≥1, indicating a non-dipping pattern. In a prospective study including 126 kidney recipients followed-up for a mean of 45 ± 11 months [32], 51.5% of patients were classified as non-dippers and 31.1% as reverse dippers.
DISCUSSION
In this systematic review in KT patients, we found a prevalence of uncontrolled HTN of 56% with ABPM and 47% with OBP, and a 44% discordance for the definition of uncontrolled HTN among the two techniques. Thus, in a population at high CV risk like KTRs, ABPM exhibited a considerable disagreement with OBP, revealing a high prevalence of MHT and the non-dipping pattern. The results of this review provide a basis for extending to transplant patients recommendations by NICE [14] and the American US Preventive Services Task Force [15] that the diagnosis of HTN is confirmed with ABPM.
Post-transplant HTN is multifactorial in nature and immunosuppressive drugs, renal transplant artery stenosis, recurrent renal disease, genetic factors, recipient’s native kidney, as well as poor-quality donor kidney all contribute to the high prevalence of HTN in this population [4]. The renal transplant population is a peculiar chronic kidney disease (CKD) population [67], characterized by the chronic use of immunosuppressive drugs and by a history of CKD and dialysis treatment of variable length in most cases.
Previous studies in CKD patients reported a prevalence of WCH ranging from 2% to 41% [68–75] and of uncontrolled MHT ranging from 6% to 51% [68–70, 72–76]. Furthermore, the non-dipping phenomenon, including actual nocturnal HTN, is progressively more frequent at more severe degrees of renal dysfunction [77] and the global prevalence of this alteration ranges from 14% to 75% [69, 70, 74, 75, 77–84]. Importantly, out-of-office BP is superior to OBP for the prediction of CKD progression in pre-dialysis CKD patients and CV outcomes in both pre-dialysis and dialysis patients [68, 70, 71, 74, 85–100]. As remarked, due to the poor diagnostic performance of OBP, both NICE [14] and the American US Preventive Services Task Force [15] recommend ABPM to confirm the diagnosis of HTN if clinic BP is between 140/90 mmHg and 180/120 mmHg, a recommendation based on a thorough literature review and cost–benefit analysis. Such a recommendation is valid for the general population and there are reasons to believe that it should be perhaps applied with more stringency to patients at high risk of CV events [101]. In our meta-analysis, the average prevalence of uncontrolled MHT (26%) was higher than that in a meta-analysis of six studies in CKD patients (8.3%) [102]. In CKD patients, WCH [103] does not pose any excess risk for adverse outcomes, while MHT predicts a 50% and 77% risk excess for CV and kidney outcomes, respectively. Thus the high prevalence of MHT in transplant patients, which is higher than in CKD patients, is of peculiar clinical relevance in this population. We believe that the risk of misdiagnosing HTN by OBP among renal transplant patients is such that the application of ABPM or home BP (if ABPM is not tolerated) for confirming HTN cannot be omitted. HTN is the most relevant modifiable risk factor for CV disease in renal transplant patients and well-controlled BP associates with longer transplant and patient survival [4–6].
Possibly, targeting nocturnal HTN may reduce the high risk for CV events in transplant patients. The prevalence of nocturnal uncontrolled HTN in 172 treated KTRs was as high as 67% [9]. Such an alteration is robustly associated with IMT, underlying a severe degree of atherosclerosis. Furthermore, a reverse dipper pattern emerged as a risk factor for CV events in a prospective study including 126 kidney recipients followed up for a mean of 45 ± 11 months [32]. These observations replicate in a specific population (renal transplant population) findings in the PAMELA (Pressioni Arteriose Monitorate E Loro Associazioni) study [104], a study in the community where the association between nighttime BP and death and CV events was stronger than that of daytime, 24 h ABPM and clinic BP with the same events.
The 2012 KDIGO BP Guideline in CKD did not provide evidence-based recommendations regarding the use of ABPM to evaluate BP in CKD patients, including renal transplant patients [105]. The issue was reconsidered in the 2021 update of the same guidelines [106]. In these guidelines, the recommended method for HTN diagnosis and monitoring is standardized BP measured by automatic recorders [as in the SPRINT (Systolic Blood Pressure Intervention Trial) study [107]]. The authors of these guidelines remarked that in many regions of the world, home BP or ABPM is impractical to be systematically recommended in the CKD population. However, this remark may not apply to renal transplantation. Renal transplantation is a complex, multi-specialty intervention usually delivered in hospitals with adequate human and instrumental resources. Even though we could not make any formal comparison between HTN as detected by standardized BP measurements (SPRINT study approach) and 24-h ABPM, other studies showed that standardized BP has a scarce agreement with 24-h ABPM [108, 109]. Therefore, we believe that the systematic application of the recommendation by NICE and the American US Preventive Services Task Force is also valid for confirming the diagnosis of HTN in high-risk population like transplant patients.
The high heterogeneity of the estimates of HTN prevalence by ABPM and OBP limits the value of the cumulative estimates of this alteration in the transplant population. This phenomenon is not unique to the transplant population. A high heterogeneity was detected also in meta-analyses focusing on the prevalence of HTN in hospital patients [110] or pre-hypertensive subjects [111] or treatment-resistant patients [112]. The prevalence of MHT [113] and WCH [114], two phenotypes common among renal transplant patients, is also very heterogeneous.
In conclusion, MHT, altered circadian BP profile and nocturnal HTN are frequent in KTRs and might contribute to the high renal and CV risk of these patients. These data suggest that the current recommendation by transplant guidelines to diagnose and monitor HTN exclusively by traditional BP measurements may need to be reconsidered.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This article has not received financial support from any institution and represents an original work of the authors.
AUTHORS’ CONTRIBUTIONS
All authors participated in the critical revision of the article for important intellectual content. F.M., J.-M.H., A.Persu, G.W., P.S., M.B., A.O., C.J.F., N.K., B.S., G.L., P.R., L.V., I.N.B. and C.Z. contributed to the study concept and design. A.Pisano and D.B. developed and ran the search strategy, and were responsible for data acquisition, interpretation and analysis. G.D. contributed to the data analysis. C.Z. and A.Pisano drafted the manuscript.
BOX 1. Definitions.
- MHT
Normal OBP but elevated out-of-clinic BP values
- WCH
High OBP but normal out-of-clinic BP values
- Controlled HTN
Optimal BP targets (OBP <140/90 and ABP <130/80 mmHg) in people with HTN (treated with antihypertensive medication)
- Dipping
Normal circadian BP variation (physiological nocturnal BP drop >10% of daytime)
- Non-dipping
Abnormal circadian BP variation, defined as <10% reduction in BP during sleep
- Reverse dipping
Abnormal circadian BP variation, defined as nocturnal BP rise
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part. Authors declare no conflicts of interest related to the present work. P.R. reports consulting for Bayer, G3P, Idorsia and KBP; honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer-Ingelheim, Corvidia, CVRx, Fresenius, Grunenthal, Novartis, Novo Nordisk, Relypsa Inc., a Vifor Pharma Group Company, Sanofi, Sequana Medical, Servier, Stealth Peptides and Vifor Fresenius Medical Care Renal Pharma; and is a Cofounder of CardioRenal. J.-M.H. reports honoraria from Bayer, Astra Zeneca, Vifor Pharma, Alexion, Servier, Boehringer Ingelheim France, MSD. L.V. is a consultant for AstraZeneca, Bayer, Ionis Pharmaceuticals, Sanofi Genzyme and Vifor Pharma Group. M.B. reports honoraria from Vifor Pharma, Servier, Boehringer Ingelheim, Menarini and Bayer. A.O. is the CKJ Editor-in-Chief. C.Z. is member of the CKJ editorial board.
Supplementary Material
Contributor Information
Anna Pisano, CNR-Institute of Clinical Physiology, Clinical Epidemiology and Physiopathology of Renal Diseases and Hypertension, Reggio Calabria, Italy.
Francesca Mallamaci, CNR-Institute of Clinical Physiology, Clinical Epidemiology and Physiopathology of Renal Diseases and Hypertension, Reggio Calabria, Italy.
Graziella D’Arrigo, CNR-Institute of Clinical Physiology, Clinical Epidemiology and Physiopathology of Renal Diseases and Hypertension, Reggio Calabria, Italy.
Davide Bolignano, CNR-Institute of Clinical Physiology, Clinical Epidemiology and Physiopathology of Renal Diseases and Hypertension, Reggio Calabria, Italy; Department of Surgical and Medical Sciences-Magna Graecia, University of Catanzaro, Italy.
Gregoire Wuerzner, Service of Nephrology and Hypertension, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Alberto Ortiz, Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Michel Burnier, Service of Nephrology and Hypertension, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Nada Kanaan, Division of Nephrology, Division of Cardiology, Cliniques Universitaires Saint-Luc and Pole of Cardiovascular Research, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium.
Pantelis Sarafidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Greece.
Alexandre Persu, Division of Cardiology, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium; Pole of Cardiovascular Research, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium.
Charles J Ferro, Department of Renal Medicine, University Hospitals Birmingham and Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK.
Charalampos Loutradis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Greece.
Ioannis N Boletis, Department of Nephrology and Renal Transplantation, Athens Medical School, Laiko Hospital.
Gérard London, FCRIN INI-CRCT Cardiovascular and Renal Clinical Trialists, Manhes Hospital and FCRIN INI-CRCT, Manhes, France.
Jean-Michel Halimi, Service de Néphrologie-Hypertension, Dialyses, Transplantation rénale, CHRU Tours,Tours, France and INSERM SPHERE U1246, Université Tours, Université de Nantes, Tours, France.
Bénédicte Sautenet, Service de Néphrologie-Hypertension, Dialyses, Transplantation rénale, CHRU Tours, Tours, France and INSERM SPHERE U1246, Université Tours, Université de Nantes, Tours, France, and FCRIN INI-CRCT, Nancy, France.
Patrick Rossignol, Université de Lorraine, Inserm 1433 CIC-P CHRU de Nancy, Inserm U1116 and FCRIN INI-CRCT, Nancy, France.
Liffert Vogt, Department of Internal Medicine, Section Nephrology, Amsterdam UMC, University of Amsterdam, The Netherlands.
Carmine Zoccali, CNR-Institute of Clinical Physiology, Clinical Epidemiology and Physiopathology of Renal Diseases and Hypertension, Reggio Calabria, Italy.
REFERENCES
- 1. Weir MR, Burgess ED, Cooper JE et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol 2015; 26: 1248–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kasiske BL, Anjum S, Shah R et al. Hypertension after kidney transplantation. Am J Kidney Dis 2004; 43: 1071–1081 [DOI] [PubMed] [Google Scholar]
- 3. Carpenter MA, John A, Weir MR et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol 2014; 25: 1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pisano A, Bolignano D, Mallamaci F et al. Comparative effectiveness of different antihypertensive agents in kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant 2019; 35: 878–887 [DOI] [PubMed] [Google Scholar]
- 5. Hillebrand U, Suwelack BM, Loley K et al. Blood pressure, antihypertensive treatment, and graft survival in kidney transplant patients. Transpl Int 2009; 22: 1073–1080 [DOI] [PubMed] [Google Scholar]
- 6. Halimi JM, Persu A, Sarafidis PA et al. Optimizing hypertension management in renal transplantation: a call to action. Nephrol Dial Transplant 2017; 32: 1959–1962 [DOI] [PubMed] [Google Scholar]
- 7. Agena F, Prado EDS, Souza PSJr et al. Home blood pressure (BP) monitoring in kidney transplant recipients is more adequate to monitor BP than office BP. Nephrol Dial Transplant 2011; 26: 3745–3749 [DOI] [PubMed] [Google Scholar]
- 8. Ahmed J, Ozorio V, Farrant M, Van Der Merwe W. Ambulatory vs office blood pressure monitoring in renal transplant recipients. J Clin Hypertens (Greenwich) 2015; 17: 46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallamaci F, Tripepi R, Leonardis D et al. Nocturnal hypertension and altered night-day BP profile and atherosclerosis in renal transplant patients. Transplantation 2016; 100: 2211–2218 [DOI] [PubMed] [Google Scholar]
- 10. Staessen JA, Thijs L, Fagard R et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999; 282: 539–546 [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Facchetti R, Bombelli M et al. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension 2006; 47: 846–853 [DOI] [PubMed] [Google Scholar]
- 12. Kayrak M, Gul EE, Kaya C et al. Masked hypertension in renal transplant recipients. Blood Press 2014; 23: 47–53 [DOI] [PubMed] [Google Scholar]
- 13. Fernandez Fresnedo G, Franco Esteve A, Gomez Huertas E et al. Ambulatory blood pressure monitoring in kidney transplant patients: RETENAL study. Transplant Proc 2012; 44: 2601–2602 [DOI] [PubMed] [Google Scholar]
- 14. McManus RJ, Caulfield M, Williams B et al. ; National Institute for Health and Clinical Excellence. NICE hypertension guideline 2011: evidence based evolution. BMJ 2012; 344: e181. [DOI] [PubMed] [Google Scholar]
- 15. Siu AL, U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015; 163: 778–786 [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42019121565
- 18. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kooman JP, Christiaans MH, Boots JM et al. A comparison between office and ambulatory blood pressure measurements in renal transplant patients with chronic transplant nephropathy. Am J Kidney Dis 2001; 37: 1170–1176 [DOI] [PubMed] [Google Scholar]
- 20. Tutal E, Erkmen Uyar M, Uyanik S et al. Hyperviscosity in renal transplant recipients. Transplant Proc 2015; 47: 1165–1169 [DOI] [PubMed] [Google Scholar]
- 21. Lipkin GW, Tucker B, Giles M et al. Ambulatory blood pressure and left ventricular mass in cyclosporin- and non-cyclosporin-treated renal transplant recipients. J Hypertens 1993; 11: 439–442 [DOI] [PubMed] [Google Scholar]
- 22. Gatzka CD, Schobel HP, Klingbeil AU et al. Normalization of circadian blood pressure profiles after renal transplantation. Transplantation 1995; 59: 1270–1274 [PubMed] [Google Scholar]
- 23. Jacobi J, Rockstroh J, John S et al. Prospective analysis of the value of 24-hour ambulatory blood pressure on renal function after kidney transplantation. Transplantation 2000; 70: 819–827 [DOI] [PubMed] [Google Scholar]
- 24. McGregor DO, Olsson C, Lynn KL. Autonomic dysfunction and ambulatory blood pressure in renal transplant recipients. Transplantation 2001; 71: 1277–1281 [DOI] [PubMed] [Google Scholar]
- 25. Marcondes AM, De Lima JJ, Giorgi DM et al. Twenty-four hour blood pressure profile and left ventricular hypertrophy early after renal transplantation. Ren Fail 2002; 24: 207–213 [DOI] [PubMed] [Google Scholar]
- 26. Haydar AA, Covic A, Jayawardene S et al. Insights from ambulatory blood pressure monitoring: diagnosis of hypertension and diurnal blood pressure in renal transplant recipients. Transplantation 2004; 77: 849–853 [DOI] [PubMed] [Google Scholar]
- 27. Baguet JP, Coste D, Bayle F et al. Ambulatory blood pressure variations relative to sitting or standing position in renal transplant patients. Blood Press Monit 2005; 10: 93–96 [DOI] [PubMed] [Google Scholar]
- 28. Covic A, Gusbeth-Tatomir P, Mardare N et al. Dynamics of the circadian blood pressure profiles after renal transplantation. Transplantation 2005; 80: 1168–1173 [DOI] [PubMed] [Google Scholar]
- 29. Stenehjem AE, Gudmundsdottir H, Os I. Office blood pressure measurements overestimate blood pressure control in renal transplant patients. Blood Press Monit 2006; 11: 125–133 [DOI] [PubMed] [Google Scholar]
- 30. Wadei HM, Amer H, Taler SJ et al. Diurnal blood pressure changes one year after kidney transplantation: relationship to allograft function, histology, and resistive index. J Am Soc Nephrol 2007; 18: 1607–1615 [DOI] [PubMed] [Google Scholar]
- 31. Paoletti E, Gherzi M, Amidone M et al. Association of arterial hypertension with renal target organ damage in kidney transplant recipients: the predictive role of ambulatory blood pressure monitoring. Transplantation 2009; 87: 1864–1869 [DOI] [PubMed] [Google Scholar]
- 32. Ibernon M, Moreso F, Sarrias X et al. Reverse dipper pattern of blood pressure at 3 months is associated with inflammation and outcome after renal transplantation. Nephrol Dial Transplant 2012; 27: 2089–2095 [DOI] [PubMed] [Google Scholar]
- 33. Czyzewski L, Wyzgal J, Kolek A. Evaluation of selected risk factors of cardiovascular diseases among patients after kidney transplantation, with particular focus on the role of 24-hour automatic blood pressure measurement in the diagnosis of hypertension: an introductory report. Ann Transplant 2014; 19: 188–198 [DOI] [PubMed] [Google Scholar]
- 34. David VG, Yadav B, Jeyaseelan L et al. Prospective blood pressure measurement in renal transplant recipients. Indian J Nephrol 2014; 24: 154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee MH, Ko KM, Ahn SW et al. The impact of kidney transplantation on 24-hour ambulatory blood pressure in end-stage renal disease patients. J Am Soc Hypertens 2015; 9: 427–434 [DOI] [PubMed] [Google Scholar]
- 36. Rojas-Vega L, Jimenez-Vega AR, Bazua-Valenti S et al. Increased phosphorylation of the renal Na+-Cl– cotransporter in male kidney transplant recipient patients with hypertension: a prospective cohort. Am J Physiol Renal Physiol 2015; 309: F836–F842 [DOI] [PubMed] [Google Scholar]
- 37. Tarta D, Denise CC, Cristina T, Frigy A et al. Office assessed blood pressure and ambulatory blood pressure monitoring in chronic kidney disease patients versus kidney transplant recipients. Acta Medica Marisiensis 2015; 61: 91–93 [Google Scholar]
- 38. Kendirlinan Demirkol O, Oruc M, Ikitimur B et al. Ambulatory blood pressure monitoring and echocardiographic findings in renal transplant recipients. J Clin Hypertens (Greenwich) 2016; 18: 766–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mallamaci F, D’Arrigo G, Tripepi R et al. Office, standardized and 24-h ambulatory blood pressure and renal function loss in renal transplant patients. J Hypertens 2018; 36: 119–125 [DOI] [PubMed] [Google Scholar]
- 40. Tiryaki O, Usalan C, Kul S et al. Urinary angiotensinogen level is increased in renal transplant recipients with masked hypertension and is correlated with left ventricular mass index and albuminuria in these patients. Clin Transplant 2018; 32: e13375. [DOI] [PubMed] [Google Scholar]
- 41. Goldsmith DJ, Covic AC, Venning MC et al. Ambulatory blood pressure monitoring in renal dialysis and transplant patients. Am J Kidney Dis 1997; 29: 593–600 [DOI] [PubMed] [Google Scholar]
- 42. Frank H, Schmieder RE, Vogt-Ladner G et al. Determinants of left ventricular structure after kidney transplantation. Transplant Proc 2000; 32: 2801–2806 [DOI] [PubMed] [Google Scholar]
- 43. Steigerwalt SP, Brar N, Dhungel A et al. Improved 24-hour blood pressure control with sirolimus versus calcineurin inhibitor based immunosuppression in renal transplant recipients. Transplant Proc 2009; 41: 4184–4187 [DOI] [PubMed] [Google Scholar]
- 44. Beltran S, Crespo J, Kanter J et al. Ambulatory blood pressure monitoring in renal transplant patients: should it be routinely performed? Transplant Proc 2010; 42: 2868–2870 [DOI] [PubMed] [Google Scholar]
- 45. Wen KC, Gourishankar S. Evaluating the utility of ambulatory blood pressure monitoring in kidney transplant recipients. Clin Transplant 2012; 26: E465–E470 [DOI] [PubMed] [Google Scholar]
- 46. Sezer S, Uyar ME, Colak T et al. Left ventricular mass index and its relationship to ambulatory blood pressure and renal resistivity index in renal transplant recipients. Transplant Proc 2013; 45: 1575–1578 [DOI] [PubMed] [Google Scholar]
- 47. Azancot MA, Ramos N, Moreso FJ et al. Hypertension in chronic kidney disease: the influence of renal transplantation. Transplantation 2014; 98: 537–542 [DOI] [PubMed] [Google Scholar]
- 48. Ozkayar N, Altun B, Yildirim T et al. Blood pressure measurements, blood pressure variability and endothelial function in renal transplant recipients. Clin Exp Hypertens 2014; 36: 392–397 [DOI] [PubMed] [Google Scholar]
- 49. Firat A, Kaya B, Balal M et al. ; The Department of Internal Medicine, Cukurova University Faculty of Medicine, Adana, Turkey. Relationship between peripheral-central blood pressure and cardiac-renal damage in kidney transplant recipients. Exp Clin Transplant 2019; 17: 188–194 [DOI] [PubMed] [Google Scholar]
- 50. Gluskin E, Tzukert K, Mor-Yosef Levi I et al. Ambulatory monitoring unmasks hypertension among kidney transplant patients: single center experience and review of the literature. BMC Nephrol 2019; 20: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sasak G, Ecder SA. Masked hypertension and obesity in renal transplant recipients. Transplant Proc 2019; 51: 2355–2357 [DOI] [PubMed] [Google Scholar]
- 52. Sennesael J, Lamote J, Violet I et al. Comparison of perindopril and amlodipine in cyclosporine-treated renal allograft recipients. Hypertension 1995; 26: 436–444 [DOI] [PubMed] [Google Scholar]
- 53. Galiatsou E, Morris ST, Jardine AG et al. Cardiac and vascular abnormalities in renal transplant patients: differential effects of cyclosporin and azathioprine. J Nephrol 2000; 13: 185–192 [PubMed] [Google Scholar]
- 54. Oliveras A, Vazquez S, Hurtado S et al. Ambulatory blood pressure monitoring in renal transplant patients: modifiable parameters after active antihypertensive treatment. Transplant Proc 2004; 36: 1352–1354 [DOI] [PubMed] [Google Scholar]
- 55. Schneider S, Promny D, Sinnecker D et al. Impact of sympathetic renal denervation: a randomized study in patients after renal transplantation (ISAR-denerve). Nephrol Dial Transplant 2015; 30: 1928–1936 [DOI] [PubMed] [Google Scholar]
- 56. Hypertension control: report of a WHO expert committee. World Health Organization Technical Report Series, Vol. 862. Geneva, Switzerland: World Health Organization, 1996; 1–83 [PubMed] [Google Scholar]
- 57. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Int Med 1997; 157: 2413–2446 [DOI] [PubMed] [Google Scholar]
- 58. Chobanian AV, Bakris GL, Black HR et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 59. Mancia G, Fagard R, Narkiewicz K et al. ; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357 [DOI] [PubMed] [Google Scholar]
- 60. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3): S1–S155 [DOI] [PubMed] [Google Scholar]
- 61. James PA, Oparil S, Carter BL et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA 2014; 311: 507–520 [DOI] [PubMed] [Google Scholar]
- 62. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053 [DOI] [PubMed] [Google Scholar]
- 63. O’Brien E, Asmar R, Beilin L et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003; 21: 821–848 [DOI] [PubMed] [Google Scholar]
- 64. Staessen JA, O’Brien ET. Development of diagnostic thresholds for automated measurement of blood pressures in adults. Blood Press Monit 1999; 4: 127–136 [PubMed] [Google Scholar]
- 65. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43 (5 Suppl 1): S1–S290 [PubMed] [Google Scholar]
- 66. Mallamaci F, Tripepi R, D’Arrigo G et al. Long-term blood pressure monitoring by office and 24-h ambulatory blood pressure in renal transplant patients: a longitudinal study. Nephrol Dial Transplant 2019; 34: 1558–1564 [DOI] [PubMed] [Google Scholar]
- 67. Levin A, Stevens P, Bilous RW et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 68. Terawaki H, Metoki H, Nakayama M et al. Masked hypertension determined by self-measured blood pressure at home and chronic kidney disease in the Japanese general population: the Ohasama study. Hypertens Res 2008; 31: 2129–2135 [DOI] [PubMed] [Google Scholar]
- 69. Pogue V, Rahman M, Lipkowitz M et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53: 20–27 [DOI] [PubMed] [Google Scholar]
- 70. Kanno A, Metoki H, Kikuya M et al. Usefulness of assessing masked and white-coat hypertension by ambulatory blood pressure monitoring for determining prevalent risk of chronic kidney disease: the Ohasama study. Hypertens Res 2010; 33: 1192–1198 [DOI] [PubMed] [Google Scholar]
- 71. Minutolo R, Agarwal R, Borrelli S et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med 2011; 171: 1090–1098 [DOI] [PubMed] [Google Scholar]
- 72. Shafi S, Sarac E, Tran H. Ambulatory blood pressure monitoring in patients with chronic kidney disease and resistant hypertension. J Clin Hypertens (Greenwich) 2012; 14: 611–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gorostidi M, Sarafidis PA, de la Sierra A et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: a 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis 2013; 62: 285–294 [DOI] [PubMed] [Google Scholar]
- 74. Cha RH, Lee H, Lee JP et al. Changes of blood pressure patterns and target organ damage in patients with chronic kidney disease: results of the APrODiTe-2 study. J Hypertens 2017; 35: 593–601 [DOI] [PubMed] [Google Scholar]
- 75. Oh YK, Chin HJ, Ahn SY et al. Discrepancies in clinic and ambulatory blood pressure in Korean chronic kidney disease patients. J Korean Med Sci 2017; 32: 772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Agarwal R, Pappas MK, Sinha AD. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol 2016; 27: 924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mojón A, Ayala DE, Piñeiro L et al. ; on behalf of the Hygia Project Investigators. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int 2013; 30: 145–158 [DOI] [PubMed] [Google Scholar]
- 78. Timio M, Venanzi S, Lolli S et al. “Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol 1995; 43: 382–387 [PubMed] [Google Scholar]
- 79. Jacob P, Hartung R, Bohlender J et al. Utility of 24-h ambulatory blood pressure measurement in a routine clinical setting of patients with chronic renal disease. J Hum Hypertens 2004; 18: 745–751 [DOI] [PubMed] [Google Scholar]
- 80. Crespo JJ, Piñeiro L, Otero A et al. ; on behalf of the Hygia Project Investigators. Administration-time-dependent effects of hypertension treatment on ambulatory blood pressure in patients with chronic kidney disease. Chronobiol Int 2013; 30: 159–175 [DOI] [PubMed] [Google Scholar]
- 81. Rahman M, Greene T, Phillips RA et al. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension 2013; 61: 82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang C, Zhang J, Liu X et al. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One 2013; 8: e55419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fedecostante M, Spannella F, Cola G et al. Chronic kidney disease is characterized by "double trouble" higher pulse pressure plus night-time systolic blood pressure and more severe cardiac damage. PLoS One 2014; 9: e86155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Che X, Mou S, Zhang W et al. The impact of non-dipper circadian rhythm of blood pressure on left ventricular hypertrophy in patients with non-dialysis chronic kidney disease. Acta Cardiol 2017; 72: 149–155 [DOI] [PubMed] [Google Scholar]
- 85. Amar J, Vernier I, Rossignol E et al. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int 2000; 57: 2485–2491 [DOI] [PubMed] [Google Scholar]
- 86. Tripepi G, Fagugli RM, Dattolo P et al. Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int 2005; 68: 1294–1302 [DOI] [PubMed] [Google Scholar]
- 87. Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69: 1175–1180 [DOI] [PubMed] [Google Scholar]
- 88. Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006; 69: 406–411 [DOI] [PubMed] [Google Scholar]
- 89. Agarwal R, Andersen MJ. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol 2006; 26: 503–510 [DOI] [PubMed] [Google Scholar]
- 90. Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2007; 2: 1228–1234 [DOI] [PubMed] [Google Scholar]
- 91. Agarwal R, Andersen MJ, Light RP. Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. Am J Nephrol 2008; 28: 210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Okada T, Nakao T, Matsumoto H, Nagaoka Y. Value of morning home blood pressure as a predictor of decline in renal function in patients with chronic kidney disease. Am J Nephrol 2008; 28: 982–989 [DOI] [PubMed] [Google Scholar]
- 93. Agarwal R, Kariyanna SS, Light RP. Prognostic value of circadian blood pressure variation in chronic kidney disease. Am J Nephrol 2009; 30: 547–553 [DOI] [PubMed] [Google Scholar]
- 94. Okada T, Nakao T, Matsumoto H et al. Prognostic significance of home blood pressure control on renal and cardiovascular outcomes in elderly patients with chronic kidney disease. Hypertens Res 2009; 32: 1123–1129 [DOI] [PubMed] [Google Scholar]
- 95. Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension 2010; 55: 762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Redon J, Plancha E, Swift PA et al. Nocturnal blood pressure and progression to end-stage renal disease or death in nondiabetic chronic kidney disease stages 3 and 4. J Hypertens 2010; 28: 602–607 [DOI] [PubMed] [Google Scholar]
- 97. McMullan CJ, Hickson DA, Taylor HA et al. Prospective analysis of the association of ambulatory blood pressure characteristics with incident chronic kidney disease. J Hypertens 2015; 33: 1939–1946 [DOI] [PubMed] [Google Scholar]
- 98. McMullan CJ, Yano Y, Bakris GL et al. Racial impact of diurnal variations in blood pressure on cardiovascular events in chronic kidney disease. J Am Soc Hypertens 2015; 9: 299–306 [DOI] [PubMed] [Google Scholar]
- 99. Turak O, Afsar B, Siriopol D et al. Morning blood pressure surge as a predictor of development of chronic kidney disease. J Clin Hypertens (Greenwich) 2016; 18: 444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Y, Deng Q, Li H et al. Prognostic value of nighttime blood pressure load in Chinese patients with nondialysis chronic kidney disease. J Clin Hypertens (Greenwich) 2017; 19: 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. de la Sierra A, Banegas JR, Segura J et al. ; CARDIORISC Event Investigators. Ambulatory blood pressure monitoring and development of cardiovascular events in high-risk patients included in the Spanish ABPM registry: the CARDIORISC Event study. J Hypertens 2012; 30: 713–719 [DOI] [PubMed] [Google Scholar]
- 102. Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol 2009; 4: 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rahman M, Wang X, Bundy JD et al. ; CRIC Study Investigators. Prognostic significance of ambulatory BP monitoring in CKD: a report from the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol 2020; 31: 2609–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sega R, Facchetti R, Bombelli M et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111: 1777–1783 [DOI] [PubMed] [Google Scholar]
- 105. KDIGO BP Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2012; Suppl 2: 337–414 [Google Scholar]
- 106. Cheung AK, Chang TI, Cushman WC et al. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021; 99: S1–S87 [DOI] [PubMed] [Google Scholar]
- 107. Wright JT Jr, Williamson Jeff; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Drawz PE, Pajewski NM, Bates JT et al. ; for the SPRINT Study Research Group. Effect of intensive versus standard clinic-based hypertension management on ambulatory blood pressure: results from the SPRINT (systolic blood pressure intervention trial) ambulatory blood pressure study. Hypertension 2017; 69: 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Agarwal R. Implications of blood pressure measurement technique for implementation of systolic blood pressure intervention trial (SPRINT). J Am Heart Assoc 2017; 6: e004536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Armitage LC, Whelan ME, Watkinson PJ et al. Screening for hypertension using emergency department blood pressure measurements can identify patients with undiagnosed hypertension: a systematic review with meta-analysis. J Clin Hypertens (Greenwich) 2019; 21: 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Guo X, Zheng L, Zhang X et al. The prevalence and heterogeneity of prehypertension: a meta-analysis and meta-regression of published literature worldwide. Cardiovasc J Afr 2012; 23: 44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Noubiap JJ, Nansseu JR, Nyaga UF et al. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart 2019; 105: 98–105 [DOI] [PubMed] [Google Scholar]
- 113. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens 2008; 21: 969–975 [DOI] [PubMed] [Google Scholar]
- 114. Noubiap JJ, Nansseu JR, Nkeck JR et al. Prevalence of white coat and masked hypertension in Africa: a systematic review and meta-analysis. J Clin Hypertens 2018; 20: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.