ABSTRACT
Background
Previous studies have suggested that long-term exposure to air pollution increases the risk of chronic kidney disease and its progression. However, the effect of air pollution on the risk of acute kidney injury (AKI) has not been studied. We aim to evaluate the transient effect of air pollution on the risk of hospital-acquired AKI (HA-AKI).
Methods
We selected from the Epidemiology of AKI in Chinese Hospitalized patients cohort AKI cases in which the onset date could be unambiguously determined. We obtained city-specific daily averages of the ambient level of particulate matter (2.5 μm and 10 μm), carbon monoxide, nitrogen dioxide (NO2), sulfur dioxide (SO2) and ozone (O3) from the Ministry of Environmental Protection of China. We used the time-stratified case-crossover approach to examine the association between the ambient level of air pollutants and the risk of HA-AKI in the selected cases.
Results
A total of 11 293 AKI cases that met the inclusion and exclusion criteria were selected. In univariable analysis, the ambient levels of NO2 and SO2 were significantly associated with the risk of HA-AKI. In the multivariable analysis that incorporated all six pollutants in the same model, NO2 was the sole pollutant whose level remained associated with the risk of AKI (P < 0.001). The relationship between the level of NO2 and the risk of HA-AKI appeared to be linear, with an estimated odds ratio of 1.063 (95% confidence interval 1.026–1.101) for each increment of 1 median absolute deviation in the exposure. The association was consistent across the subgroups stratified by age, gender, baseline estimated glomerular filtration rate, AKI severity, need for intensive care and season.
Conclusions
Higher ambient levels of NO2 are associated with an increased risk of HA-AKI in hospitalized adults in China.
Keywords: air pollution, case-crossover, hospital-acquired acute kidney injury, nitrogen dioxide
INTRODUCTION
The burden of disease and death attributable to air pollution is becoming a public health challenge worldwide, especially in developing countries [1, 2]. Numerous experimental and epidemiological studies have demonstrated that air pollution adversely affects respiratory and cardiocerebral vascular health [3–5]. Recently, long-term exposure to air pollution has also been linked to an increased risk of chronic kidney disease (CKD) in different populations [6–8]. However, the transient effect of air pollution on acute kidney diseases has not been fully studied in humans.
Acute kidney injury (AKI), characterized by an abrupt loss of kidney function, is a common and serious complication observed in hospitalized patients and is associated with increased mortality and adverse outcomes, including development and progression of CKD [9–12]. Maladaptive repairs after repeated AKIs have been recognized as a major pathological cause for CKD [13, 14]. Thus we hypothesized that exposure to air pollution may also increase the risk of hospital-acquired AKI (HA-AKI). Case-crossover is a design for investigating the transient effects of an exposure on the onset of acute outcomes [15] and is frequently used in studying the acute adverse effects of air pollution on health [16, 17]. A key feature of this design is that it requires only cases and each case serves as its own control, thus eliminating confounding by subject characteristics such as overall health status and presence of risk factors.
In this study we used a time-stratified case-crossover design to study the association between the ambient level of air pollutants, including particulate matter [2.5 μm (PM2.5) and 10 μm (PM10)], nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and ozone (O3) and AKI in 11 293 AKI cases identified from the Epidemiology of AKI in Chinese Hospitalized patients (EACH) study [18, 19].
MATERIALS AND METHODS
Study population
The study population was drawn from the EACH study, a retrospective cohort of 3 044 224 inpatients admitted from 2013 to 2015 at 25 tertiary academic medical centers across China [18, 19]. We obtained patient-level data from the electronic hospitalization databases and laboratory databases from the participating centers. The hospitalization records consisted of the patients’ age, sex, date of admission, diagnosis at admission, date of discharge, operational procedures, total cost incurred during the hospitalization and survival status at discharge. The laboratory data included the values and time of serum creatinine tests. The current study included 11 293 hospitalized adults with AKI whose onset dates of AKI could be unambiguously determined. The flowchart of the study population and the patients selected for subsequent analyses is presented in Supplementary data, Figure S1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Nanfang Hospital (NFEC-2014-098) and individual consent for this retrospective analysis was waived.
Identification of AKI cases and determination of the onset dates
Assessment of the transient effect of air pollution on HA-AKI requires accurate determination of the onset date of AKI, which is only possible when serum creatinine is measured daily. In many AKI cases, the onset date cannot be accurately determined due to long intervals between serum creatinine tests. We screened patients’ creatinine data for HA-AKI events of which the onset date could be determined within ±1 day. Specifically, the creatinine data of every qualified event met the following criteria: having three consecutive creatinine tests, with values of c1, c2 and c3 and testing times of t1, t2 and t3, respectively; the time interval was ≤30 days between the first two tests (t2 − t1 ≤ 30 days) and ≤48 h between the second and third test (t3 − t2 ≤ 48 h); there was no evidence of AKI between t1 and t2, indicated by |c2 – c1| < 26.5 μmol/L and c2/c1 < 1.5 and c1/c2 < 1.5; and there was evidence of AKI between t2 and t3, indicated by c3 − c2 ≥ 26.5 μmol/L or c3/c2 ≥ 1.5. The onset date of AKI was defined as the midpoint between the second and third test (t2 and t3). Since the true onset time of these AKI cases was bounded by t2 and t3 and the time interval between t2 and t3 was ≤48 h, the estimated onset time (midpoint between t2 and t3) should be within 1 day of the true onset time. For patients with multiple qualified events, only the earliest one was selected.
Exposure to air pollution
We obtained air pollution data for each city from 1 January 2014 to 31 December 2015, including daily average concentrations of PM2.5, PM10, NO2, CO, SO2 and O3, from the Ministry of Environmental Protection of China (http://zhb.gov.cn/hjzl/). Since daily air pollution data before 1 January 2014 were not available for most cities, we only included HA-AKI cases with onset dates within 2014–2015 in the analysis. We used the daily average of each air pollutant in the city where the hospital resided as the exposure of the inpatients. We also obtained the daily average temperature data of each city over the same period from the Chinese weather website (http://www.tianqi.com).
We staged AKI using the peak creatinine level within 14 days after the onset date, according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, and classified Stages 2 and 3 AKI as severe AKI.
Statistical analysis
We used the time-stratified case-crossover approach [15] to examine the association between the daily average of each air pollutant (PM2.5, PM10, NO2, SO2, CO and O3) and HA-AKI in the selected AKI cases. In the analysis, we excluded the AKI cases that fell into one of the following categories: age <18 or ≥100 years, baseline estimated glomerular filtration rate (eGFR) <30 mL/min and extremely low baseline creatinine values (<40 μmol/L). We used the onset dates of AKI to index the case days and selected the days that matched the onset dates by the year, the month and the day of the week as controls. We performed conditional Poisson regression analyses to estimate the effects of each air pollutant on AKI with different lag times (Lag 0, 1, 2 or 3 days) and with adjustment for the daily temperature (single-pollutant models). We used these values of lag time to accommodate the possible lags between the time of exposure and AKI onset. We standardized the estimated effect size using the median absolute deviation (MAD) of each air pollutant, because the distributions of air pollutants were far from normal and MAD is a more robust statistic for the spread of distribution than standard deviation. We plotted the smoothed curves of the ambient level of each air pollutant and the risk of HA-AKI. We also performed analyses that included the six air pollutants in a single regression model (six-pollutant model).
We further performed analyses to examine possible modifications of the effects of the air pollutant by age (>60 years and ≤60 years), gender (male and female), baseline eGFR (>60 and ≤60 mL/min/1.73 m2), AKI severity (mild and severe), need for intensive care and season (quarter of the year).
We performed all statistical analyses using R software, version 3.6.1 with package gnm version 1.1-1 (R Foundation for Statistical Computing, Vienna, Austria. All statistical tests were two-sided and P-values <0.05 were considered statistically significant.
RESULTS
We screened the serum creatinine data in inpatients of the EACH cohort and identified a total of 16 886 HA-AKI cases with unambiguous onset time (±1 day) according to the KDIGO criteria. We excluded the AKI cases with any of the following conditions: age <18 or ≥100 years, baseline eGFR <30 mL/min/1.73 m2 or baseline creatinine ≤40 μmol/L (Supplementary data, Figure S1). A total of 11 293 cases that met the inclusion and exclusion criteria were selected and their characteristics were summarized in Table 1. Among these, 3175 (28.1%) were severe AKI (Stage 2 or 3). The median age was 61.6 years, 67.5% were males, the median eGFR at baseline was 82 mL/min/1.73 m2 and 32.3% required intensive care during hospitalization. The number of HA-AKI cases distributed evenly among the four quarters of the year.
Table 1.
Characteristics of the selected HA-AKI cases by AKI severitya
| Variable | Mild AKI | Severe AKI | Total |
|---|---|---|---|
| (n = 8118) | (n = 3175) | (n = 11 293) | |
| Age (years), median (q25–q75) | 61.4 (50.0–72.0) | 61.9 (50.0–72.8) | 61.6 (50.0–72.1) |
| Male, n (%) | 5480 (67.5) | 2144 (67.5) | 7624 (67.5) |
| Baseline creatinine (μmol/L), median (q25–q75) | 85 (66–111) | 76 (58–100) | 82 (63–108) |
| eGFR (mL/min), median (q25–q75) | 78 (55–97) | 86 (62–102) | 80 (57–99) |
| Need intensive care, n (%) | 2691 (33.1) | 962 (30.3) | 3653 (32.3) |
| Season of onset, n (%) | |||
| Q1 | 1982 (24.4) | 802 (25.3) | 2784 (24.7) |
| Q2 | 2086 (25.7) | 796 (25.1) | 2882 (25.5) |
| Q3 | 1995 (24.6) | 763 (24.0) | 2758 (24.4) |
| Q4 | 2055 (25.3) | 814 (25.6) | 2869 (25.4) |
| Temperature at onsetb, °C | 21 (13, 26) | 20 (12, 26) | 21 (13, 26) |
q25, 25th quantile; q75, 75th quantile.
Severe AKI is defined as Stage 2 or 3 AKI.
The distributions of the exposure level in the study population, measured as the daily average concentration of the air pollutants, are summarized in Table 2. The correlations were high between PM2.5 and PM10 (r = 0.91); intermediate among PM, NO2, SO2 and CO (r = 0.5–0.7); and low between O3 and other pollutants (r < 0.2) (Supplementary data, Table S1).
Table 2.
Distribution of daily average concentration of air pollutants
| Air pollutant | Minimum | q25 | Median | q75 | Maximum | MAD |
|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 6 | 32 | 49 | 72 | 545 | 27.9 |
| PM10 (μg/m3) | 4 | 52 | 93 | 118 | 977 | 46.7 |
| NO2 (μg/m3) | 5 | 34 | 45 | 57 | 146 | 16.9 |
| SO, (μg/m3) | 3 | 11 | 16 | 24 | 145 | 8.5 |
| CO (mg/m3) | 0.37 | 0.79 | 0.97 | 1.22 | 6.01 | 0.30 |
| O3 (μg/m3) | 5 | 63 | 100 | 152 | 496 | 62.3 |
q25, 25th quantile; q75, 75th quantile.
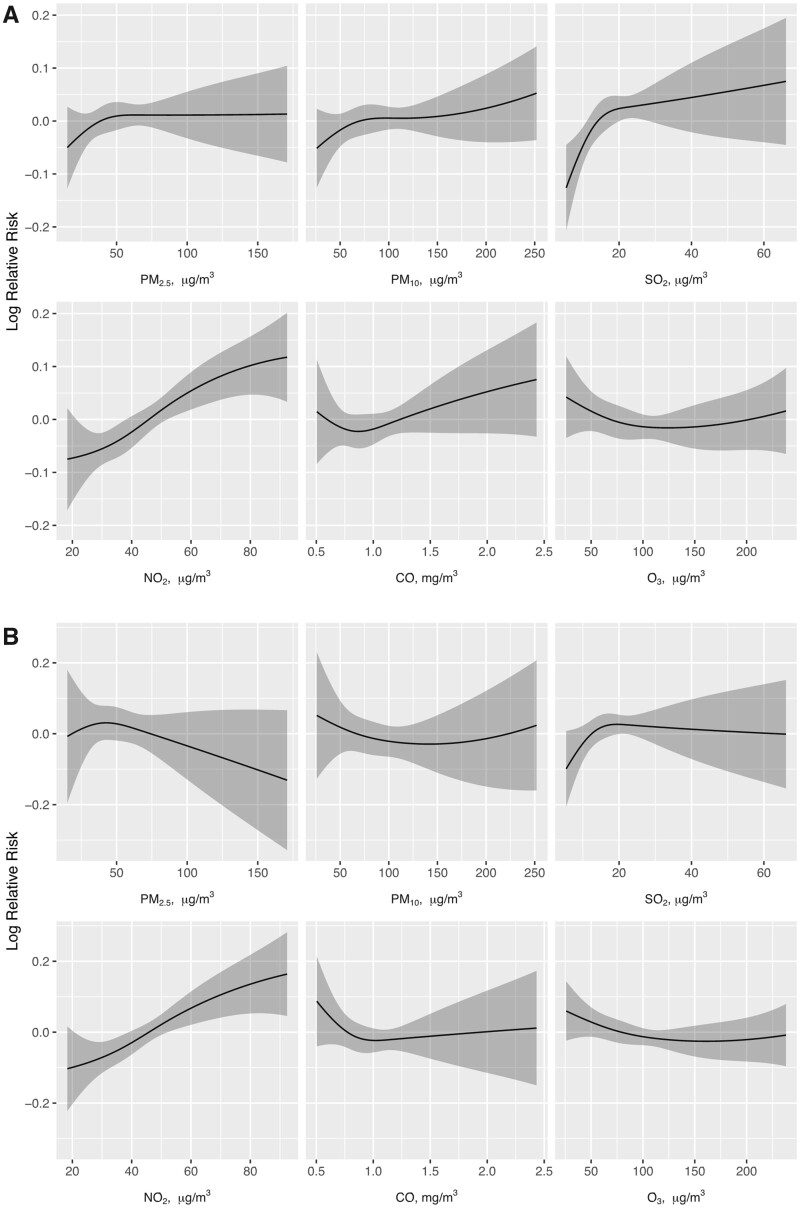
In the analyses of single-pollutant models, daily average levels of NO2 and SO2 were significantly associated with the risk of HA-AKI (Table 3). The association was the strongest when the lag time was 1–2 days and substantially attenuated with increased lag time. PM2.5, PM10, CO and O3 were not significantly associated with the risk of HA-AKI regardless of the lag time. In the analysis that included all six pollutants in a single model, NO2 was the sole pollutant whose concentration remained to be associated with the risk of HA-AKI, with an estimated relative risk (RR) of 1.063 [95% confidence interval (CI) 1.026–1.101] for each increment of one MAD in the exposure (P < 0.001). The relationship between the level of NO2 and the risk of HA-AKI appeared to be linear (Figure 1).
Table 3.
RRs of HA-AKI by a 1 MAD increase in the ambient level of air pollutants
| Pollutant | RR (95% CI) from single-pollutant modelsa |
RR (95% CI) from six-pollutant model with 1-day lag | |||
|---|---|---|---|---|---|
| No lag | 1-day lag | 2-day lag | 3-day lag | ||
| PM2.5 | 1.000 (0.981, 1.020) | 1.008 (0.987, 1.028) | 1.006 (0.986, 1.026) | 1.006 (0.986, 1.026) | 0.963 (0.922, 1.007) |
| PM10 | 1.009 (0.986, 1.032) | 1.022 (0.999, 1.047) | 1.018 (0.994, 1.042) | 1.023 (0.999, 1.047) | 1.029 (0.980, 1.082) |
| NO2 | 1.042 (1.016, 1.067) | 1.051 (1.025, 1.077) | 1.046 (1.020, 1.072) | 1.036 (1.011, 1.062) | 1.063 (1.026, 1.101) |
| SO2 | 1.021 (1.001,1.041) | 1.022 (1.002, 1.043) | 1.030 (1.010, 1.051) | 1.017 (0.997, 1.038) | 1.000 (0.975, 1.026) |
| CO | 1.009 (0.987, 1.031) | 1.014 (0.992, 1.036) | 1.009 (0.987, 1.032) | 1.008 (0.986, 1.030) | 0.996 (0.964, 1.029) |
| O3 | 1.003 (0.971, 1.036) | 0.997 (0.965, 1.030) | 1.031 (0.998, 1.065) | 1.012 (0.979, 1.046) | 0.988 (0.955, 1.022) |
RRs (95% CIs) were estimated from the conditional Poisson regression models with adjustment for ambient temperature and scaled by 1 MAD of the daily concentration of the air pollutant. Since a 1-day lag produced the strongest association in the single-pollutant models, the six-pollutant model was analyzed with a 1-day lag only.
FIGURE 1:
The smooth curves of the ambient levels of air pollutants and the RR of HA-AKI. (A) The effect of the daily average of each air pollutant on the risk of HA-AKI was estimated from single-pollutant models. (B) The effect of the daily average of each air pollutant on the risk of HA-AKI was estimated from the six-pollutant model that included all six air pollutants in a single regression model. The 95% CIs of the estimates are given by the shaded areas.
In the subgroup analyses, the effect of exposure to NO2 on the risk of HA-AKI did not significantly differ among the subgroups stratified by age (≤60 years), gender, baseline eGFR (≤60 mL/min), AKI severity, need for intensive care and season (Table 4).
Table 4.
Subgroup analyses of the effect of ambient NO2 on the RR of HA-AKI
| Variable | Subgroup | n | RR (95% CI)a | P for interaction |
|---|---|---|---|---|
| Age (years) | ≤60 | 5118 | 1.082 (1.036–1.130) | – |
| >60 | 6175 | 1.054 (1.010–1.099) | 0.29 | |
| Gender | Male | 7624 | 1.052 (1.012–1.095) | – |
| Female | 3669 | 1.098 (1.044–1.154) | 0.11 | |
| Baseline eGFR (mL/min/1.73 m2) | ≤60 | 8137 | 1.068 (1.027–1.110) | – |
| >60 | 3156 | 1.066 (1.011–1.123) | 0.95 | |
| AKI severity | Mild | 8118 | 1.061 (1.021–1.103) | – |
| Severe | 3175 | 1.082 (1.026–1.141) | 0.50 | |
| Intensive care | Yes | 3653 | 1.081 (1.030–1.135) | – |
| No | 7640 | 1.058 (1.016–1.102) | 0.42 | |
| Season | Q1 | 2784 | 1.085 (1.032–1.141) | – |
| Q2 | 2882 | 1.096 (1.027–1.169) | 0.80 | |
| Q3 | 2758 | 1.037 (0.959–1.121) | 0.31 | |
| Q4 | 2869 | 1.045 (0.992–1.100) | 0.22 |
RRs (95% CIs) were scaled by 1 MAD of the daily concentration of NO2.
DISCUSSION
To the best of our knowledge, this is the first population study to evaluate the transient effect of air pollution on the risk of HA-AKI. We observed a linear relationship between the ambient level of NO2 and the risk of AKI. Every MAD increase in ambient NO2 was associated with a 6.3% increase in the risk of AKI. The association was consistent across the subgroups stratified by age, gender, baseline eGFR, AKI severity, need for intensive care and season. The ambient level of SO2 was also associated with the risk of AKI in the single-pollutant analysis. However, the association disappeared after adjusting for the level of NO2.
More than 90% of the AKI events are attributed to ischemic injuries [20]. Previous studies have shown that exposure to air pollution increases the risk of ischemic cardiocerebral vascular disease, such as acute myocardial infraction (AMI) and stroke [21, 22]. Similar to what we observed in the cases of HA-AKI, a recent retrospective analysis found that moderate to high levels of NO2 were significantly associated with a higher risk of AKI hospitalization, while O3 was not shown to be a risk factor for AKI [23]. A large study in Alberta, Canada [24] found that a higher ambient level of NO2, but not CO, NO, O3 or PM2.5, was associated with an elevated risk of AMI, and the association was the strongest with a 1-day lag. Meanwhile, the ambient level of NO2 has also been linked to the risk of ischemic stroke [25] and vascular dementia [26]. In animal models, there is a dose–response effect of exposure to NO2 on endothelial dysfunction and inflammatory response [27]. We speculate that ischemic AKI, together with AMI and ischemic stroke, share similar etiological pathways in acute response to changes in ambient NO2. It is worth noting that an elevated ambient level of NO2 may not directly cause AKI, but rather increases an individual’s susceptibility to AKI when a causing factor is present.
Previous studies have implicated long-term exposure to air pollution in the development and progression of CKD [6, 7]. Repeated AKIs have been recognized as an important cause in the etiology of CKD. Thus it is plausible that the observed effect of air pollution on the risk of CKD may be partially mediated by the increased risk of AKI as a result of elevated exposure. Among the six pollutants examined in our study, only the ambient NO2 level appeared to be independently associated with the risk of AKI. In comparison, the associated patterns between air pollution and the risk of CKD are more complex, as multiple pollutants, including PM2.5, PM10, NO2 and CO, are implicated. Further studies are needed to fully understand these differences and the underlying mechanisms.
Our study has several strengths. First, accurate determination of the onset time of AKI is crucial for estimating the transient effect of air pollution on AKI. The large size of the EACH cohort with time-stamped serum creatinine data allowed us to identify a sufficient number of AKI cases with well-defined onset dates. Second, we used a time-stratified case-crossover design in the association analysis, which is robust to possible confounding by the characteristics of the patients, such as overall health status and the presence of risk factors. Our study also has limitations. First, since all AKI cases occurred during hospitalization, use of the exposure level at the exact locations of the participating hospitals would be desirable. Instead, we used the average levels of the air pollutants in the cities where the participating hospitals were located as surrogates for the exposure. This may result in an underestimation of the effect of air pollution. Second, our study cohort was from China, where the level of air pollution is much higher than in many developed countries. Whether our findings can be generalized to other populations needs further investigation.
In summary, higher ambient levels of NO2 were significantly associated with an increased risk of AKI in hospitalized patients in China. Ambient NO2 is mainly derived from traffic and industrial fuel combustion and has been recognized as an important driver of increasing greenhouse gas emissions globally [28, 29]. Our findings call for more awareness among policymakers, industries, medical researchers and the public of the adverse effects of air pollution on kidney health.
SUPPLEMENTARY MATERIAL
Supplementary data are available at ckj online.
FUNDING
This work was supported by the National Natural Science Foundation of China (Key Program 82030022), National Natural Science Foundation of China (81770683, 81970586 and 81900626), Major International (Regional) Joint Research Project (81620108003), Guangzhou Regenerative Medicine and Health–Guangdong Laboratory Research Grant (2018GZR0201003) and Major Scientific and Technological Planning Project of Guangzhou (201607020004).
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participant centers of the EACH study for their important contributions. Participant centers: Nanfang Hospital, Southern Medical University, Guangzhou, China; First Affiliated Hospital of Zhengzhou University, Zhengzhou, China; West China Second University Hospital, Sichuan University, Chengdu, China; Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China; Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China; Children’s Hospital of Chongqing Medical University, Chongqing, China; Guizhou Provincial People’s Hospital, Guizhou University, Guiyang, China; Second Affiliated Hospital, Zhejiang University, Hangzhou, China; Guilin Medical University Affiliated Hospital, Guilin, China; Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; First Affiliated Hospital, Zhejiang University, Hangzhou, China; First Affiliated Hospital of Shenzhen University, Shenzhen University, Shenzhen, China; Second Affiliated Hospital of Dalian Medical University, Dalian, Chin; Huashan Hospital, Fudan University, Shanghai, China; Institute of Nephrology, Zhong Da Hospital, Nanjing, China; Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China; Children’s Hospital of Nanjing Medical University, Nanjing, China; Children’s Hospital of Zhejiang University, Hangzhou, China; Anhui Provincial Children’s Hospital, Hefei, China; Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China; Children’s Hospital of Fudan University, Shanghai, China; Chengdu Women and Children’s Central Hospital, Chengdu, China; Shanghai Children’s Medical Center, Shanghai Jiaotong University, Shanghai, China; Jinan Children’s Hospital, Jinan, China; and Lanzhou University Second Hospital, Lanzhou, China.
Contributor Information
Pinghong He, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China; Renal Division, Department of Medicine, Guizhou Provincial People’s Hospital, Guizhou Provincial Institute of Nephritic and Urinary Disease, Guiyang, China.
Ruixuan Chen, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Liping Zhou, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Yanqin Li, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Licong Su, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Jin Dong, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Yan Zha, Renal Division, Department of Medicine, Guizhou Provincial People’s Hospital, Guizhou Provincial Institute of Nephritic and Urinary Disease, Guiyang, China.
Yuxin Lin, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Sheng Nie, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Fan Fan Hou, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
Xin Xu, Renal Division, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, State Key Laboratory of Organ Failure Research, Guangzhou, China.
REFERENCES
- 1. Xu X, Nie S, Ding H et al. Environmental pollution and kidney diseases. Nat Rev Nephrol 2018; 14: 313–324 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1459–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bourdrel T, Bind MA, Béjot Y et al. Cardiovascular effects of air pollution. Arch Cardiovasc Dis 2017; 110: 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manisalidis I, Stavropoulou E, Stavropoulos A et al. Environmental and health impacts of air pollution: a review. Front Public Health 2020; 20: 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burney P, Amaral AFS. Air pollution and chronic airway disease: is the evidence always clear? Lancet 2019; 394: 2198–2200 [DOI] [PubMed] [Google Scholar]
- 6. Xu X, Wang G, Chen N et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol 2016; 27: 3739–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowe B, Xie Y, Li T et al. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2018; 29: 218–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowe B, Xie Y, Li T et al. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: a cohort study. Lancet Planet Health 2017; 1: e267–e276 [DOI] [PubMed] [Google Scholar]
- 9. Ostro B, Malig B, Broadwin R et al. Chronic PM2.5 exposure and inflammation: determining sensitive subgroups in mid-life women. Environ Res 2014; 132: 168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sørensen M, Daneshvar B, Hansen M et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect 2003; 111: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atkinson RW, Carey IM, Kent AJ et al. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 2013; 24: 44–53 [DOI] [PubMed] [Google Scholar]
- 12. Madrigano J, Kloog I, Goldberg R et al. Long-term exposure to PM2.5 and incidence of acute myocardial infarction. Environ Health Perspect 2013; 121: 192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiorentino M, Grandaliano G, Gesualdo L et al. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol 2018; 193: 45–54 [DOI] [PubMed] [Google Scholar]
- 14. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 2015; 11: 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991; 133: 144–153 [DOI] [PubMed] [Google Scholar]
- 16. Peters A, Dockery DW, Muller JE et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001; 103: 2810–2815 [DOI] [PubMed] [Google Scholar]
- 17. Peters A, Von Klot S, Heier M et al. Cooperative health research in the region of Augsburg study group: exposure to traffic and the onset of myocardial infarction. N Engl J Med 2004; 351: 1721–1730 [DOI] [PubMed] [Google Scholar]
- 18. Xu X, Nie S, Liu Z et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 2015; 10: 1510–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Nie S, Zhang A et al. A new criterion for pediatric AKI based on the reference change value of serum creatinine. J Am Soc Nephrol 2018; 29: 2432–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colvin R, Anthony C. Diagnostic Pathology: Kidney Diseases, 2nd edn. Amsterdam: Elsevier Health Sciences, 2015
- 21. Wang Z, Peng J, Liu P et al. Association between short-term exposure to air pollution and ischemic stroke onset: a time-stratified case-crossover analysis using a distributed lag nonlinear model in Shenzhen, China. Environ Health 2020; 19: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishii M, Seki T, Kaikita K et al. Short-term exposure to desert dust and the risk of acute myocardial infarction in Japan: a time-stratified case-crossover study. Eur J Epidemiol 2020; 35: 455–464 [DOI] [PubMed] [Google Scholar]
- 23. Mohammad KN, Chan EYY, Lau SY et al. Relationship between acute kidney injury, seasonal influenza, and environmental factors: a 14-year retrospective analysis. Environ Int 2021; 153: 106521. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Kindzierski W, Kaul P. Air pollution and acute myocardial infarction hospital admission in Alberta, Canada: a three-step procedure case-crossover study. PLoS One 2015; 10: e0132769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu N, Li H, Han M et al. Environmental nitrogen dioxide (NO2) exposure influences development and progression of ischemic stroke. Toxicol Lett 2012; 214: 120–130 [DOI] [PubMed] [Google Scholar]
- 26. Li H, Xin X. Nitrogen dioxide (NO2) pollution as a potential risk factor for developing vascular dementia and its synaptic mechanisms. Chemosphere 2013; 92: 52–58 [DOI] [PubMed] [Google Scholar]
- 27. Mirowsky JE, Dailey LA, Devlin RB. Differential expression of pro-inflammatory and oxidative stress mediators induced by nitrogen dioxide and ozone in primary human bronchial epithelial cells. Inhal Toxicol 2016; 28: 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levy I, Mihele C, Lu G et al. Evaluating multipollutant exposure and urban air quality: pollutant interrelationships, neighborhood variability, and nitrogen dioxide as a proxy pollutant. Environ Health Perspect 2014; 122: 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Intergovernmental Panel on Climate Change. Climate Change 2014: Mitigation of Climate Change—Working Group III Contribution to the IPCC Fifth Assessment Report. Cambridge: Cambridge University Press, 2015: 351–412 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.