Synopsis
The natural history of moderate alcoholic hepatitis (AH) is not well known. It is a frequent disease with a probable underestimated incidence compared to its severe form. Among the different prognostic scores to predict short-term mortality in AH, MELD seems to be the most accurate one. The mortality of moderate AH is up to 3–7% in the short-medium term and 13–20% at one year, mainly due to liver-related complications including severe infections. Long-term abstinence is the main goal of the treatment. There is still a dire need for the development of new therapies for AH, including the less severe forms.
Keywords: alcoholic hepatitis, moderate, non-severe, MELD, survival, abstinence
1. Introduction
Alcohol-related liver disease (ALD) represents one of the leading etiologies of chronic liver disease in Europe and the United States. According to the Global Status Report on Alcohol and Health 2018 from the World Health Organization, ALD accounts for 50% of cirrhosis cases and 50% of liver-related deaths worldwide1–3. ALD encompasses a range of disorders including simple liver steatosis, alcoholic steatohepatitis (ASH), fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)4 (Figure 1). In addition, patients with underlying ALD and active heavy drinking can develop an episode of acute-on-chronic liver injury characterized by the rapid onset of jaundice, infiltration of polymorphonuclear leukocytes, hepatocellular damage and liver-related complications called “alcoholic hepatitis” (AH), which portends a poor prognosis. The severe form of this disease is associated with a high short-term mortality up to 30% at 28 days and a long-term mortality exceeding 50% at one year5. The initial attempts to assess the severity of this disease were performed by Maddrey in 19786. Patients with higher prothrombin time and serum bilirubin at admission showed increased mortality. Therefore, a scoring system including these two parameters named Discriminant Function (DF) was developed. The initial threshold to consider the disease as severe was set at 93 and subsequently adjusted to 32 to overcome disparities secondary to inter-laboratory variations7. Thus, patients with a DF score < 32 have been traditionally considered as having a “non-severe” or a “moderate” AH. Nevertheless, other clinical, histological and hemodynamic scores aimed at better characterizing the prognosis of patients with AH have been developed in recent years8–12. Among the clinical scores, the Model for End-Stage Liver Disease (MELD) has emerged as one of the most accurate at establishing the prognosis of the disease with a threshold set at ≥ 21 for those with severe AH8. Therefore, a MELD < 21 is increasingly accepted as the best scoring system to define a moderate AH.
Figure 1. The spectrum of alcoholic liver disease.

Abbreviations: ASH, Alcoholic steatohepatitis; HCC, Hepatocellular carcinoma.
The natural history of moderate AH is not well known but involves significant morbidity and mortality. Most observational studies and clinical trials have focused on severe AH, while only few original studies have recently delineated the natural history of the moderate form. In particular, a recent systematic review and meta-analysis along with a couple of observational studies have specifically focused on patients with moderate AH. These studies show a non-negligible mortality up to 6% and 13% at 28-days and 1-year respectively, challenging the “non-severe” nomenclature13–15. Finally, there is a gap in knowledge regarding the specific therapeutic strategies for patients with less severe forms of the disease since they have been traditionally excluded from clinical trials. This review is aimed at summarizing the available evidence concerning the natural history, diagnosis and management options in patients with moderate AH.
2. Natural history
2.1. Epidemiology
Patients with underlying ALD (with or without cirrhosis) and sustained alcohol abuse are at risk of developing AH. The incidence of AH in patients with underlying liver injury at less advanced stages of the disease is not well known. In patients with established cirrhosis, it may occur in up to 40% of cases5. The incidence of moderate AH is probably underestimated given that some of these patients may present with mild symptoms such as jaundice. Nonetheless, data from the STOPAH trial16 suggest that it might be even more common than the severe form. Specifically, a total of 2006 patients out of the 3109 that were evaluated for the trial, were considered screen failures because they did not fulfill the inclusion criteria (DF < 32 or bilirubin < 4.68 mg/dL), which represents nearly 65% of the entire population screened14. Similarly, a recent study that included patients with AH from the TREAT Consortium showed that the incidence of moderate AH in this cohort, defined as MELD score ≤ 20 at presentation, was 39% (100 patients with moderate AH out of 255 patients recruited with diagnosis of AH)13. Thus, it is very likely that this is an underestimated entity in terms of incidence.
The demographic characteristics of patients with moderate AH are not well described. Only inconclusive information regarding age and gender can be extracted from previous observational studies and clinical trials. In only 6 out of 25 studies included in a recent review, gender and age ranges were reported14. However, a recent study aimed at describing the clinical characteristics and outcomes of patients with mild to moderate AH showed that they are more likely to be male (68% vs 55%) and older (49 vs 44 years) as compared with patients with severe AH. In addition, these patients seem to have a lower body mass index (BMI) compared to those with severe AH (27 vs 31 kg/m2), which is in accordance with previous information showing that obesity is independently associated with mortality at 3 months in patients with AH. No differences were found regarding the amount and duration of alcohol consumption between the groups suggesting that other involved factors such as demographic, genetic and environmental may play an important role in disease severity. Actually, due to the differences seen in coffee consumption and PNPLA3 genotype between patients with moderate and severe AH in this study, the authors hypothesized that there might be an interaction between this environmental and genetic factor that impacts severity13,17. Similar results regarding gender and age have been reported in a series of 121 patients with acutely decompensated alcohol-related liver disease, with histological steatohepatitis and DF < 32. Interestingly, up to 84% of the patients in this series had underlying histologically proven cirrhosis, a higher prevalence than that described for the whole spectrum of AH5,15.
2.2. Prognostic scores to define moderate AH
Stratification of patients according to the severity of the disease is crucial to identifying those with the highest risk of death in the short-term that could benefit from therapies beyond the general management and supportive care, such as corticosteroids, investigational drugs from clinical trials or liver transplantation.
To date, four different scores are available to predict short-term mortality in patients with AH with reasonable accuracy viz. Maddrey’s DF6, MELD score8, Age, serum Bilirubin, INR, and serum Creatinine (ABIC) Score11 and Glasgow Alcoholic Hepatitis Score (GAHS)9. Each score was generated from different variables independently associated with survival in each cohort. Lille score18, is a dynamic score developed to evaluate the response to corticosteroid therapy and it is associated with a particular risk of 1-month mortality according to that response. Thus, it is only applicable in patients with severe AH who are candidates to receive prednisolone.
As previously addressed, patients with a DF score < 32 are considered as having a “non-severe” or a “moderate” AH. Patients with a DF < 32 have a 30-day survival of > 90% with supportive care19. MELD score, which was initially developed to predict survival following transjugular intrahepatic portosystemic shunt (TIPS) for refractory variceal bleeding or refractory ascites20, has shown to be useful for predicting 90-day mortality in patients with AH. It has both a sensitivity and specificity of 75% using a threshold of 21 points. The overall accuracy of GAHS in its original description was 81% and 75% to predict 28-day and 84-day outcome. A cutoff of 9 was selected to optimize the specificity to predict mortality at 28 days (89%) and 84 days (90%). That threshold showed a mortality in patients with AH and GAHS < 9 of 13% and 21% at 28 days and 84 days, respectively. Finally, the ABIC score can identify patients with low (< 6.71), intermediate (6.71–8.99), and high risk (≥ 9.0) of death. Patients with an ABIC score < 6.71 had an excellent short- and long-term prognosis in the original cohort with survival rates of 100% and 97.1% at 90 days and 1 year, respectively.
The accuracy of these scores to predict short-term mortality has been previously explored and validated in external cohorts without significant differences among them21–23, except for a poor performance of DF in severe AH patients recruited to the STOPAH trial compared to MELD, ABIC and GAHS scores24. Nevertheless, in a large worldwide cohort (abstract presented in AASLD 2020, manuscript in preparation) we showed that MELD score has the best performance in predicting short-term mortality compared to other scores. A total of 2,581 patients from 85 centers were included in this study aimed at assessing the accuracy of the different scores to predict short-term mortality in AH at a worldwide scale. In this cohort, the performance of GAHS and ABIC was inferior to MELD but significantly better than Maddrey’s DF, which had the worst accuracy to predict death, with significant differences between all scores and DF. The sample size along with the inclusion of AH patients of different severity and from different geographic regions, confers robustness to our results compared with previous validation series. These results suggest that MELD score should be the elective prognostic score to stratify the severity of the disease. Table 1 shows a summary of the main characteristics of the static prognostic scores and observed mortality according to their severity cutoffs.
Table 1.
Static prognostic scores in AH.
| Score | Variables | Significance | Severity cutoff | Mortality observed | ||
| Maddrey’s DF | Bilirubin, PT | Evaluates risk of mortality at 30 days | <32: non-severe | ≥32: severe |
At 30 days:
• DF <32: < 10% • DF ≥32: > 20–30% |
|
| MELD | Bilirubin, INR, creatinin | Evaluates risk of mortality at 90 days | <21: non-severe | ≥21: severe |
At 90 days:
• MELD <21: <20% • MELD ≥21: ≥20% The probability of 90-day mortality according to MELD score can be calculated with the next formula: P = e(−4.3+0.16 × MELD) / [1+e(−4.3+0.16 × MELD)]) |
|
| GAHS | Bilirubin, INR, urea, WBC, age | Evaluates risk of mortality at 28 days and 84 days | <9: nonsevere | ≥9: severe |
At 28 days:
• GAHS <9: 13% • GAHS ≥9: 54% At 84 days: • GAHS <9: 21% • GAHS ≥9: 60% |
|
| ABIC | Bilirubin, INR, creatinin, age | Evaluates risk of mortality at 90 days and 1 year | <6.71: low risk | 6.71–8.99: intermediate risk | ≥9: high risk |
At 90 days:
• ABIC <6.71: 0% • ABIC 6.71–8.99: 30% • ABIC ≥9: 75% At 1 year: • ABIC <6.71: 2.9% • ABIC 6.71–8.99: 35.7% • ABIC ≥9: 66.7% |
Abbreviations: ABIC, Age, serum Bilirubin, INR, and serum Creatinine; DF, Discriminant Function; GAHS, Glasgow Alcoholic Hepatitis Score; INR, International Normalized Ratio; MELD, Model for End-Stage Liver Disease; PT, prothrombin time; WBC, white blood cell count
Besides the clinical scores based mainly on laboratory parameters associated with mortality in patients with AH, the histological features of the disease have shown to be a complementary prognostic tool in patients who undergo liver biopsy to confirm the diagnosis. The Alcoholic Hepatitis Histologic Score (AHHS) was developed in a series of 121 patients with histologically proven AH and further validated in an independent cohort of 109 patients. AHHS adequately stratifies patients with a low (0–3 points), moderate (4–5 points), and high (6–9 points) risk of death at 90 days (100%, 83%, and 64% survival, respectively)12.
2.3. Mortality associated to moderate AH
The mortality associated with moderate AH has been described in a recent systematic review and meta-analysis and two observational studies specifically focused on this phenotype. Bennet et al compiled the data from 25 studies (n = 1372 patients) including prospective and retrospective observational studies and clinical trials available in literature that reported mortality of patients with non-severe AH either at 28 days, 90 days or 1 year. The definition used for non-severe AH was based on previously accepted cutoffs of the available prognostic scores as follows: DF < 32, MELD < 218, ABIC < 6.7111 or bilirubin < 4.97 mg/dL with histological confirmation. Even if there was substantial heterogeneity in their primary analysis due to differences in study design, inclusion criteria or severity definitions, the results showed a non-negligible mortality of 6% at 28 days (n=993 patients), 7% at 90 days (n=775 patients) and 13% at 1 year (n= 224 patients). A similar mortality of 2%, 3% and 10.3% at 30 days, 90 days and 1 year respectively has been recently reported in patients with moderate AH defined as MELD score ≤ 20 at presentation13. There are few data available regarding the prognosis of moderate AH patients at long-term beyond 12 months. In a recently published series of patients with alcoholic steatohepatitis and acutely decompensated ALD with a DF < 32, the mortality at 2 and 5 years was up to 30% and 49%, respectively. Similar long-term survival data have been previously reported in patients that survived a first episode of AH with a median MELD of 16 at presentation in whom the overall mortality was 38% with a median follow-up of 55 months25. As consistently reported in all these studies, the cause of death in patients with moderate AH was secondary to liver related complications including severe infections in up to 80% of cases13–15,25. These mortality rates strongly suggest that the so called “non-severe AH” should no longer be considered as a benign form of the disease since it entails a short-term mortality comparable to other acute life-threatening diseases (i.e. acute myocardial infarction) and a 1-year mortality similar to that of cirrhotic patients with moderately impaired liver function26. Table 2 summarizes the reported short and long-term mortality rates in patients with moderate AH available in literature.
Table 2.
Reported short and long-term mortality in published studies focused on moderate AH.
| Study | Definition of moderate AH | 28 or 30-day mortality | 90-day mortality | 1-year mortality | 2-year mortality | 5-year mortality |
|---|---|---|---|---|---|---|
| Samala et al13 | MELD score ≤20 at presentation | 2% | 3% | 10.3% | - | - |
| Bennett at al14 | DF < 32, MELD < 21, ABIC < 6.7 or bilirubin < 4.97 mg/dL with histological confirmation | 6% | 7% | 13% | - | - |
| Degré et al15 | Acutely decompensated ALD with histologically proven ASH and Maddreýs DF <32 | 3.3% | 5.8% | 20% | 30% | 49% |
Abbreviations: ABIC, Age, serum Bilirubin, INR, and serum Creatinine; ALD, Alcoholic-related liver disease; ASH, Alcoholic steatohepatitis; DF, Discriminant Function; MELD, Model for End-Stage Liver Disease
Interestingly, a sensitivity analysis comparing the estimated mortality between studies published in or prior 2010 and those published after 2010 showed no differences14. This is in agreement with previous meta-analysis of mortality in all spectrum of AH which did not show a change in mortality at 28 and 90 days over time with a small but statistically significant increase in mortality in 180-day mortality27. These data support that despite the improvement in the management of the disease over the last decades, there is still a dire need for the development of new therapies for AH patients, including for those with less severe forms.
The main factors likely to impact the long-term prognosis of patients with moderate AH that present with jaundice at admission are alcohol abstinence (HR 0.39) and overt hepatic encephalopathy at the moment of presentation (HR 3.84). Patients with encephalopathy at presentation have a significantly reduced survival at 5 years compared to those without (21.4 ± 9.3% and 62.8 ± 7.5%, respectively). Similarly, those patients that do not remain completely abstinent during follow-up after an episode of moderate AH have a notably reduced survival compared to those with total cessation of alcohol use without any relapse episode (39.2 ± 7.4 % and 71.7 ± 10.9%, respectively)15.
3. Diagnosis
Regardless of the severity, AH should be suspected in individuals with history of sustained (> 6 months) heavy alcohol use considered as 3 drinks (~ 40 g) per day for women and 4 drinks (~ 50–60 g) per day for men and acute onset of jaundice that can be accompanied by other signs and symptoms such as malaise, tender hepatomegaly, and liver related complications (ascites, encephalopathy, bacterial infection, and variceal bleeding), especially in patients with underlying cirrhosis. History of heavy and sustained alcohol use is mandatory to suspect an AH episode, nevertheless, it is important to bear in mind that some patients may be intermittently abstinent (up to 8 weeks before the jaundice onset) and alcohol consumption underreporting is frequent19.
Serum bilirubin is usually elevated (> 3 mg/dL), as well as the aspartate aminotransferase (AST) (> 50 IU/mL), and AST to alanine aminotransferase (ALT) ratio of > 1.5 (usually > 2). It is important to take into consideration that the AST and ALT do not typically exceed 400 IU/mL, and when that is the case, an alternative cause with similar clinical presentation such as drug-induced liver injury (DILI) and ischemic hepatitis, should be considered. Screening of other potential causes of jaundice should be part of the general differential diagnosis work-up (biliary obstruction, HCC, viral hepatitis, severe autoimmune liver disease, Wilson disease). A histological confirmation is recommended in those cases in which other confounding factors or concomitant diseases cannot be excluded by clinical criteria, laboratory findings and imaging procedures19. Typical histological findings include macrovesicular steatosis, neutrophil infiltration, hepatocyte ballooning and Mallory-Denk bodies. Fibrosis is always present with pericellular or perisinusoidal distribution showing a classic chicken-wire fence pattern as it extends outward, sometimes all the way to the portal tract28. The majority of patients have underlying cirrhosis12,15. Other common findings include the presence of megamitochondria and bilirubinostasis, that along with fibrosis and polymorphonuclear neutrophils infiltration are associated with prognosis12.
In 2016, a panel of experts from the US National Institute on Alcohol Abuse and Alcoholism (NIAAA) Alcoholic Hepatitis Consortia, proposed a set of standard definitions to address diagnostic criteria of AH and practical issues to homogenize the research in the AH scenario. In summary, a “definite” AH is that with compatible clinical diagnosis and histological confirmation. In the absence of histological assessment, the diagnosis of AH should be considered as “probable” if no other confounding factors are present (i.e autoimmune markers, metabolic liver diseases, sepsis, shock, cocaine use, DILI). In the presence of potential confounding factors, even if the clinical picture is compatible with AH, the diagnosis should be considered as “possible” and histological confirmation is strongly advised19.
Finally, there is an interesting question that arises from recent literature in patients with moderate AH in which two different phenotypes of patients that fulfilled the selected criteria to consider the disease as non-severe or moderate were described. Jaundiced patients with clinical diagnosis of AH and patients with histological alcoholic steatohepatitis that may not have had jaundice admitted to the hospital and with similar mortality rates14,15. Even if they have similar outcomes in terms of survival, it is difficult to determine in those patients with compatible histology but without jaundice, if mortality is actually driven by an AH episode or, what seems more likely, by the underlying liver disease. This reflects the diagnostic challenge that is represented in distinguishing these two clinically and histologically overlapped entities.
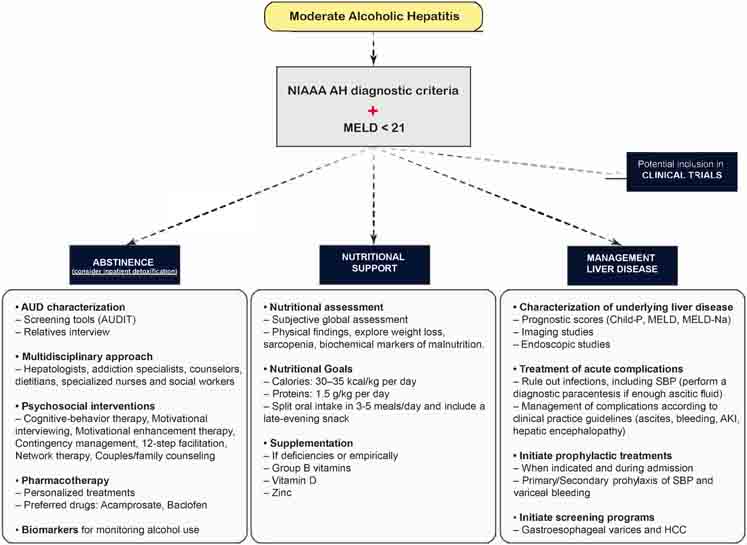
Figure 2 depicts an algorithm with the general diagnostic approach of AH.
Figure 2. Algorithm of the general diagnostic approach in AH.
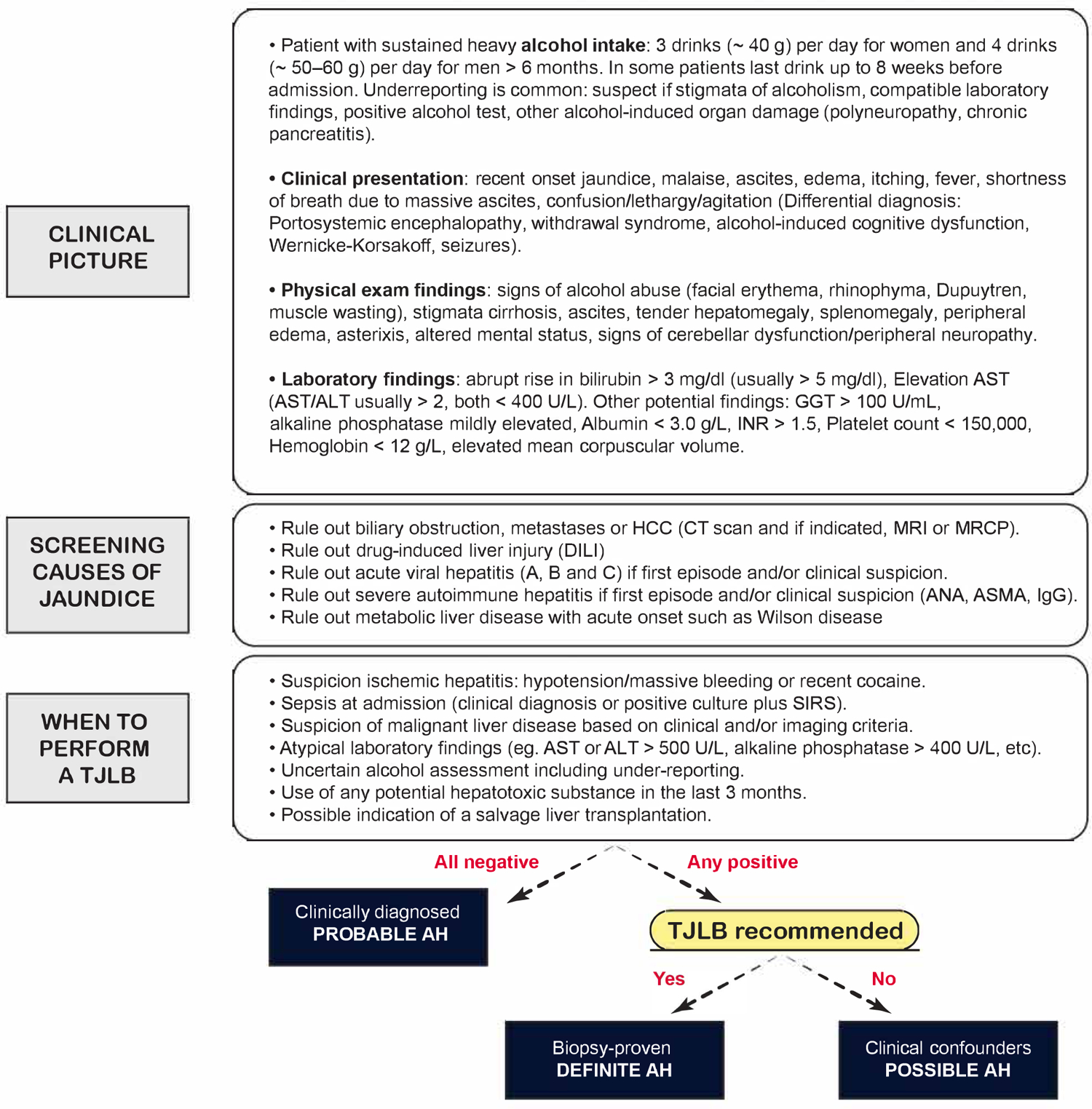
Abbreviations: AH, Alcoholic hepatitis; ALT, Alanine aminotransferase; ANA, Antinuclear antibody; ASMA, Anti smooth-muscle antibody; AST, Aspartate aminotransferase; CT, Computed tomography; DILI, Drug-induced liver injury; GGT, Gamma-glutamyl transferase; HCC, hepatocellular carcinoma; IgG, Immunoglobulin G; MRCP, Magnetic resonance cholangiopancreatography; MRI, Magnetic resonance imaging; SIRS, Systemic inflammatory response syndrome; TJLB, Transjugular liver biopsy.
4. Management options
The management of moderate AH has not evolved in the last few decades since most studies assessing specific therapies are performed in patients with severe forms. Corticosteroids, due to their non-selective systemic anti-inflammatory effect, have been traditionally used to treat severe AH patients when there are no contraindications. Nevertheless, there is robust evidence showing that the benefit of corticosteroids is modest improving the mortality only in the short-term, with a considerable proportion of adverse events, mainly serious infections16,29. Whether corticosteroids could be effective in patients with moderate AH is unknown and in most centers these patients do not receive any specific therapy.
There is intense research activity aimed at developing new therapies that specifically target different pathways involved in the pathophysiology of the disease and, as a result, several clinical trials are underway. Unfortunately, patients with moderate AH have usually been excluded from clinical trials, since they have more favourable outcomes when alcohol abstinence and conservative general measures are implemented. As previously discussed, recent data have shown that mortality in this subgroup of patients should not be underestimated and the paradigm of systematically excluding them from clinical trials should be reconsidered. Some ongoing clinical trials are actually recruiting patients with MELD scores within the current definition of moderate AH30.
To date, the cornerstones in the treatment of moderate AH are long-term abstinence, nutritional support and management of the underlying liver disease. In this review we provide a summary of the evidence related to these therapeutic modalities as well as some information regarding the ongoing clinical trials including patients with less severe forms of the disease.
4.1. Alcohol abstinence
Alcohol abstinence is the main goal of management since in the long-term, it increases survival in patients with AH. Conversely, a return to alcohol consumption after an episode of AH has a dose dependent effect on mortality25,31,32. Recent studies focused on identifying predictors of long-term survival in patients with AH have shown that alcohol abstinence is independently associated to long-term survival in patients with moderate AH15. Besides being the most important risk factor for ALD progression and precluding patient eligibility for an eventual liver transplant33–35, patients that continue drinking after an episode of AH are at high risk of developing recurrent episodes. Such episodes have a more severe clinical course and higher morbidity and mortality (60%)36. Therefore, alcohol abstinence must be closely monitored in all patients after discharge of an AH episode regardless of the severity, since approximately up to two-thirds of patients who survive the episode will relapse in the long-term25,37–39. Abstinence treatment should be based on an integrated approach involving not only the patients and their relatives, but a multidisciplinary team of healthcare professionals including hepatologists, addiction specialists, counselors, dietitians, specialized nurses and social workers, to provide and to closely monitor psychosocial and pharmacological interventions as well as referral to rehabilitation programs4,5,40. In fact, there are data that suggest that an early intervention within 30 days after discharge of an episode of AH is associated with a lower risk of hospital readmissions, alcohol relapse and death41. Inpatient detoxification is strongly advised in those patients with heavy active drinking or those who have experienced previous alcohol withdrawal syndrome5,42. Regarding pharmacological interventions, several drugs have been shown to prevent alcohol relapse in patients with alcohol use disorder (AUD), but most of them have either not been tested in patients with underlying ALD or have known potential of hepatotoxicity, which may preclude their use in this population. Among them, the ones with a safer profile seem to be acamprosate42, due to the absence of hepatic metabolism, and baclofen, which is the only drug that has been studied in a randomized double-blind placebo-controlled trial in patients with decompensated ALD43 and even in a small series of AH patients44. However, data from a large pharmacoepidemiological study in France have recently raised some concerns about baclofen safety45. The pharmaceutical therapies for maintaining alcohol abstinence in patients with ALD have been further reviewed elsewhere30,40,42. The use of biomarkers for monitoring alcohol use may be a useful complementary tool to detect early alcohol relapse episodes since due to the stigma associated to this disease, many patients under-report their alcohol consumption40,46.
4.2. Nutritional support
Malnutrition is a common feature in patients with ALD and it is especially relevant in patients with AH in whom decreased oral intake and underlying hypermetabolic state can lead to nutritional deficiencies. Therefore, a thorough nutritional assessment in patients with an AH episode is mandatory. Current recommendations are based on ensuring an oral intake of 30–35 kcal/kg per day and 1.5 g/kg per day of protein split into three to five meals, including a late-evening snack, which may contribute to ameliorate catabolism adverse effects and muscle mass loss30,40,47. Evaluation and empirical supplementation of micronutrients and vitamins might also be recommended since deficiencies are common in these patients. Special attention should be paid to group B vitamins like thiamine to prevent Wernicke encephalopathy, liposoluble vitamins such as vitamin D since its deficiency has been associated with increased liver damage and mortality in ALD48 and zinc, which seems to improve in combination with lactulose mild hepatic encephalopathy manifestations49. Intensive supplemental enteral nutrition via a nasogastric tube has only been tested in severe cases, and even if it could improve liver function, it has not proven benefitial in survival and should be cautiously considered due to the risk of aspiration pneumonia50.
4.3. Management of the underlying liver disease
Up to 70% of patients with ALD that develop an episode of AH will have underlying cirrhosis5. In a recent retrospective study in patients with moderate AH with histological assessment, an even higher prevalence of cirrhosis (84%) has been reported15. As previously discussed, the cause of death in patients with moderate AH is secondary to liver-related complications, including severe infections in up to 80% of patients13–15,25. Therefore, assessing the severity of the underlying liver disease at presentation of an AH episode with prognostic purposes and to establish the most appropriate follow-up strategy should be part of the general management in all patients regardless of the severity. Patients with less severe forms of AH may also present or develop complications related to the underlying liver disease such as ascites, portal hypertensive bleeding, acute kidney injury (AKI) or hepatic encephalopathy that should be managed according to the current recommendations51–54. Special attention should be paid to infections. In patients with severe AH, the prevalence of infection within 90 days of diagnosis is as high as 42% with an increased risk in those who receive corticosteroids55,56. The prevalence of infection in patients with less severe forms of AH is not well described, but they represent one of main causes of death in this population as is the case with other liver-related complications. Distinguishing between infection and systemic inflammatory response syndrome (SIRS) in the absence of infection can be challenging. In a series of AH patients with histological confirmation and a mean MELD at presentation of 21 (48.1% of patients had MELD > 21), the prevalence of SIRS without infection was 32%. SIRS has been shown as a major determinant of multiorgan failure and mortality in patients within the whole severity spectrum of AH55. Besides the treatment of acute liver-related complications, prophylactic therapies, either to prevent new decompensations or the recurrence of current complications such as antibiotics for spontaneous bacterial peritonitis or beta-blockers for variceal bleeding, should be initiated during admission when indicated54. Those patients with underlying cirrhosis or advanced fibrosis, especially with continued alcohol consumption after the episode, should be enrolled in screening programs for the early detection of gastroesophageal varices54,57 and hepatocellular carcinoma58,59 following the current guidelines.
Early liver transplantation for AH is restricted to highly selected patients with severe forms who do not respond to medical therapy. Nevertheless, patients with less severe forms are at risk for developing life-threatening liver-related complications during follow-up secondary to the underlying advanced liver disease, even after maintaining alcohol abstinence. When indicated, liver transplantation should be considered as part of the management of ALD on a case by case basis after a sobriety period (usually 6 months) in patients with a prior episode of moderate AH according to the latest evidence60. Figure 3 depicts a suggested algorithm for the management of moderate AH.
Figure 3. Suggested algorithm for the management of moderate AH.
Abbreviations: AH, Alcoholic hepatitis; AKI, Acute kidney injury; AUD, Alcohol Use Disorder; AUDIT, Alcohol Use Disorders Inventory Test; Child-P, Child-Pugh; HCC, Hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease; NIAAA, National Institute on Alcohol Abuse and Alcoholism; SBP, Spontaneous bacterial peritonitis.
4.4. Novel therapies
Many new therapies for AH have been evaluated in recent decades in different clinical trials, however most of them were in patients with severe AH. Nevertheless, patients with less severe forms of the disease have recently become the target population of a few ongoing clinical trials, which represents an encouraging turnaround in the research activity of the field. Pathophysiologically-oriented therapies aimed at improving the outcomes in this population are urgently needed given the mortality associated with the so called non-severe or moderate AH as recently shown13–15.
The gut-liver dysfunction has become one of the most attractive and studied pathophysiological mechanisms of ALD in the last years. Bacterial translocation as a result of the gut barrier dysfuntion among other mechanisms, may play a relevant role in the progression of the disease, including in patients with AH. An open-label single arm trial aimed at investigating the effect of gut sterilisation on macrophage activation in patients with AH with a combination of orally formulated antibiotics (vancomycin, gentamycin and meropenem) has recently concluded the recruitment (NCT03157388). According to the available information, patients with AH without any specific cutoff of severity have been included, however, there is no published data available at the moment. A different approach to target the gut-liver axis via administration probiotic supplements containing Lactobacillus Rhamnosus GG versus placebo is also currently underway. The study is aimed at evaluating the effects of probiotic supplements on improvement in MELD score and gut mucosal integrity in patients with MELD < 21 and will collect valuable information that will potentially help to document the natural history of moderate AH (NCT01922895).
Targeting liver regeneration to counteract apoptosis and necrosis phenomena that take place in AH as a potentially new approach to treating the disease has also received considerable attention. In that regard, a phase 2a open-label single-arm clinical trial to test the safety and efficacy of F-652, has already been completed. F-652 is a recombinant fusion protein containing human interleukin 22 (IL-22) and human Immunoglobulin G2 (IgG2) fragment crystallizable with anti-oxidant, anti-apoptotic, anti-steatotic, anti-microbial and proliferative effect. In this dose escalating study, nine patients with moderate AH defined as MELD score 11–20 were included and received the investigational drug at different doses. Preliminary results already published suggest that F-652 is safe in doses up to 45 μg/kg and it is associated with a high rate of improvement as determined by Lille and MELD score, a reduction in markers of inflammation and an increase in markers of hepatic regeneration61. A phase 2b is currently underway (NCT02655510).
Obeticholic acid (OCA) has been a drug that has received special interest from the liver research community in the past years. It is a potent selective agonist of the farnesoid X receptor (FXR), a nuclear receptor which is believed to be a key regulator of bile acids and of the inflammatory, fibrotic, and metabolic pathways62. Due to its ameliorating effect on cholestasis in other liver diseases such as primary biliary cholangitis (PBC) and non-alcoholic steatohepatitis (NASH), OCA has been proposed as a new therapy for AH. A phase 2 double-blind, placebo controlled trial of OCA in patients with moderate AH defined as MELD score > 11 and < 20, has already been completed. The study is primarily aimed at evaluating the impact on MELD score as well as its safety in this population, but the results have yet to be reported (NCT02039219).
Finally, a small molecule called DUR-928, is being tested in an open-label dose escalation phase 2 multicentric trial in patients with AH, including less severe forms of the disease (MELD 11–30, inclusive). DUR-928 acts as an epigenetic modulator regulating multiple biological pathways involved in metabolic homeostasis, inflammatory response, cell survival and tissue regeneration. The study is aimed at assessing the dose related safety, pharmacokinetics, and pharmacodynamics of DUR 928 in patients with moderate and severe AH. The trial is currently under recruitment (NCT03432260).
The ongoing clinical trials evaluating new therapies in all the spectrum of patients with AH have been further reviewed elsewhere30. Table 3 shows a summary of the clinical trials that are underway recruiting patients with moderate AH.
Table 3.
Ongoing clinical trials in patients with moderate alcoholic hepatitis.
| NCT identifier | Treatment | Mechanism | Study design | Definition of moderate AH | Primary endpoint |
|---|---|---|---|---|---|
| 03157388 | Combination of vancomycin, gentamycin and meropenem in oral formulation | Microbiome modulation | Open-label. Single-arm treatment | All spectrum of AH, potentially including patients with moderate disease. Not severity cutoff specified. | Evaluate the effect of gut sterilisation on macrophage activation (Difference in serum levels of macrophage activation markers sCD163) |
| 01922895 | Lactobacillus rhamnosus GG versus placebo | Microbiome modulation | Double-blind, randomised controlled trial with parallel assignment | MELD < 20 | Evolution of MELD score at 30 days |
| 02655510 | IL-22 (F-652) | Hepatocyte regeneration | Open-label. Single-arm treatment. Dose escalating study | All spectrum of AH. Includes a subgroup of patients with MELD 11–20 |
Safety and tolerability |
| 02039219 | Obeticholic acid versus placebo | Amelioration of bile-acid injury | Double-blind, randomised controlled trial with parallel assignment | MELD > 11 and < 20 | Evolution of MELD score at 6 weeks along with safety and tolerability |
| 03432260 | DUR-928 | Small molecule. Epigenetic modulator regulating multiple biological pathways involved in metabolic homeostasis, inflammatory response, cell survival and tissue regeneration. | Open-label. Single-arm treatment. Dose escalating study | All spectrum of AH. MELD 11–30, inclusive |
Safety and tolerability |
5. Conclusions
Moderate AH is a frequent disease with a probable underestimated incidence compared to its severe form. Recent evidence supports that it is not a benign disease since it has a mortality in the short-medium term of up to 3–7% and as high as 13–20% at 1 year. Accordingly, a different nomenclature such as “moderate alcoholic hepatitis”, “alcoholic hepatitis of intermediate severity” or “moderately severe alcoholic hepatitis”, has been proposed. A homogeneous definition of severity according to the different available prognostic scores is lacking as well. The use of a single score to determine the severity of the disease could help to better classify these patients and fully characterize this less severe form of the disease in terms of epidemiology and natural history. Additionally, the systematic use of a single prognostic score could potentially identify new cutoffs to categorize these patients into three groups of risk viz. non-severe, moderate and severe. In this regard, MELD seems to be the most accurate prognostic score. Long-term abstinence is the main goal of the treatment since it is the main driver for disease progression and an independent predictor of long-term survival in these patients. Along with adequate nutritional support, the management of the underlying liver disease is critical, considering that up to 80% of patients will die during follow-up due to liver-related complications, including severe infections. New pathophysiological-oriented therapies for the treatment of less severe forms of AH are being evaluated in several different ongoing clinical trials. While the results of these investigations have yet to be reported, hopefully they will provide new insights about the natural history of the disease as well as new effective treatments that improve the outcomes of these patients in terms of morbidity and mortality.
Clinics Care Points.
AH should be suspected in patients with history of sustained heavy alcohol use and acute onset of jaundice. Other signs and symptoms may be present (mal-aise, tender hepatomegaly, and liver-related complications such as ascites, en-cephalopathy, infections or variceal bleeding).
Tipical laboratory findings are elevation of serum bilirubin (>3 mg/dL), AST >50 IU/mL and AST/ALT >1.5 (usually >2). AST and ALT do not typically exceed 400 IU/mL, and when that is the case, an alternative cause should be considered. Screening of other potential causes of jaundice should be part of the general dif-ferential diagnosis workup.
A histologic confirmation is recommended when other confounding factors or concomitant diseases cannot be excluded by clinical criteria, laboratory findings, and imaging procedures.
MELD score should be the elective prognostic score to stratify the severity of the disease. A cut-off of 21 discriminates severe (≥21) from nonsevere or moderate (<21) forms of the disease.
Cirrhosis is present in up to 70% of cases and 80% of patients will die during follow-up because of liver-related complications. Besides the treatment of acute liver-related complications, prophylactic therapies, to prevent new decompensa-tions or the recurrence of current complications, should be initiated during admission when indicated. Enrollement in screening programs for the early detection of gastroesophageal varices or HCC should be considered as well.
There are not specific therapies for moderate AH. Long-term abstinence is the main goal of the treatment since it is an independent predictor of long-term sur-vival. It must be closely monitored after discharge and based on a multidisci-plinary approach. Inpatient detoxification should be considered as well as pharmacologic treatment to prevent alcohol relapse, being acamprosate and baclofen the preferred drugs for their safety profile. The use of biomarkers to detect relapse episodes may be useful since alcohol consumption is often underreported.
Key points:
Moderate AH is a frequent disease with a probable underestimated incidence compared to its severe form.
A homogeneous definition of severity according to the different available prognostic scores is lacking. MELD seems to be most accurate score.
Patients with moderate AH have a mortality in the short-medium term up to 3–7% and as high as 13–20% at one year.
Long-term abstinence is the main goal of the treatment since it is an independent predictor of long-term survival in these patients.
Pathophysiologically-oriented therapies aimed at improving the outcomes in this population are needed.
Disclosure statement
- Ana Clemente-Sánchez is supported by an international scholarship sponsored by the Spanish Association of the Study of the Liver (AEEH).
- Ramón Bataller is supported by NIAAA grants 1U01AA026978-01, 1U01AA026972-02, 5U01AA026264-02, NIH R01 (ENaC regulation by biliary factors), NIDDK 1R01DK117881-01 and NIEHS R35 (Xenobiotic Receptors in the Crossroad of Xenobiotic Metabolism and Endobiotic Metabolism).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019. doi: 10.1016/j.jhep.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 2.Hammer JH, Parent MC, Spiker DA, World Health Organization. Global Status Report on Alcohol and Health 2018. Vol 65.; 2018. doi: 10.1037/cou0000248 [DOI] [Google Scholar]
- 3.Rehm J, Samokhvalov A V, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013. doi: 10.1016/j.jhep.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Altamirano J, Bataller R. Alcoholic liver disease: Pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8(9):491–501. doi: 10.1038/nrgastro.2011.134 [DOI] [PubMed] [Google Scholar]
- 5.Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Prim. 2018. doi: 10.1038/s41572-018-0014-7 [DOI] [PubMed] [Google Scholar]
- 6.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978. doi: 10.1016/0016-5085(78)90401-8 [DOI] [PubMed] [Google Scholar]
- 7.Carithers RL, Herlong F, Diehl AM, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989. doi: 10.7326/0003-4819-110-9-685 [DOI] [PubMed] [Google Scholar]
- 8.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005. doi: 10.1002/hep.20503 [DOI] [PubMed] [Google Scholar]
- 9.Forrest EH, Evans CDJ, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005. doi: 10.1136/gut.2004.050781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rincon D, Lo Iacono O, Ripoll C, et al. Prognostic value of hepatic venous pressure gradient for in-hospital mortality of patients with severe acute alcoholic hepatitis. Aliment Pharmacol Ther. 2007. doi: 10.1111/j.1365-2036.2007.03258.x [DOI] [PubMed] [Google Scholar]
- 11.Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008. doi: 10.1111/j.1572-0241.2008.02104.x [DOI] [PubMed] [Google Scholar]
- 12.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014. doi: 10.1053/j.gastro.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samala N, Gawrieh S, Tang Q, et al. Clinical Characteristics and Outcomes of Mild to Moderate Alcoholic Hepatitis. GastroHep. 2019;1(4):161–165. doi: 10.1002/ygh2.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett K, Enki DG, Thursz M, Cramp ME, Dhanda AD. Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment Pharmacol Ther. 2019. doi: 10.1111/apt.15376 [DOI] [PubMed] [Google Scholar]
- 15.Degre D, Stauber RE, Englebert G, et al. Long-term outcomes in patients with decompensated alcohol-related liver disease, steatohepatitis and Maddrey’s discriminant function <32. J Hepatol. 2020;72(4):636–642. doi: 10.1016/j.jhep.2019.12.023 [DOI] [PubMed] [Google Scholar]
- 16.Thursz MR, Richardson P, Allison M, et al. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N Engl J Med. 2015. doi: 10.1056/nejmoa1412278 [DOI] [PubMed] [Google Scholar]
- 17.Parker R, Kim SJ, Im GY, et al. Obesity in acute alcoholic hepatitis increases morbidity and mortality. EBioMedicine. 2019. doi: 10.1016/j.ebiom.2019.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007. doi: 10.1002/hep.21607 [DOI] [PubMed] [Google Scholar]
- 19.Crabb DW, Bataller R, Chalasani NP, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients with Alcoholic Hepatitis: Recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016. doi: 10.1053/j.gastro.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, Ter Borg PCJ. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000. doi: 10.1053/he.2000.5852 [DOI] [PubMed] [Google Scholar]
- 21.Sandahl TD, Jepsen P, Ott P, Vilstrup H. Validation of prognostic scores for clinical use in patients with alcoholic hepatitis. Scand J Gastroenterol. 2011. doi: 10.3109/00365521.2011.587200 [DOI] [PubMed] [Google Scholar]
- 22.Papastergiou V, Tsochatzis EA, Pieri G, et al. Nine scoring models for short-term mortality in alcoholic hepatitis: Cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther. 2014. doi: 10.1111/apt.12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palaniyappan N, Subramanian V, Ramappa V, Ryder SD, Kaye P, Aithal GP. The Utility of Scoring Systems in Predicting Early and Late Mortality in Alcoholic Hepatitis: Whose Score Is It Anyway? Int J Hepatol. 2012. doi: 10.1155/2012/624675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrest EH, Atkinson SR, Richardson P, et al. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J Hepatol. 2018. doi: 10.1016/j.jhep.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 25.Altamirano J, López-Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology. 2017. doi: 10.1002/hep.29338 [DOI] [PubMed] [Google Scholar]
- 26.Infante-Rivard C, Esnaola S, Villeneuve J-P. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987. doi: 10.1002/hep.1840070408 [DOI] [PubMed] [Google Scholar]
- 27.Hughes E, Hopkins LJ, Parker R. Survival from alcoholic hepatitis has not improved over time. PLoS One. 2019. doi: 10.1371/journal.pone.0192393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theise ND. Histopathology of alcoholic liver disease. Clin Liver Dis. 2013. doi: 10.1002/cld.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louvet A, Thursz MR, Kim DJ, et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo—a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology. 2018. doi: 10.1053/j.gastro.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 30.Sehrawat TS, Liu M, Shah VH. The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol Hepatol. 2020. doi: 10.1016/S2468-1253(19)30326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louvet A, Labreuche J, Artru F, et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology. 2017. doi: 10.1002/hep.29240 [DOI] [PubMed] [Google Scholar]
- 32.Atkinson SR, Hamesch K, Spivak I, et al. Serum Transferrin Is an Independent Predictor of Mortality in Severe Alcoholic Hepatitis. Am J Gastroenterol. 2020. doi: 10.14309/ajg.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pessione F, Ramond MJ, Peters L, et al. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003. doi: 10.1034/j.1600-0676.2003.01804.x [DOI] [PubMed] [Google Scholar]
- 34.Chedid A, Mendenhall CL, Gartside P, French SW, Chen T, Rabin L. Prognostic factors in alcoholic liver disease. VA Cooperative Study Group. Am J Gastroenterol. 1991. [PubMed] [Google Scholar]
- 35.Lackner C, Spindelboeck W, Haybaeck J, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017. doi: 10.1016/j.jhep.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 36.Potts JR, Howard MR, Verma S. Recurrent severe alcoholic hepatitis: Clinical characteristics and outcomes. Eur J Gastroenterol Hepatol. 2013. doi: 10.1097/MEG.0b013e32835d83d9 [DOI] [PubMed] [Google Scholar]
- 37.Potts JR, Goubet S, Heneghan MA, Verma S. Determinants of long-term outcome in severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013. doi: 10.1111/apt.12427 [DOI] [PubMed] [Google Scholar]
- 38.Deltenre P, Trépo E, Fujiwara N, et al. Gene signature-MELD score and alcohol relapse determine long-term prognosis of patients with severe alcoholic hepatitis. Liver Int. 2020. doi: 10.1111/liv.14265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan A, Tansel A, White DL, et al. Efficacy of Psychosocial Interventions in Inducing and Maintaining Alcohol Abstinence in Patients With Chronic Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol. 2016. doi: 10.1016/j.cgh.2015.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonetto DA, Shah VH, Kamath PS. Outpatient management of alcohol-related liver disease. Lancet Gastroenterol Hepatol. 2020. doi: 10.1016/S2468-1253(19)30415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peeraphatdit T (Bee), Kamath PS, Karpyak VM, et al. Alcohol Rehabilitation Within 30 Days of Hospital Discharge Is Associated With Reduced Readmission, Relapse, and Death in Patients With Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2020. doi: 10.1016/j.cgh.2019.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellinger JL, Winder GS. Alcohol Use Disorders in Alcoholic Liver Disease. Clin Liver Dis. 2019. doi: 10.1016/j.cld.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007. doi: 10.1016/S0140-6736(07)61814-5 [DOI] [PubMed] [Google Scholar]
- 44.Yamini D, Lee SH, Avanesyan A, Walter M, Runyon B. Utilization of baclofen in maintenance of alcohol abstinence in patients with alcohol dependence and alcoholic hepatitis with or without cirrhosis. Alcohol Alcohol. 2014. doi: 10.1093/alcalc/agu028 [DOI] [PubMed] [Google Scholar]
- 45.Chaignot C, Zureik M, Rey G, Dray-Spira R, Coste J, Weill A. Risk of hospitalisation and death related to baclofen for alcohol use disorders: Comparison with nalmefene, acamprosate, and naltrexone in a cohort study of 165 334 patients between 2009 and 2015 in France. Pharmacoepidemiol Drug Saf. 2018. doi: 10.1002/pds.4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabezas J, Lucey MR, Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis. 2016. doi: 10.1002/cld.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merli M, Berzigotti A, Zelber-Sagi S, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019. doi: 10.1016/j.jhep.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trépo E, Ouziel R, Pradat P, et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013. doi: 10.1016/j.jhep.2013.03.024 [DOI] [PubMed] [Google Scholar]
- 49.Shen YC, Chang YH, Fang CJ, Lin YS. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: A systematic review and meta-analysis. Nutr J. 2019. doi: 10.1186/s12937-019-0461-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno C, Deltenre P, Senterre C, et al. Intensive Enteral Nutrition Is Ineffective for Patients with Severe Alcoholic Hepatitis Treated with Corticosteroids. Gastroenterology. 2016. doi: 10.1053/j.gastro.2015.12.038 [DOI] [PubMed] [Google Scholar]
- 51.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: Alcoholic liver disease. Am J Gastroenterol. 2018. doi: 10.1038/ajg.2017.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020. doi: 10.1002/hep.30866 [DOI] [PubMed] [Google Scholar]
- 53.Thursz M, Gual A, Lackner C, et al. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018. doi: 10.1016/j.jhep.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 54.Angeli P, Bernardi M, Villanueva C, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018. doi: 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 55.Michelena J, Altamirano J, Abraldes JG, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015. doi: 10.1002/hep.27779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vergis N, Atkinson SR, Knapp S, et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology. 2017. doi: 10.1053/j.gastro.2016.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017. doi: 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 58.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 59.Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 60.Mathurin P, Lucey MR. Liver transplantation in patients with alcohol-related liver disease: current status and future directions. Lancet Gastroenterol Hepatol. 2020. doi: 10.1016/S2468-1253(19)30451-0 [DOI] [PubMed] [Google Scholar]
- 61.Arab JP, Sehrawat TS, Simonetto DA, et al. An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients With Alcohol-associated Hepatitis. Hepatology. 2020. doi: 10.1002/hep.31046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiorucci S, Di Giorgio C, Distrutti E. Obeticholic Acid: An Update of Its Pharmacological Activities in Liver Disorders. In: Handbook of Experimental Pharmacology. 2019. doi: 10.1007/164_2019_227 [DOI] [PubMed] [Google Scholar]
- 63.Thursz M, Gual A, Lackner C, et al. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018. doi: 10.1016/j.jhep.2018.03.018 [DOI] [PubMed] [Google Scholar]