This review highlights the importance of volatile organic compounds (VOCs) in plant–plant interactions, covering the defence, growth, and reproduction responses in recipient plants upon VOC exposure.
Keywords: Defence, green leaf volatiles, growth, photosynthesis, plant–plant communication, primary metabolism, priming, reproduction, secondary metabolism, terpenes, volatile organic compounds
Abstract
It is firmly established that plants respond to biotic and abiotic stimuli by emitting volatile organic compounds (VOCs). These VOCs provide information on the physiological status of the emitter plant and are available for detection by the whole community. In the context of plant–plant interactions, research has focused mostly on the defence-related responses of receiver plants. However, responses may span hormone signalling and both primary and secondary metabolism, and ultimately affect plant fitness. Here we present a synthesis of plant–plant interactions, focusing on the effects of VOC exposure on receiver plants. An overview of the important chemical cues, the uptake and conversion of VOCs, and the adsorption of VOCs to plant surfaces is presented. This is followed by a review of the substantial VOC-induced changes to receiver plants affecting both primary and secondary metabolism and influencing plant growth and reproduction. Further research should consider whole-plant responses for the effective evaluation of the mechanisms and fitness consequences of exposure of the receiver plant to VOCs.
Introduction
Plants produce and emit a diverse array of volatile organic compounds (VOCs) as defences against biotic and abiotic stresses and to interact with their environment (Dudareva et al., 2013). Upon damage by herbivores, plants emit de novo-produced VOCs to defend against the attacker; these VOCs can also act as a rapid warning signal to prime defences in undamaged parts of the same plant and undamaged neighbouring plants (McCall et al., 1994; Engelberth et al., 2004; Heil and Silva Bueno, 2007). This phenomenon, referred to as a plant–plant interaction or plant–plant communication, was first documented in the early 1980s in studies that were the subject of extensive debate (Baldwin and Schultz, 1983; Rhoades, 1983). The scientific discourse that ensued threw doubt on the conclusions (Fowler and Lawton, 1985) and temporarily slowed progress in the field (Karban et al., 2014a). However, a wealth of studies since has provided a solid literature base supporting the paradigm that plants release VOCs that can be detected by and elicit responses in their neighbours (Karban et al., 2014b).
A common observation has been that exposure to herbivore-induced plant volatiles (HIPVs) elicits changes in a receiver plant that decreases the cumulative seasonal herbivore damage to that receiver (Dolch and Tscharntke, 2000; Tscharntke et al., 2001; Karban and Maron, 2002; Karban et al., 2006). The frequency with which this observation has been reported provides compelling evidence that volatile-mediated plant–plant interactions are ecologically significant (Karban et al., 2014b), and great progress has been made in elucidating the integration of VOCs into plant defence mechanisms (Erb, 2019; Ye et al., 2019). These mechanisms encompass the detection of VOCs and the triggering of sophisticated molecular pathways (Ye et al., 2019), and the associational resistance facilitated through chemical camouflage (Himanen et al., 2010; Mofikoya et al., 2017; Bui et al., 2021). There is now less debate about the phenomenon of volatile-mediated plant–plant interactions, but there remain a lot of questions about how sophisticated, flexible, or specific the interactions are.
Many studies of biotic-stress-induced plant–plant interactions have focused on defence-related responses of receiver plants—the common assumption being that plants can pre-empt a potential herbivore attack and ready or initiate their defences in preparation (e.g. Heil and Kost, 2006; Kost and Heil, 2006; Frost et al., 2007). The rationale behind this strategy is logical if we consider that volatile-mediated plant–plant interactions could have evolved as an artifact of volatile-mediated within-plant defence coordination via volatiles (Heil and Silva Bueno, 2007; Frost et al., 2007; Heil and Karban, 2010; Li and Blande, 2017). Within-plant signalling via volatiles could have evolved to overcome vascular constraints, which may be extensive in highly branched modular plants (Frost et al., 2007). However, responses may be broader than being defence related, and may span receiver plant hormone signalling and primary and secondary metabolism, and ultimately affect plant fitness. To gain a complete picture of biotic-stress-induced plant–plant interactions, we need to expand research beyond the common focus on plant defence to encompass the effects of VOC exposure on non-defence-related parameters.
In this review, we present an overview of plant–plant interactions with an emphasis on receiver plants. The review begins with a brief description of recent advances in the perception, uptake, and conversion of VOCs and then addresses recent research on passive volatile-mediated plant–plant interactions. The focus then develops to cover defence-related changes induced or primed by VOCs in receiver plants, before synthesizing literature that addresses how VOCs influence non-defence-related parameters. We found that few studies have addressed the effect of VOCs on non-defence-related parameters, with those studies suggesting that VOCs might affect gas exchange and nutrient assimilation, influencing the resources allocated to primary and secondary metabolism, and ultimately affecting plant growth, reproduction, and defence. We finish with a consideration of the ecological consequences of responding to VOCs and conclude with a summary and suggestions for future research. Our aim is to emphasize the need to consider whole-plant responses for effective evaluation of the mechanisms and consequences of volatile-mediated plant–plant interactions across mechanistic and ecological levels of organization.
Perception, uptake, and conversion of VOCs
It has typically been concluded in the literature that the mechanisms of detection of volatiles by plants are poorly understood (e.g. Hemachandran et al., 2017); however, there has been a lot of progress in this area and knowledge of at least partial mechanisms has been accumulating. Information on the identities of molecules eliciting responses in receiver plants has steadily grown (see Table 1), while the early stages of responses have become increasingly clear (Erb, 2019). One of the reasons that mechanisms have been viewed as elusive is the general lack of VOC receptors that have been identified in plants. Nevertheless, at least 38 different VOCs have been implicated as providing a form of between-plant cue (Table 1). This suggests that there are likely to be mechanisms other than through specific molecule receptors that initiate the process of volatile detection by plants. It has been proposed that VOCs could be considered as damage-associated molecular patterns, which are tissue-derived signals of damage that initiate cellular signalling cascades (reviewed by Quintana-Rodriguez et al., 2018; Meents and Mithöfer, 2020). In this capacity, VOCs may trigger plant immunity and have roles in protection against herbivores and disease.
Table 1.
Known VOCs inducing direct responses in plants and/or priming of defences upon biotic or abiotic stress
| Compound | Species | Exposure time | Induced responses in the receiver plants | Primed responses upon stress in the receiver plants | Refs |
|---|---|---|---|---|---|
| Aldehydes | |||||
| Acrolein | Arabidopsis thaliana | 3 h | Increases the expression of defensive genes similar to those expressed upon Pseudomonas syringae infection and induces the production of jasmonates | Alméras et al., 2003 | |
| Methacrolein | Nicotiana attenuata | 72 h | Enhances the production of trypsin proteinase inhibitors in response to subsequent herbivory by Manduca sexta caterpillars | Kessler et al., 2006 | |
| Nonanal | Phaseolus lunatus | 6h and 24 h | All concentrations and times tested reduce infection by the bacterial pathogen P. syringae | Girón-Calva et al., 2012 | |
| Benzenoids | |||||
| Benzothiadiazole | P. lunatus | Spraying | Increases expression of PR-2 gene and increases resistance to P. syringae | Yi et al., 2009 | |
| Indole | Camellia sinensis | 48 h | Directly induces salicylic acid (SA) production | Increases resistance to Ectropis obliqua larvae. Up-regulates genes involved in Ca2+ signalling and mitogen-activated protein kinase and increases the production of phytohormones after herbivory | Ye et al., 2021 |
| Oryza sativa | 12 h | Increases the accumulation of 12-oxophytodienoic acid and up-regulates LRR-RLKs gene expression | Increases the expression of early defence signalling genes and enhances jasmonic acid (JA) production upon herbivory. Decreases Spodoptera frugiperda larval growth and damage | Ye et al., 2019 | |
| Zea mays | 12 h | Increases abscisic acid (ABA) production | Increases ABA and jasmonic acid isoleucine production upon wounding and application of Spodoptera littoralis regurgitant | Erb et al., 2015 | |
| Z. mays | 16 h | Increases jasmonate production and volatile abundance, and induces defence gene expression after herbivory | Hu et al., 2019 | ||
| (+)-Menthofuran | Cucumis sativus | Perfusion | Induces Vm depolarization | Maffei et al., 2001 | |
| Methyl salicylate (MeSA) | P. lunatus | 6h and 24 h | Reduces P. syringae infection | Girón-Calva et al., 2012 | |
| Green leaf volatiles | |||||
| (E)-2-Hexenal | A. thaliana | 15–20 min | Rapidly promotes cytosolic calcium ([Ca2+]cyt) transients and induces superoxide production | Asai et al., 2009 | |
| A. thaliana | 24 h | Up-regulates defence-related genes | Increases plant resistance to the necrotrophic fungal pathogen Botrytis cinerea | Kishimoto et al., 2005 | |
| Lolium temulentum | 1–60 min | Activates mitogen-activated protein kinases | Dombrowski et al., 2018 | ||
| N. attenuata | 72 h | Enhances the production of trypsin proteinase inhibitors to subsequent M. sexta caterpillar feeding | Kessler et al., 2006 | ||
| Solanum lycopersicum | Perfusion | Induces Vm depolarization and increases [Ca2+]cyt | Zebelo et al., 2012 | ||
| (Z)-3-Hexenal | A. thaliana | 24 h | Up-regulates defence-related genes | Increases plant resistance to the necrotrophic fungal pathogen B. cinerea | Kishimoto et al., 2005 |
| S. lycopersicum | Perfusion | Induces Vm depolarization and increases [Ca2+]cyt | Zebelo et al., 2012 | ||
| Z. mays | 30 min | Increases JA production and quantities of VOCs emitted | Increases JA production and quantities of VOCs emitted after Spodoptera exigua regurgitant application | Engelberth et al., 2004 | |
| Z. mays | 3–48 h | Up-regulates defensive genes and increases MeSA emissions | Farag et al., 2005 | ||
| (E)-2-Hexenol | A. thaliana | 15–20 min | Promotes [Ca2+]cyt transients | Asai et al., 2009 | |
| L. temulentum | 1–60 min | Activates mitogen-activated protein kinases | Dombrowski et al., 2018 | ||
| (Z)-3-Hexenol(E)-3-Hexenol | L. temulentum | 1–60 min | Activates mitogen-activated protein kinases | Dombrowski et al., 2018 | |
| Z. mays | 30 min | Increases JA production and quantities of VOCs emitted | Increases JA production and quantities of VOCs emitted after S. exigua regurgitant application | Engelberth et al., 2004 | |
| Z. mays | 20–60 min | Up-regulates genes involved in transcriptional regulation and Ca2+ and lipid signalling | Engelberth et al., 2013 | ||
| Z. mays | 14 h | Increases the quantity of VOCs and induces emissions of VOCs associated with herbivory, such as (Z)-3-hexenyl acetate, (3E)-DMNT, and sesquiterpenes | Ruther and Kleier, 2005 | ||
| (Z)-3-Hexenyl acetate(E)-3-Hexenyl acetate | P. lunatus | 24 h | Increases the production of extrafloral nectar | Heil et al., 2008 | |
| Populus deltoides × nigra | 16 h | Induces higher concentrations of JA and linolenic acid upon feeding by Lymantria dispar larvae. Up-regulates the expression of direct defence-related genes, and induces stronger and quicker emissions of terpenes upon larvae feeding | Frost et al., 2008 | ||
| S. lycopersicum | Perfusion | Induces Vm depolarization and increases [Ca2+]cyt | Zebelo et al., 2012 | ||
| Triticum aestivum | 16 h | Increases H2O2 production and increases activity levels of ROS-scavenging enzymes. Lowers infection rate by the fungus Fusarium graminearum and necrotic lesions induced by the fungus | Ameye et al., 2015; Van Meulebroek et al., 2020 | ||
| Z. mays | 30 min | Increases JA production and the quantities of VOCs emitted | Increases JA production and quantities of VOCs emitted after S. exigua regurgitant application | Engelberth et al., 2004 | |
| Z. mays | 1.5–4 h | Increases the expression of cold-stress-related genes | Reduces damage after cold stress | Cofer et al., 2018 | |
| Z. mays | 24 h | Directly induces defence gene expression in the absence of herbivory | Increases jasmonate production and volatile abundance, and induces defence gene expression after herbivory | Hu et al., 2019 | |
| (E)-2-Hexenyl acetate5-Hexenyl acetate(Z)-3-Hexenyl isovalerate(Z)-3-Hexenyl butyrate | P. lunatus | 24 h | Increases the production of extrafloral nectar | Heil et al., 2008 | |
| Jasmonates | |||||
| Methyl jasmonate (MeJA) | Gossypium hirsutum | 16 h | Increase emissions of (Z)-3-hexenyl acetate, (E)-β-ocimene, linalool, (3E)-DMNT, (E,E)-α-farnesene, (E)-β-farnesene, and (E,E)-TMNT | Rodriguez-Saona et al., 2001 | |
| S. lycopersicum | 24 h | Induces proteinase inhibitor I and II proteins | Farmer and Ryan, 1990 | ||
| Z. mays | 3–24 h | Increases the expression of defensive genes | Farag et al., 2005 | ||
| Ketones | |||||
| (Z)-Jasmone | Z. mays | 24 h | Reduces susceptibility to the leafhopper Cicadulina storeyi | Increases emission of the sesquiterpenes (E)-(1R,9S)-caryophyllene, (E)-α-bergamotene, (E)-β-farnesene, and (E)-DMNT upon insect feeding | Oluwafemi et al., 2013 |
| (–)-Menthone | C. sativus | Perfusion | Induces Vm depolarization | Maffei et al., 2001 | |
| Methyl vinyl ketone | A. thaliana | 3 h | Increases the expression of defensive genes upon P. syringae infection | Alméras et al., 2003 | |
| (+)-Pulegone | C. sativus | Perfusion | Induces Vm depolarization | Maffei et al., 2001 | |
| Vinyl ketone | A. thaliana | 3 h | Increases the expression of defensive genes upon P. syringae infection | Alméras et al., 2003 | |
| Terpenes | |||||
| Isoprene | A. thaliana | 72 h | Functions through SA signalling to prime plant defence and reduce the growth of the biotrophic pathogen P. syringae | Frank et al., 2021 | |
| α-Pinene | A. thaliana | 2 h | Increases pinII-promoter activity | Godard et al., 2008 | |
| A. thaliana | 15–20 min | Promotes [Ca2+]cyt transients | Asai et al., 2009 | ||
| A. thaliana | 72 h | Induces the accumulation of reactive oxygen species (ROS), and the expression of salicylic acid (SA)-and systemic acquired resistance (SAR)-associated genes | Riedlmeier et al., 2017 | ||
| S. lycopersicum | Perfusion | Induces Vm depolarization | Zebelo et al., 2012 | ||
| β-Pinene | A. thaliana | 72 h | Induces the accumulation of ROS, and the expression of SA-and SAR-associated genes | Riedlmeier et al., 2017 | |
| A. thaliana | 15–20 min | Promotes [Ca2+]cyt transients | Asai et al., 2009 | ||
| Cumene | Brassica nigra | 5 d | Reduces larval biomass | Pashalidou et al., 2020 | |
| Myrcene | A. thaliana | 2 h | Increases pinII-promoter activity and up-regulates genes associated with response to biotic or abiotic stress, defence, and transcription factors | Godard et al., 2008 | |
| A. thaliana | 15–20 min | Promotes [Ca2+]cyt transients | Asai et al., 2009 | ||
| Limonene | A. thaliana | 2 h | Increases pinII-promoter activity | Godard et al., 2008 | |
| 1,8-Cineole | A. thaliana | 2 h | Increases pinII-promoter activity | Godard et al., 2008 | |
| Artemisia tridentata | 24 h | Reduces herbivore damage | Shiojiri et al., 2015 | ||
| Linalool | A. thaliana | 2 h | Increases pinII-promoter activity | Godard et al., 2008 | |
| S. lycopersicum | 24h | Plants from the variety Moneymaker increased JA levels and expression of the PI-I gene 6 and 12 h after S. exigua caterpillar regurgitant treatment | Zhang et al., 2020 | ||
| (E)-β-Ocimene | A. thaliana | 15–20 min | Promotes [Ca2+]cyt transients | Asai et al., 2009 | |
| A. thaliana | 2 h | Increases pinII-promoter activity and up-regulates genes associated with response to biotic or abiotic stress, defence, and transcription factors | Godard et al., 2008 | ||
| Brassica pekinensis | 24 h | Increases the concentration of glucosinolates in leaves upon Myzus persicae infection and reduces the performance of M. persicae | Kang et al., 2018 | ||
| P. lunatus | 3–72 h | Up-regulates defence-related genes | Induces greater emissions of VOCs such as MeSA and (E)-DMNT after 1 d of feeding by Tetranychus urticae. Increases plant attraction to the predatory mite Phytoseiulus persimilis | Arimura et al., 2000a; Arimura et al., 2012, Muroi et al., 2011 | |
| S. lycopersicum | 24 h | Plants from the variety Moneymaker exposed to linalool showed increased expression of the PI-I and PI-II genes at 12 h after S. exigua caterpillar regurgitant treatment | Zhang et al., 2020 | ||
| S. lycopersicum | 72 h | Induces emissions of VOCs. Decreases susceptibility to Macrosiphum euphorbiae aphids and increases attractivity to Aphidius ervi parasitoids | Cascone et al., 2015 | ||
| Z. mays | 72 h | Induces greater emissions of VOCs after 1 d of feeding by Mythimna separata larvae and reduces larval weight gain | Muroi et al., 2011 | ||
| allo-Ocimene | A. thaliana | 24 h | Up-regulates defence-related genes | Increases plant resistance to the necrotrophic fungal pathogen B. cinerea | Kishimoto et al., 2005, 2006 |
| (E)-DMNT | A. thaliana | 15–20 min | Promotes [Ca2+]cyt transients | Asai et al., 2009 | |
| C. sinensis | 6 h | Decreases the amount of leaf eaten by Ectropis obliqua. Increases JA and SA contents and up-regulates defence-related genes | Jing et al., 2021 | ||
| Ipomoea batatas | 3 h | Increases trypsin inhibitory activity induced by Spodoptera litura feeding and reduces larval weight | Meents et al., 2019 | ||
| P. lunatus | 3h and 24 h | Increases the expression of defence-related genes | Arimura et al., 2000b | ||
| (E)-TMTT | P. lunatus | 24 h | Increases the expression of defence-related genes | Arimura et al., 2000b | |
| (–)-Menthol | C. sativus | Perfusion | Induces Vm depolarization and increases [Ca2+]cyt | Maffei and Camusso, 2001; Maffei et al., 2001 | |
| (+)-Neomenthol | C. sativus | Perfusion | Induces Vm depolarization | Maffei et al., 2001 | |
| (E)-Nerolidol | C. sinensis | 0.5–2 h | Activates mitogen-activated protein kinase, induces H2O2 burst, and increases JA and SA contents. Reduces susceptibility to the pathogen Colletotrichum fructicola | Increases resistance to Empoasca onukii | Chen et al., 2020 |
| β-Caryophyllene | S. lycopersicum | Perfusion | Induces Vm depolarization | Zebelo et al., 2012 | |
| A. tridentata | 24 h | Reduces herbivore damage | Shiojiri et al., 2015 | ||
| A. thaliana | 72 h | Functions through JA signalling to prime plant defence and reduce growth of the biotrophic pathogen P. syringae | Frank et al., 2021 | ||
An active volatile-mediated interaction between plants requires the signalling VOCs to traverse the plant cuticle. The cuticle is the final barrier for VOCs to cross for release to the atmosphere (Liao et al., 2021); it is, similarly, a barrier to the entry of VOCs into receiver plants (Noe et al., 2008). The uptake of VOCs depends on their physicochemical properties and the properties of the plant surface. For example, limonene, a characteristic hydrophobic monoterpene that is readily incorporated into cell membranes, is taken up by plants, with the degree of uptake scaling positively with lipid content (Noe et al., 2008).
A series of studies has shown that VOCs taken up by plants can be converted into new compounds, with alterations to compounds from several different groupings described so far. Green leaf volatiles (GLVs), which have been implicated as mediators of plant–plant interactions in several studies (see Table 1), were shown to be taken up by tomato plants (Sugimoto et al., 2014). Interestingly, exposure to tomato volatiles induced by common cutworm feeding, a major component of which is (Z)-3-hexanol, resulted in receiver plants having higher concentrations of (Z)-3-vicianoside. Labelling (Z)-3-hexanol, an aglycone of (Z)-3-vicianoside, with deuterium enabled the passage of the compound in receiver plants to be followed and showed that glycosylation of the up-taken aglycone was the mechanism underpinning (Z)-3-vicianoside accumulation (Sugimoto et al., 2014). A recent study of wheat plants demonstrated that another GLV, (Z)-3-hexenyl acetate, a compound frequently linked to plant–plant interactions, is also taken up and metabolized by plants (Ameye et al., 2020). The authors used metabolomics to show that exposure to (Z)-3-hexenyl acetate resulted in an increase in oxidative stress, modulation of the phenylpropanoid pathway, and a subsequent induction of glycosylation processes. These observations provide strong indications that GLV uptake and conversion could be involved in the overall responses of receiver plants as a result of plant–plant interactions.
In addition, it has been observed that exposure to (E)-nerolidol, a volatile sesquiterpene alcohol and precursor to (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), increases the DMNT emissions of Achyranthes bidentata receiver plants under conditions of herbivore feeding or exposure to methyl jasmonate (MeJA) (Tamogami et al., 2011). DMNT is a common HIPV, which has been shown to be primed in volatile-mediated plant–plant interactions (Giron-Calva et al., 2014). Deuterium labelling showed that (E)-nerolidol taken up by receiver plants was converted into DMNT (Tamogami et al., 2011). Interestingly, nerolidol uptake and conversion into nerolidol glucoside has been observed in tea plants (Camellia sinensis) (Zhao et al., 2020). Nerolidol exposure also correlated with a decrease in malondialdehyde content and increased resistance to cold stress (Zhao et al., 2020). These studies suggest an important role of uptake and conversion of VOCs on the defence properties of receiver plants to both abiotic and biotic stressors, with responses potentially underpinning the observations of defence priming in some species.
The plant hormone jasmonic acid (JA) and its methyl ester, MeJA, are important regulators of plant responses to stress. Airborne MeJA has been shown to be taken up by plants and converted into JA, jasmonoyl isoleucine, and jasmonoyl leucine (Tamogami et al., 2008). Additional conversion products were described by Oki et al. (2019). These conversions initiate signal transduction leading to the emission of VOCs (Tamogami et al., 2008). The role of jasmonates in plant stress responses, growth, and development have been extensively reviewed (e.g. Wasternack, 2007; Wang et al., 2021) and, although integral to mechanisms of plant–plant interactions, will not be considered in detail here.
Interestingly, similar mechanisms of uptake and conversion of VOCs have also been observed in roots. A study of co-cultivated rye (Secale cereale) and hairy vetch (Vicia villosa) showed that benzoxazinoids released into the rhizosphere by rye are taken up by neighbouring vetch plants and translocated to shoots (Hazrati et al., 2020). However, the full mechanism underlying this observation is currently lacking. Literature documenting the uptake of chemical compounds by above- and belowground plant parts and conversion into biologically active molecules highlights the entry of chemical compounds into plants as an important part of an active plant–plant interaction.
Passive deposition of VOCs on receiver leaves and chemical camouflage
Volatile-mediated plant–plant interactions can occur via active and passive mechanisms (Li and Blande, 2015). By definition, an active interaction requires a response by the receiver plant, which can usually be observed as molecular or physiological changes (see below, Defence responses induced and primed by VOCs) (Arimura et al., 2000a). Passive interactions involve the sequestration or adherence of volatiles to the surfaces of a receiver plant, with further changes not required (Himanen et al., 2010; Camacho-Coronel et al., 2020). Both mechanisms of interaction may result in a change in the volatile profiles released by receiver plants, as either an immediate response, an effect of changes in abiotic conditions, or a primed response to stress (Himanen et al., 2015; Li, 2016; Mofikoja et al., 2018). Adsorption of VOCs to plant surfaces can occur between conspecific plants (Li and Blande, 2015) or in heterospecific associations (e.g. Himanen et al., 2010). Where VOCs from a strongly emitting plant adsorb to the surfaces of weaker emitting plants and provide associational resistance to pests or disease, the term ‘chemical camouflage’ may be used (Bui et al., 2021).
It is notable that several studies have shown important ecological roles for passively mediated interactions, which involve the adsorption of chemicals to surfaces of plants. The chemicals most frequently linked to passive interactions are the sesquiterpenes, which are often referred to as semi-volatile compounds (Mofikoya et al., 2019). Passive interactions have been shown to occur in conspecific and heterospecific associations, which can lead to beneficial or detrimental effects on receiver plants. Herbivore-induced sesquiterpenes emitted by broccoli plants (Brassica oleracea var. italica) have been shown to render conspecific neighbours more susceptible to oviposition (Li and Blande, 2015), whereas sesquiterpenes and sesquiterpene alcohols emitted by Rhododendron tomentosum have been shown to stick to neighbouring birch and confer greater resistance to herbivores (Himanen et al., 2010).
Glandular trichomes are significant reservoirs of sesquiterpenes, which are stored in high concentrations and prevented from entering the subcellular space, where they could be toxic (Tissier et al., 2017). When the trichomes are broken, for example, by insect feeding, or when temperatures are high, large quantities of sesquiterpenes can be emitted (Mofikoya et al., 2019). After release, these volatiles can reach a receiver plant and be deposited on the plant surface. When temperatures are adequate for their volatility, the newly acquired volatiles can be re-emitted by the receiver plant in addition to its own characteristic blend of volatiles (Mofikoya et al., 2019). Leaf surface characteristics, air temperature, and leaf surface temperature, as well as the physico-chemical properties of volatiles, are important factors in determining the deposition and re-release of VOCs from leaf surfaces (Schaub et al., 2010; Niinemets et al., 2014).
The adherence and release of R. tomentosum volatiles by neighbouring mountain birch trees in the subarctic has an important temperature-related component (Himanen et al., 2010; Mofikoya et al., 2019). The re-release of volatiles could potentially have a camouflaging effect, making plants less attractive to foraging herbivores. However, the adsorption or sequestration of volatiles by surface waxes has been shown to have some direct effects on receiver plant defences. Using cuticular-wax-covered microscope slides, Li and Blande (2015) showed that sesquiterpenes adsorb to surfaces and affect the oviposition choices by Plutella xylostella moths, whereas Camacho-Coronel et al. (2020) showed that 20 different VOCs were sequestered by wax-covered slides, with 18 of them significantly reducing conidia germination of the fungal pathogen Colletotrichum lindemuthianum. These studies indicate that passive volatile-mediated interactions can have significant roles in structuring the interactions of receiver plants with other organisms in the community.
Defence responses induced and primed by VOCs
VOC-induced responses in receiver plants
Early response events
Early studies on volatile-mediated plant–plant interactions focused on induced responses, the changes occurring in receiver plants in response to a volatile cue. A number of defence-related responses were observed in above- and belowground plant parts, including both those related to direct defence against the invader (e.g. Arimura et al., 2000a) and indirect defence through the attraction of beneficial insects (e.g. Kost and Heil, 2006) (Table 1).
The earliest detectable event in the plant response to VOC exposure is a change in the plasma membrane potential of exposed cells (Maffei et al., 2004). The plasma membrane of cells is the only cellular compartment to have direct contact with the extracellular medium through which a volatile arrives at a receiver plant. Once the volatiles are recognized at the membrane a further cascade of events can be initiated, leading to adapted responses. VOCs have been shown in a series of electrophysiological studies to elicit changes in the transmembrane potential, either through hyperpolarization (increases) or depolarization (decreases), which correlate with the activation of signal transduction pathways that lead to changes in gene expression (Zebelo and Maffei, 2016). Membrane depolarization is a fast electrical signal (action potential) that travels through the entire plant from the point of origin of the perceived input (Maffei and Bossi, 2006). It can function in systemic responses, allowing quick but non-specific signals to propagate through the entire plant (Maffei et al., 2007). Changes in plasma transmembrane potential (Vm) result in a change in ion fluxes through the membrane (Maffei et al. 2004; Zebelo and Maffei, 2015), most notably the movement of calcium (Ca2+) into the cytosol. Zebelo et al. (2012) exposed tomato leaves to different products of the lipoxygenase pathway (commonly known as GLVs)—monoterpenes and sesquiterpenes—and found that all volatiles tested depolarized the Vm. However, determination of Ca2+ influx showed that (Z)-3-hexenyl acetate and (E)-2-hexenal prompted a strong Ca2+ signature in treated leaves, whereas α-pinene and β-caryophyllene did not induce a Ca2+ flux (Zebelo et al., 2012). The Ca2+ ion is a secondary messenger in numerous plant signalling pathways and is rigorously regulated across the plasma membrane by passive fluxes (Ca2+ channels) and active transport (Ca2+ transporters) (Lecourieux et al., 2006). It is an important secondary messenger of plant immune responses, and import of Ca2+ into the cytosol acts as a signal that may induce cellular responses involving the production of hydrogen peroxide (H2O2) (Hepler, 2005). Exposure to several GLVs and terpenes was found to increase cytosol Ca2+ concentrations prior to herbivore feeding (Table 1). Hence, Ca2+ is an important indicator of a significant active plant–plant interaction.
Similar electrophysiological changes in the plasma cell membranes were found in plant roots. For example, several compounds, including (+)-menthofuran, (+)-pulegone, (+)-neomenthol, (–)-menthol, and (–)-menthone, were found to depolarize root cell membranes of cucumber seedlings (Maffei et al., 2001). Of these compounds, (–)-menthol was found to increase the cytosolic Ca2+ concentration of the root cells (Maffei and Camusso, 2001). This observation suggests a similar detection mechanism in the root apices to that observed in the leaves, whereby high concentrations of VOCs triggered a depolarization of membranes.
In response to herbivore wounding, pathogen attack, or insect-derived elicitors, the production of superoxide (O2–) and H2O2 can act as a local signal for hypersensitive cell death and as a systemic signal inducing defensive genes in adjacent cells (Orozco-Cárdenas et al., 2001; Maffei et al., 2006; Smirnoff and Arnaud, 2019). Similar responses were found when Arabidopsis thaliana plants were exposed to α-pinene and β-pinene, which induced the accumulation of O2– and up-regulated systemic acquired resistance (SAR)-mediated genes (Riedlmeier et al., 2017).
Late response events
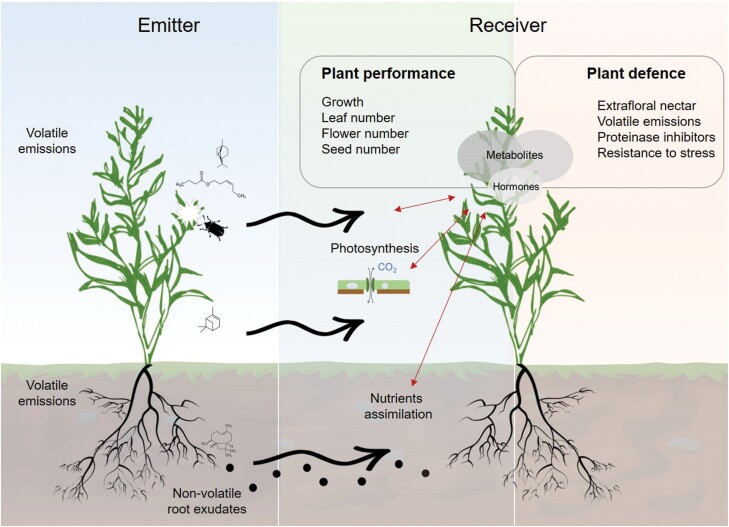
A later component of the plant response to VOC cues is the regulation of the phytohormone network that leads to the induction or priming of plant defences, especially by promoting JA signalling (Arimura et al., 2000b; Rodriguez-Saona et al., 2009; Frank et al., 2021). JA signalling plays a key role in inducing defensive responses locally and systemically upon insect feeding (Farmer et al., 1992). Many GLVs have been shown to promote JA levels in receiver plants, which prepare defences for subsequent herbivore attack (Engelberth et al., 2004; Frost et al., 2008). (Z)-3-hexenol and (Z)-3-hexenyl acetate enhanced JA production in Zea mays after short-term exposure periods (Engelberth et al., 2004). Several terpenes were also found to promote the JA pathways. The first observation was made in the year 2000 in a study in which several terpenes induced up-regulation of the lipoxygenase (LOX) gene as well as multiple defence genes mediated by JA in receiver plants (Arimura et al., 2000b). More recently, (E)-nerolidol was found to increase JA and salicylic acid (SA) levels in C. sinensis (Chen et al., 2020). SA signalling was also induced by exposure to volatilized α-pinene and β-pinene, which activated the expression of SAR-associated genes (Riedlmeier et al., 2017). To date, several studies have shown that VOCs modulate JA, SA, and auxin phytohormonal pathways to enhance plant defences (Erb, 2018). Phytohormones are key elements of the signal transduction leading to the control of gene expression and the production of primary and secondary metabolites (Zhao et al., 2005). Consequently, VOCs may act as modulators of receiver plant homeostasis and could trigger reconfiguration of receiver plant primary and secondary metabolism, thus enabling fine-tuning of responses in accordance with the situation represented by the VOC blend (Fig. 1).
Fig. 1.
Summary of known changes occurring in receiver plants induced by volatile cues. By influencing the rate of photosynthesis, nutrient assimilation, and hormone signalling, VOCs might reconfigure the primary and secondary metabolism to support physiological adjustments in receiver plants. Physiological adjustments to VOCs are characterized by an increase in defences before and upon stress in receivers, such as a greater production of extrafloral nectar (Kost and Heil, 2006; Choh et al., 2006), volatile emissions (Engelberth et al., 2004; Li and Blande, 2017), and proteinase inhibitors (Farmer and Ryan, 1990; Kessler et al., 2006). VOCs can also influence receiver plant performance by affecting root and shoot growth (Ninkovic, 2003; Engelberth and Engelberth, 2019) and their reproduction (Kost and Heil, 2006; Pashalidou et al., 2020).
Further events in the defence response include the enhancement of defensive gene expression, and an increase in secondary metabolites, including VOC emissions (Table 1). Several studies have reported the up-regulation of defence-related genes (Table 1), indicating that plants have detected and responded to a volatile cue, but falling short of demonstrating a tangible defence-related response. Moreover, gene expression may be more likely to be observed if analyses are conducted on excised leaves rather than whole plants, which was the case in several of the early studies (Baldwin et al., 2002), although it should be noted that many more recent studies utilizing whole plants or seedlings have also shown the up-regulation of defence-related genes in response to volatile cues (Farag et al., 2005; Godard et al., 2008). For example, cabbage (B. oleracea) plants exposed to neighbours infested with Pieris brassicae larvae had higher levels of LOX transcripts than control plants (Peng et al., 2011). After subsequent infestation with larvae, those same plants were more attractive to Cotesia glomerata parasitoids than controls, showing that an increase in gene expression can indicate a defence-related response. Increases in secondary metabolites such as proteinase inhibitors (Kessler et al. 2006) and Sporamin protease inhibitor (Meents et al., 2019) were also reported. Several studies also showed that exposure to VOCs induced the de novo production of VOCs (Engelberth et al., 2004; Yan and Wang, 2006; Cascone et al., 2015) and increased secretion of extrafloral nectar as a source of sugar to attract more predators of herbivores (Kost and Heil, 2006; Heil and Silva Bueno, 2007; Li et al., 2012). In a study of hybrid aspen (Populus tremula × tremuloides), the secretion of extrafloral nectar was directly induced by VOCs from herbivore-infested conspecifics, whereas increased emission of volatiles was primed and increased only upon subsequent herbivore feeding (Li et al., 2012).
Priming: responses to VOCs upon stress
Defence priming in plants was reported by Engelberth et al. (2004), whereby corn seedlings exposed to GLVs emitted by damaged neighbours increased their production of JA and volatile sesquiterpenes upon exposure to mechanical damage and regurgitant of Spodoptera exigua, the beet armyworm. This was a landmark study showing that plants can respond to an environmental cue to prepare themselves for defence without costly investment into manufacturing defence-related compounds in the absence of attack (Frost et al., 2008). It was quickly suggested that utilizing herbivore-induced plant volatiles to prime agricultural plants for augmented defence expression could be an ecologically and environmentally sustainable method of combating pests (Turlings and Ton, 2006). Indeed, since the study by Engelberth et al. (2004) there has been a proliferation of work on defence priming (Table 1). Exposure to stress-induced volatiles has been shown to lead to quicker and stronger defence responses upon subsequent herbivory (e.g. Frost et al., 2008; Cascone et al., 2015; Shiojiri et al., 2015) and increased resistance to herbivores, abiotic stresses (e.g. Cofer et al., 2018), and pathogen infection (e.g. Girón-Calva et al., 2012; Ameye et al., 2015). Several studies have also shown that exposure to stress-induced volatiles makes receiver plants more attractive to beneficial insects or enhances the production of extrafloral nectar (Muroi et al., 2011; Choh et al., 2006). Focusing specifically on coverage of volatile-mediated priming, several reviews have provided detailed syntheses of current knowledge and insight into mechanisms and consequences (Frost et al., 2008; Kant et al. 2009; Kim and Felton, 2013; Gonzalez-Bosch, 2018). However, there has been a rapid increase in reports of priming in the past few years, and knowledge on the intricacies of primed plant responses is increasing. Studies of maize have shown aboveground volatile emission and receipt of cues to be more important in volatile-mediated plant–plant interactions than belowground processes (van Doan et al., 2021), while indole has been shown to be an important cue in priming of maize (Erb et al., 2015) and tea (Ye et al., 2021). Interestingly, priming in maize is induced independently by indole and the GLV (Z)-3-hexenyl acetate, but there is stronger priming when both molecules are present, in line with a synergistic effect (Hu et al., 2019). A study by Michereff et al. (2021) indicated that priming in maize also appears to be genotype specific, although this observation was likely to be due to differences in the capacities of the two genotypes tested for induced volatile emission.
Interestingly, a recent study showed that whitefly infestation of tomato plants induces the release of a blend of volatiles that renders neighbouring plants more susceptible to whitefly infestation (Zhang et al., 2019). The whitefly-induced volatiles prime SA-dependent defences and suppress JA-dependent defences, which renders the plants more suitable for whitefly development. Volatiles induced by the chewing herbivore S. exigua primed the plants for better defence against S. exigua larvae, indicated by lower weight gain of larvae. This suggests that the enhanced susceptibility induced by whitefly-induced volatiles is not a general response but could be part of an elaborate manipulation of the plants through low-molecular-weight proteins in the whitefly saliva (Xu et al., 2019).
There is substantially less documented evidence of belowground volatile-mediated plant–plant interactions, although there is significant evidence indicating roles for root exudates and mycorrhizae in providing between-plant cues and signals (e.g. Babikova et al., 2013). Recent studies have shown that sesquiterpenes constitutively released from roots of spotted knapweed, Centaurea stoebe, have no significant effect on the secondary metabolites of Taraxacum officinale plants (Huang et al., 2019). A similar observation was observed in maize roots, whereby volatiles induced by the root-feeding banded cucumber beetle did not induce responses in neighbouring conspecifics (van Doan et al., 2021). Studies of plant–plant interactions, and particularly priming, are potentially sensitive to the timing of the cue or signal, the longevity of the receiver plant response, and the plant organ receiving the cue. Consequently, to accurately detect priming there is a need to acquire temporal and spatial measurements of metabolic change. Non-invasive methods to detect physiological plant responses are required. Observing changes in VOC emission patterns over time can be done using proton transfer reaction mass spectrometry, which offers a means to monitor rapid changes in volatile emissions (Misztal et al., 2016). Moreover, there is some potential for hyperspectral imaging to be utilized to correlate leaf reflectance to phytochemical data (Ribeiro et al., 2018), which could be a useful tool for comparing plants exposed to different treatments, including volatile exposure and herbivore feeding. Non-destructive tools could be highly advantageous for measuring primed plant responses.
Effect of VOCs on plant performance
Effects on growth and reproduction
Typically, research on plant–plant interactions based on VOCs has focused on defence-related responses, which is a logical approach if it is considered that the VOCs emitted by herbivore-damaged plants are indicative of a potential attack (Douma et al., 2019). The most appropriate response in a receiver plant could be to initiate or prepare defences to repel or minimize damage (Frost et al., 2008). In this context, growth and reproduction have received less attention (Table 2). Growth responses could indicate possible competition-related or strengthening responses, whereas advanced flowering could indicate the preparation of damage-limitation strategies based on ensuring reproduction (Lucas-Barbosa et al. 2013).
Table 2.
Summary of studies published on the effects of exposure to VOCs on plant primary metabolism and performance
| Volatiles | Species | Exposure time | Effects on plant primary metabolism and performance | References |
|---|---|---|---|---|
| (Z)-3-Hexenyl acetate (E)-3-Hexenyl acetate | Zea mays | 1.5 h | Increased growth | Cofer et al., 2018 |
| Z. mays | 16 h | Enhanced overall growth | Engelberth and Engelberth, 2019 | |
| Phaseolus lunatus | 7 d | Increased growth and flower production | Freundlich et al., 2021 | |
| Capsicum annuum | 7 d | Reduced growth | Freundlich et al., 2021 | |
| Clipped Artemisia tridentata | A. tridentata | 24 h | Reduced growth in height | Karban, 2017 |
| Clipped A. tridentata | Nicotiana attenuata | Growing season | Increased seed production | Karban and Maron, 2002 |
| VOCs from Solidago altissima primed by Eurosta solidaginis volatiles | S. altissima | 24 h | Increased growth rate and reduced rhizome production, indicating a reduction in clonal reproductive capacity | Yip et al., 2019 |
| Synthetic VOC blend | P. lunatus | 3 d | Increased number of leaves and inflorescences | Kost and Heil, 2006 |
| Pieris brassicae oviposition-induced volatile cues | Brassica nigra | 5 d | Reduced growth and increased number of flowers and seeds | Pashalidou et al., 2020 |
| Salt-induced VOCs | Vicia faba | 2 weeks | Reduced net photosynthesis; increased growth and resilience upon salt stress | Caparrotta et al., 2018 |
| VOCs from Hordeum vulgare cv. Alva | H. vulgare var. Kara | Throughout the vegetative growth phase | Increased root biomass and leaf area | Ninkovic, 2003 |
| VOCs from another cultivar | H. vulgare | 5 d | Reduced leaf temperature | Pettersson et al., 1999 |
| Jasmonic acid (25mM) | Brassica napus | Exogenous applications | Increased net photosynthesis, stomatal conductance, transpiration rate, and intracellular CO2 concentration | Ali et al., 2018 |
| Methyl salicylate (10mM) | Vaccinium myrtillus | Exogenous applications | Reduced net photosynthesis and down-regulated genes associated with growth, photosynthesis, and reproduction | Benevenuto et al., 2019 |
| Root VOCs of Centaurea stoebe | Several sympatric neighbours | Throughout growth | Increased germination and growth of sympatric neighbours | Gfeller et al., 2019 |
| Root VOCs of C. stoebe | Taraxacum officinale | 7 weeks | Increased root protein, fructose, and sucrose concentrations | Huang et al., 2019 |
Growth and reproduction are the most important components of plant fitness and constitute key physiological parameters to estimate the allocation of carbon. Since priming is an inducible phenomenon, theories predict a cost to inducing physiological changes (Douma et al., 2017). This cost, although less expensive than induced defence, could reduce the resources allocated to growth and reproduction and in so doing affect plant fitness (Paul-Victor et al., 2010; Orrock et al., 2018). We previously stated that VOCs modulate JA signalling involved in the induction of defences, and it is mostly assumed that by enhancing JA signalling and defences, VOCs would inhibit growth (Zhang and Turner, 2008).
The cost of priming has been studied by assessing plant growth after the application of a low-dose of (Z)-3-hexenyl acetate to lima bean (Phaseolus lunatus) and pepper (Capsicum annuum) plants (Freundlich et al., 2021). Whereas (Z)-3-hexenyl acetate-treated pepper plants showed reduced growth but had no difference in resistance to herbivores relative to controls, (Z)-3-hexenyl acetate-treated lima bean plants grew more, produced more flowers, and suffered less chewing herbivory compared with control plants (Freundlich et al., 2021). Similarly, Karban (2017) found differential effects of exposure to volatile cues on plant growth depending on the species exposed. Young focal sagebrush (Artemisia tridentata subsp. vaseyana) plants exposed to clipped conspecific neighbours grew less in height than controls not exposed to these cues, whereas Nicotiana attenuata exposed to clipped sagebrush produced more seeds relative to control plants (Karban and Maron, 2002). These studies support the hypothesis that exposure to plant volatiles affects plant fitness (i.e. growth and reproduction) but that the extent to which fitness is affected differs depending on the species and the volatile chemical. This idea is corroborated in tall goldenrod with priming cues derived from Eurosta solidaginis; in a semi-natural field experiment, primed plants were shown to grow faster than unprimed plants but they produced fewer rhizomes, indicating a reduction in clonal reproductive capacity (Yip et al., 2019).
Additional studies have provided evidence that plant volatiles might affect plant performance. Lima bean shoots exposed to HIPVs produced more leaves and inflorescences than untreated control shoots (Kost and Heil, 2006). More recently, it was reported that (Z)-3-hexenyl acetate-treated maize seedlings had a significant reduction in growth over the first 16h of treatment (Engelberth and Engelberth, 2019). However, the growth rate of seedlings increased on the second day after treatment, and their resistance against subsequent herbivory was not affected. At the end of the experiment, treated plants had similar or even slightly enhanced overall growth compared with control plants. A recent study on brassicaceous plants showed that oviposition-induced volatile cues ramp up plant defences; this was evaluated through performance assays with larvae of the specialist Brassica-feeding herbivore P. brassicae (Pashalidou et al., 2020). There was a concurrent reduction in the growth of receiver plants, but an increase in the number of flowers and seeds (Pashalidou et al., 2020).
Effects on gas exchange and nutrient uptake
The literature suggests that plants may have developed several physiological mechanisms to compensate for the cost of inducing defences (Engelberth and Engelberth, 2019). Plants might increase their photosynthesis or stomatal conductance (to take up more CO2) or increase their root length (to take up more nutrients). Interestingly, salt stress has been shown to induce volatile emissions that can prime neighbouring plants for greater resilience to salt in Arabidopsis (Lee and Seo, 2014), broad bean (Vicia faba) (Caparrotta et al., 2018), and sweet basil (Ocimum basilicum) (Landi et al., 2020) plants. Broad bean plants showed reduced photosynthesis in response to VOCs but gained greater resilience to salt and grew more than controls upon salt exposure (Caparrotta et al., 2018). Sweet basil did not reduce photosynthesis upon receipt of a volatile cue, but when subsequently exposed to salt it reduced photosynthesis more dramatically than controls, maintained a greater water use efficiency, and, while not differing from controls in growth, showed earlier senescence and flowering, and a greater seed set (Landi et al., 2020).
Ninkovic (2003) investigated the effect of volatiles from two barley (Hordeum vulgare) cultivars (Alva and Kara) on plant root and shoot biomass, and found that Kara plants exposed to Alva volatiles allocated significantly more biomass to roots and increased their leaf area compared with Kara plants exposed to volatiles from Kara or to clean air. Another study on different barley cultivars reported a reduction in leaf temperature when some cultivars were exposed to volatiles from other cultivars (Pettersson et al., 1999). A reduction in leaf temperature is generally correlated with a higher transpiration rate and greater stomatal conductance. A greater stomatal conductance assumes an increase in CO2 uptake, which is required for a higher photosynthetic activity. Recent work investigating the effect of HIPVs on the photosynthesis and stomatal conductance of receiver plants has indicated increases of both relative to controls (Blande and Yu, 2021). With the exception of this ongoing research, the effect of plant volatiles on photosynthesis has been observed in connection with exogenous application of the signalling phytohormones SA and its volatile derivative methyl salicylate (MeSA), and JA and its derivative MeJA. The exogenous application of JA (25mM) was found to increase net photosynthesis, stomatal conductance, transpiration rate, and intracellular CO2 concentration (Ali et al., 2018). However, the application of MeSA (10mM) and MeJA (150mM) decreased photosynthesis and down-regulated genes associated with growth, photosynthesis, and reproduction (Rahnamaie-Tajadod et al., 2017; Benevenuto et al., 2019).
Ecological implications of responding to VOCs
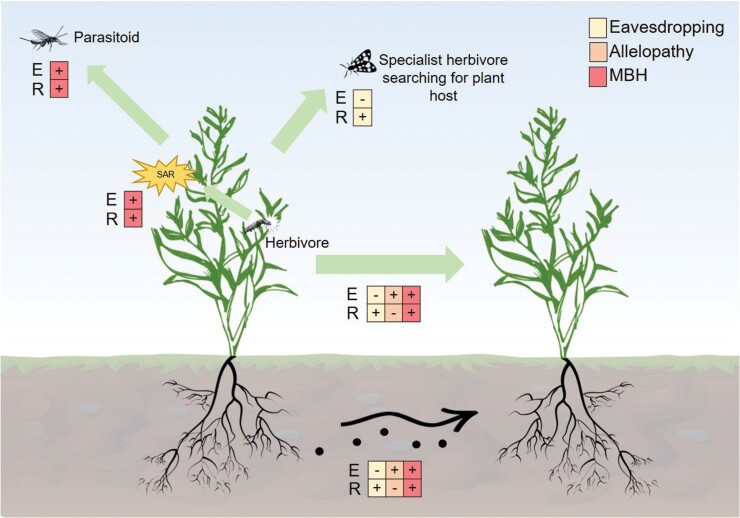
In the above sections we have provided an overview of mechanisms underpinning volatile-mediated plant–plant interactions and the variety of responses induced by VOCs in relation to both plant defences and performance. It is evident that a number of responses induced in receiver plants can affect the behaviour and performance of herbivorous insects (e.g. Grof-Tisza et al., 2020) and plant resistance to other biotic or abiotic stressors (e.g. Cofer et al., 2018). Therefore, the role of volatile-mediated plant–plant interactions can be of ecological importance (Fig. 2). A number of studies conducted under field conditions have shown that plants receiving damage-induced volatile cues can gain a greater level of resistance to pests during the course of a season (e.g. Tscharntke et al., 2001; Karban et al., 2006). It has been argued that receiver plants ‘eavesdrop’ on cues that have evolved for different recipients, for example, predatory or parasitic insects, or different parts of the emitting plant (Fig. 2). However, a number of nuances in plant–plant interactions have been observed indicating that there could be a higher than expected level of complexity and sophistication. Sagebrush plants have been shown to produce cues that are responded to more strongly by self-cloned plants than by non-self alternatives (Karban and Shiojiri, 2009). Sagebrush chemotypes can also respond more effectively to volatiles emitted by individuals of the same, rather than a different, chemotype (Karban et al., 2014a). Moreover, it seems that some chemotypes may be better at receiving cues than others. Maize genotypes that differ in traits related to volatile emission capacity also appear to differ in their abilities to interact (Michereff et al., 2021). Interspecific interactions have also indicated that some plants are better senders and receivers of cues than others (Karban et al., 2006; Pearse et al., 2012). It appears that although the quantity of volatiles emitted could play a significant role in determining what makes a good emitter, it does not follow that all plants receiving the cues from such an emitter will respond.
Fig. 2.
Ecological consequences of emitting and receiving volatile cues. The arrows indicate the direction of VOC transport. The boxes indicate the potential outcomes of the response and whether the emitter or receiver gains a beneficial (+) or a detrimental (-) effect on fitness. SAR indicates that the emitting plant may gain systemic acquired resistance. We refer to allelopathy as a phenomenon whereby the emitter releases chemicals that have detrimental effects on the performance of the receiver plant (Inderjit and Duke, 2003). Eavesdropping is the process whereby a receiver intercepts and uses information encoded in chemical cues that evolved to provide information to a different recipient (Karban, 2015). MBH indicates the mutual-benefits hypothesis, whereby the emitter and receiver benefit from the transport of VOC cues, irrespective of their relatedness, through the responses of receivers reducing the risk of herbivory (Kalske et al., 2019).
A recent study by Kalske et al. (2019) tested the predictions of two hypotheses, the kin-selection and mutual-benefits hypotheses, to determine the selective criteria that would favour the occurrence of volatile-mediated plant–plant interactions. The kin-selection hypothesis dictates that emitter plants would indirectly benefit from volatile-mediated interactions by providing a cue that improves the fitness of their kin more than that of other genotypes. The mutual-benefits hypothesis requires that the emitter plant benefits from providing cues to plants irrespective of their relatedness, through the responses of receiver plants reducing the risk of herbivory (Fig. 2). Using tall goldenrod (Solidago altissima) as a model system, the communication of plants in an area with regular herbivore infestation was compared with that of those in an area from which herbivores were excluded. The authors observed that when selection occurred under the condition in which herbivores were excluded, interactions between related plants were more effective, whereas when selection occurred with herbivores present, communication was more uniform throughout the population (Kalske et al., 2019). An earlier study showed that plant–plant interactions in tall goldenrod led to herbivores spending less time on a receiver plant and causing less damage (Morrell and Kessler, 2017). These observations provide some of the most compelling evidence for plant–plant interactions to be considered a true communication process. While there have been a number of field studies investigating volatile-mediated plant–plant interactions, relatively few have extended to the point of examining the fitness costs and benefits. There is a clear need for a better integration of responses based on growth or reproduction with the modulation of direct and indirect defences.
Assessing the fitness benefits of plant–plant interactions provides a technical challenge due to the difficulties in manipulating plants that can and cannot interact via volatiles, while maintaining ecologically realistic scenarios. Advances in genomics in the past couple of decades have enabled the development of ‘deaf’ or ‘mute’ plants that cannot detect volatile cues (deaf) or do not release detectable cues (mute) (Baldwin et al., 2006). It was proposed that these advancements would enable the research community to test whether volatile-mediated interactions between plants enhance the fitness of plants in natural communities (Baldwin et al., 2006; Dicke and Baldwin, 2010). A study conducted with N. attenuata in nature showed that mute plants, those silenced in HIPV emission, have lower fitness than HIPV-emitting plants, based on the recruitment of Geocoris spp. predators (Schuman et al., 2012). However, field studies utilizing such mute or deaf plants to develop understanding of the ecological implications of volatile-mediated plant–plant interactions remain mostly lacking. Indeed, mechanistic studies to explicitly determine the roles of chemical mediators of plant interactions under field conditions in general are typically lacking (Schuman and Baldwin, 2018).
Since the chemotype of plants appears to be an important factor in determining the outcome of intraspecific plant–plant interactions, there is scope for increasing our understanding of the intricacies of plant–plant interactions through reciprocal experiments exposing numerous different chemotype receivers to numerous different chemotype emitters. Combining the use of chemotypes with the genomic tools for creating mute and deaf plants would provide an excellent resource for future studies on the ecology and mechanisms of variation in intraspecific plant–plant interactions.
Conclusions and future directions
Here, we have shown that the perception of VOCs by plants triggers a series of events leading to changes in plant defence and performance. The induction or priming of defences depends on the intake or storage of energy, and the availability of carbon, nitrogen, and sulfur provided by primary metabolism. In this review, we highlighted that VOCs have the potential to affect the photosynthesis rate and nutrient uptake and therefore modulate primary and secondary metabolisms, leading to change in overall plant performance (Fig. 1).
Evidence is accumulating that volatile-mediated interactions are nuanced and highly variable based on the capacity of plants to produce and emit volatiles, but there are as yet several poorly elucidated factors. The trade-off between growth and defence is important, particularly given that rapid growth is a desirable trait in many agricultural crop species that have been models in studies of plant–plant interactions. Our literature searches indicated that most studies that have looked at the growth of plants after their exposure to plant VOCs concern the phenomenon of allelopathy (Fig. 2). Relatively few studies have focused on this goal in the context of other plant–plant interactions. This indicates that an excessively strong focus on defence-related pathways could miss important responses in primary metabolism, growth, and reproduction. Measurements of plant reproductive capacity or success are critical for determining the potential fitness benefits of plant–plant interactions. There are some non-invasive techniques for monitoring photosynthesis and stomatal conductance, which can indicate changes in the primary metabolism of plants. Future research should in general capitalize on technological advancements for the non-destructive measurement of plant responses, which will enable a deeper understanding of temporal and spatial receiver plant responses. It has been shown that volatile-mediated plant–plant interactions occur both above and below ground, but there is significant variation in the efficiency of each for different species, and even for different genotypes of the same species. Hence, finally, we propose that future research focuses more on integrating concurrent above- and belowground processes and the trade-offs in each occurring under different environmental conditions.
Acknowledgements
We acknowledge two anonymous reviewers for their comprehensive reviews and insightful comments, which helped us to improve the manuscript.
Contributor Information
Agnès Brosset, Department of Environmental and Biological Sciences, University of Eastern Finland, Yliopistonranta 1 E, P.O. Box 1627, Kuopio FIN-70211, Finland.
James D Blande, Department of Environmental and Biological Sciences, University of Eastern Finland, Yliopistonranta 1 E, P.O. Box 1627, Kuopio FIN-70211, Finland.
Maaria Rosenkranz, Helmholtz Zentrum München, Germany.
Conflict of interest
The authors have no conflict of interest to declare.
Funding
We acknowledge funding by the Academy of Finland, decision numbers 309425 and 311925. AB was supported by grants from the Niemi Foundation, project number 20190091, and Kone Foundation, project number 201905451.
References
- Ali E, Hussain N, Shamsi IH, Jabeen Z, Siddiqui MH, Jiang LX. 2018. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. Journal of Zhejiang University Science B 19, 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alméras E, Stolz S, Vollenweider S, Reymond P, Mène-Saffrané L, Farmer EE. 2003. Reactive electrophile species activate defense gene expression in Arabidopsis. The Plant Journal 34, 205–216. [DOI] [PubMed] [Google Scholar]
- Ameye M, Audenaert K, De Zutter N, Steppe K, Van Meulebroek L, Vanhaecke L, De Vleesschauwer D, Haesaert G, Smagghe G. 2015. Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium graminearum but boosts deoxynivalenol production. Plant Physiology 167, 1671–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameye M, Van Meulebroek L, Meuninck B, Vanhaecke L, Smagghe G, Haesaert G, Audenaert K. 2020. Metabolomics reveal induction of ROS production and glycosylation events in wheat upon exposure to the green leaf volatile Z-3-hexenyl acetate. Frontiers in Plant Science 11, 596271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Muroi A, Nishihara M. 2012. Plant–plant–plant communications, mediated by (E)-β-ocimene emitted from transgenic tobacco plants, prime indirect defense responses of lima beans. Journal of Plant Interaction 7, 193– 196. [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. 2000b. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406, 512–515. [DOI] [PubMed] [Google Scholar]
- Arimura G, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J. 2000a. Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochemical and Biophysical Research Communications 277, 305–310. [DOI] [PubMed] [Google Scholar]
- Asai N, Nishioka T, Takabayashi J, Furuichi T. 2009. Plant volatiles regulate the activities of Ca2+-permeable channels and promote cytoplasmic calcium transients in Arabidopsis leaf cells. Plant Signaling & Behavior 4, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikova Z, Gilbert L, Bruce TJ, Birkett M, Caulfield JC, Woodcock C, Pickett JA, Johnson D. 2013. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecology Letters 16, 835–843. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. 2006. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311, 812–815. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Kessler A, Halitschke R. 2002. Volatile signaling in plant–plant–herbivore interactions: what is real? Current Opinion in Plant Biology 5, 351–354. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schultz JC. 1983. Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221, 277–279. [DOI] [PubMed] [Google Scholar]
- Benevenuto RF, Seldal T, Hegland SJ, Rodriguez-Saona C, Kawash J, Polashock J. 2019. Transcriptional profiling of methyl jasmonate-induced defense responses in bilberry (Vaccinium myrtillus L.). BMC Plant Biology 19, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blande JD, Yu H. 2021. Volatile-mediated interactions in polluted environments: consequences for chemical communication in conifers. In: SEB 2021 Annual Conference Abstract Book, P6.19. https://www.sebiology.org/digital-magazine/abstract-book/#p=246 [Google Scholar]
- Bui TNT, Himanen SJ, Holopainen JK. 2021. Environmentally acquired chemical camouflage affects Pieris brassicae L. host plant selection and orientation behaviour of a larval parasitoid. Arthropod-Plant Interactions 15, 299–312. [Google Scholar]
- Camacho-Coronel X, Molina-Torres J, Heil M. 2020. Sequestration of exogenous volatiles by plant cuticular waxes as a mechanism of passive associational resistance: a proof of concept. Frontiers in Plant Science 11, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparrotta S, Boni S, Taiti C, Palm E, Mancuso S, Pandolfi C. 2018. Induction of priming by salt stress in neighboring plants. Environmental and Experimental Botany 147, 261–270. [Google Scholar]
- Cascone P, Iodice L, Maffei ME, Bossi S, Arimura G, Guerrieri E. 2015. Tobacco overexpressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. Journal of Plant Physiology 173, 28–32. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang L, Cai X, et al. 2020. (E)-Nerolidol is a volatile signal that induces defenses against insects and pathogens in tea plants. Horticulture Research 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choh Y, Takabayashi J. 2006. Herbivore-induced extrafloral nectar production in lima bean plants enhanced by previous exposure to volatiles from infested conspecifics. Journal of Chemical Ecology 32, 2073–2077. [DOI] [PubMed] [Google Scholar]
- Cofer TM, Engelberth M, Engelberth J. 2018. Green leaf volatiles protect maize (Zea mays) seedlings against damage from cold stress. Plant, Cell & Environment 41, 1673–1682. [DOI] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. 2010. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends in Plant Science 15, 167–175. [DOI] [PubMed] [Google Scholar]
- Dolch R, Tscharntke T. 2000. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia 125, 504–511. [DOI] [PubMed] [Google Scholar]
- Dombrowski JE, Martin RC. 2018. Activation of MAP kinases by green leaf volatiles in grasses. BMC Research Notes 11, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma JC, Ganzeveld LN, Unsicker SB, Boeckler GA, Dicke M. 2019. What makes a volatile organic compound a reliable indicator of insect herbivory? Plant, Cell & Environment 42, 3308–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma JC, Vermeulen PJ, Poelman EH, Dicke M, Anten NPR. 2017. When does it pay off to prime for defense? A modeling analysis. New Phytologist 216, 782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Klempien A, Muhlemann JK, Kaplan I. 2013. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytologist 198, 16–32. [DOI] [PubMed] [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. 2004. Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences, USA 101, 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Contreras CF, Dalvi C, Li T, Engelberth M. 2013. Early transcriptome analyses of Z-3-hexenol-treated Zea mays revealed distinct transcriptional networks and anti-herbivore defense potential of green leaf volatiles. PLoS One 8, e77465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Engelberth M. 2019. The costs of green leaf volatile-induced defense priming: Temporal diversity in growth responses to mechanical wounding and insect herbivory. Plants 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M. 2018. Volatiles as inducers and suppressors of plant defense and immunity – origins, specificity, perception and signaling. Current Opinion in Plant Biology 44, 117–121. [DOI] [PubMed] [Google Scholar]
- Erb M. 2019. Plant biology: evolution of volatile-mediated plant–plant interactions. Current Biology 29, R873–R875. [DOI] [PubMed] [Google Scholar]
- Erb M, Veyrat N, Robert CM, et al. 2015. Indole is an essential herbivore-induced volatile priming signal in maize. Nature Communication 6, 6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Fokar M, Abd H, Zhang H, Allen RD, Paré PW. 2005. (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta 220, 900–909. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Johnson RR, Ryan CA. 1992. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiology 98, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. 1990. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proceedings of the National Academy of Sciences, USA 87, 7713–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SV, Lawton JH. 1985. Rapidly induced defenses and talking trees: the devil’s advocate position. American Naturalist 126, 181–195. [Google Scholar]
- Frank L, Wenig M, Ghirardo A, van der Krol A, Vlot AC, Schnitzler JP, Rosenkranz M. 2021. Isoprene and β-caryophyllene confer plant resistance via different plant internal signalling pathways. Plant, Cell & Environment 44, 1151–1164. [DOI] [PubMed] [Google Scholar]
- Freundlich GE, Shields M, Frost CJ. 2021. Dispensing a synthetic green leaf volatile to two plant species in a common garden differentially alters physiological responses and herbivory. Agronomy 11, 958. [Google Scholar]
- Frost CJ, Appel HM, Carlson JE, De Moraes CM, Mescher MC, Schultz JC. 2007. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecology Letters 10, 490–498. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM. 2008. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiology 146, 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM. 2008. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytologist 180, 722–734. [DOI] [PubMed] [Google Scholar]
- Gfeller V, Huber M, Förster C, Huang W, Köllner TG, Erb M. 2019. Root volatiles in plant–plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant, Cell & Environment 42, 1950–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón-Calva PS, Li T, Koski TM, Klemola T, Laaksonen T, Huttunen L, Blande JD. 2014. A role for volatiles in intra- and inter-plant interactions in birch. Journal of Chemical Ecology 40, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Girón-Calva PS, Molina-Torres J, Heil M. 2012. Volatile dose and exposure time impact perception in neighboring plants. Journal of Chemical Ecology 38, 226–228. [DOI] [PubMed] [Google Scholar]
- Godard KA, White R, Bohlmann J. 2008. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry 69, 1838–1849. [DOI] [PubMed] [Google Scholar]
- González-Bosch C. 2018. Priming plant resistance by activation of redox-sensitive genes. Free Radical Biology & Medicine 122, 171–180. [DOI] [PubMed] [Google Scholar]
- Grof-Tisza P, Karban R, Pan VS, Blande JD. 2020. Assessing plant-to-plant communication and induced resistance in sagebrush using the sagebrush specialist Trirhabda pilosa. Arthropod-Plant Interactions 14, 327–332. [Google Scholar]
- Hazrati H, Fomsgaard IS, Kudsk P. 2020. Root-exuded benzoxazinoids: uptake and translocation in neighboring plants. Journal of Agricultural and Food Chemistry 68, 10609–10617. [DOI] [PubMed] [Google Scholar]
- Heil M, Karban R. 2010. Explaining evolution of plant communication by airborne signals. Trends in Ecology & Evolution 25, 137–144. [DOI] [PubMed] [Google Scholar]
- Heil M, Kost C. 2006. Priming of indirect defences. Ecology Letters 9, 813–817. [DOI] [PubMed] [Google Scholar]
- Heil M, Lion U, Boland W. 2008. Defense-inducing volatiles: in search of the active motif. Journal of Chemical Ecology 34, 601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC. 2007. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proceedings of the National Academy of Sciences, USA 104, 5467–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemachandran H, Doss CGP, Siva R. 2017. Plant communication: an unresolved mystery. Current Science 112, 1990–1991. [Google Scholar]
- Hepler PK. 2005. Calcium: a central regulator of plant growth and development. The Plant Cell 17, 2142–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen SJ, Blande JD, Klemola T, Pulkkinen J, Heijari J, Holopainen JK. 2010. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants – a mechanism for associational herbivore resistance? New Phytologist 186, 722–732. [DOI] [PubMed] [Google Scholar]
- Himanen SJ, Bui TN, Maja MM, Holopainen JK. 2015. Utilizing associational resistance for biocontrol: impacted by temperature, supported by indirect defence. BMC Ecology 15, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Ye M, Erb M. 2019. Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defence and resistance. Plant, Cell & Environment 42, 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Gfeller V, Erb M. 2019. Root volatiles in plant–plant interactions II: Root volatiles alter root chemistry and plant-herbivore interactions of neighbouring plants. Plant, Cell & Environment 42, 1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderjit, Duke SO. 2003. Ecophysiological aspects of allelopathy. Planta 217, 529–539. [DOI] [PubMed] [Google Scholar]
- Jing T, Du W, Gao T, et al. 2021. Herbivore-induced DMNT catalyzed by CYP82D47 plays an important role in the induction of JA-dependent herbivore resistance of neighboring tea plants. Plant, Cell & Environment 44, 1178–1191. [DOI] [PubMed] [Google Scholar]
- Kalske A, Shiojiri K, Uesugi A, Sakata Y, Morrell K, Kessler A. 2019. Insect herbivory selects for volatile-mediated plant-plant communication. Current Biology 29, 3128–3133.e3. [DOI] [PubMed] [Google Scholar]
- Kang ZW, Liu FH, Zhang ZF, Tian HG, Liu TX. 2018. Volatile β-ocimene can regulate developmental performance of peach aphid Myzus persicae through activation of defense responses in Chinese cabbage Brassica pekinensis. Frontiers in Plant Science 9, 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant MR, Bleeker PM, Van Wijk M, Schuurink RC, Haring MA. 2009. Plant volatiles in defence. Plant Innate Immunity 51, 613–666. [Google Scholar]
- Karban R. 2015. Plant sensing and communication. Chicago: University of Chicago Press. [Google Scholar]
- Karban R. 2017. Tradeoff between resistance induced by volatile communication and over-topping vertical growth. Plant Signaling & Behavior 12, e1309491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R, Maron J. 2002. The fitness consequences of interspecific eavesdropping between plants. Ecology 83, 1209–1213. [Google Scholar]
- Karban R, Shiojiri K. 2009. Self-recognition affects plant communication and defense. Ecology Letters 12, 502–506. [DOI] [PubMed] [Google Scholar]
- Karban R, Shiojiri K, Huntzinger M, McCall AC. 2006. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87, 922–930. [DOI] [PubMed] [Google Scholar]
- Karban R, Wetzel WC, Shiojiri K, Ishizaki S, Ramirez SR, Blande JD. 2014a. Deciphering the language of plant communication: volatile chemotypes of sagebrush. New Phytologist 204, 380–385. [DOI] [PubMed] [Google Scholar]
- Karban R, Yang LH, Edwards KF. 2014b. Volatile communication between plants that affects herbivory: a meta-analysis. Ecology Letters 17, 44–52. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT. 2006. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuatta. Ocecologia 148, 280– 292. [DOI] [PubMed] [Google Scholar]
- Kim J, Felton GW. 2013. Priming of antiherbivore defensive responses in plants. Insect Science 20, 273–285. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Matsui K, Ozawa R, Takabayashi J. 2005. Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant & Cell Physiology 46, 1093–1102. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Matsui K, Ozawa R, Takabayashi J. 2006. Analysis of defensive responses activated by volatile allo-ocimene treatment in Arabidopsis thaliana. Phytochemistry 67, 1520–1529. [DOI] [PubMed] [Google Scholar]
- Kost C, Heil M. 2006. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. Journal of Ecology 94, 619–628. [Google Scholar]
- Landi M, Araniti F, Flamini G, Piccolo EL, Trivellini A, Abenavoli MR, Guidi L. 2020. “Help is in the air”: volatiles from salt-stressed plants increase the reproductive success of receivers under salinity. Planta 251, 48. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. 2006. Calcium in plant defence-signalling pathways. New Phytologist 171, 249–269. [DOI] [PubMed] [Google Scholar]
- Lee K, Seo PJ. 2014. Airborne signals from salt-stressed Arabidopsis plants trigger salinity tolerance in neighboring plants. Plant Signaling & Behavior 9, e28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. 2016. Neighbour recognition through volatile-mediated interactions. In: Blande JD, Glinwood R, eds. Deciphering the chemical language of plant communication, Vol. 1. Cham: Springer International Publishing, 153–174. [Google Scholar]
- Li T, Blande JD. 2015. Associational susceptibility in broccoli: mediated by plant volatiles, impeded by ozone. Global Change Biology 21, 1993–2004. [DOI] [PubMed] [Google Scholar]
- Li T, Blande JD. 2017. Volatile-mediated within-plant signaling in hybrid aspen: required for systemic responses. Journal of Chemical Ecology 43, 327–338. [DOI] [PubMed] [Google Scholar]
- Li T, Holopainen JK, Kokko H, Tervahauta AI, Blande JD. 2012. Herbivore-induced aspen volatiles temporally regulate two different indirect defences in neighbouring plants. Functional Ecology 26, 1176–1185. [Google Scholar]
- Liao P, Ray S, Boachon B, Lynch JH, Deshpande A, McAdam S, Morgan JA, Dudareva N. 2021. Cuticle thickness affects dynamics of volatile emission from petunia flowers. Nature Chemical Biology 17, 138–145. [DOI] [PubMed] [Google Scholar]
- Lucas-Barbosa D, van Loon JJ, Gols R, van Beek TA, Dicke M. 2013. Reproductive escape: annual plant responds to butterfly eggs by accelerating seed production. Functional Ecology 27, 245–254. [Google Scholar]
- Maffei M, Bossi S, Spiteller D, Mithöfer A, Boland W. 2004. Effects of feeding Spodoptera littoralis on lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiology 134, 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Bossi S. 2006. Electrophysiology and plant responses to biotic stress. In: Volkov A, eds. Plant electrophysiology – theory and methods. Berlin, Heidelberg: Springer, 461–481. [Google Scholar]
- Maffei M, Camusso W, Sacco S. 2001. Effect of Mentha × piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 58, 703–707. [DOI] [PubMed] [Google Scholar]
- Maffei ME, Mithöfer A, Arimura G, et al. 2006. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiology 140, 1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Mithöfer A, Boland W. 2007. Before gene expression: early events in plant–insect interaction. Trends in Plant Science 12, 310–316. [DOI] [PubMed] [Google Scholar]
- McCall PJ, Turlings TC, Loughrin J, Proveaux AT, Tumlinson JH. 1994. Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L.) seedlings. Journal of Chemical Ecology 20, 3039–3050. [DOI] [PubMed] [Google Scholar]
- Meents AK, Chen SP, Reichelt M, Lu HH, Bartram S, Yeh KW, Mithöfer A. 2019. Volatile DMNT systemically induces jasmonate-independent direct anti-herbivore defense in leaves of sweet potato (Ipomoea batatas) plants. Scientific Reports 9, 17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents AK, Mithöfer A. 2020. Plant–plant communication: is there a role for volatile damage-associated molecular patterns? Frontiers in Plant Science 11, 583275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michereff MFF, Grynberg P, Togawa RC, et al. 2021. Priming of indirect defence responses in maize is shown to be genotype-specific. Arthropod-Plant Interactions 15, 313–328. [Google Scholar]
- Misztal PK. 2016. Measuring rapid changes in plant volatiles at different spatial levels. In: Blande JD, Glinwood R, eds. Deciphering the chemical language of plant communication, Vol. 1. Cham: Springer International Publishing, 95–116. [Google Scholar]
- Mofikoya AO, Bui TNT, Kivimaenpaa M, Holopainen JK, Himanen SJ, Blande JD. 2019. Foliar behaviour of biogenic semi-volatiles: potential applications in sustainable pest management. Arthropod-Plant Interactions 13, 193–212. [Google Scholar]
- Mofikoya AO, Kim TH, Abd El-Raheem AM, Blande JD, Kivimäenpää M, Holopainen JK. 2017. Passive adsorption of volatile monoterpene in pest control: aided by proximity and disrupted by ozone. Journal of Agricultural and Food Chemistry 65, 9579–9586. [DOI] [PubMed] [Google Scholar]
- Mofikoya AO, Miura K, Ghimire RP, Blande JD, Kivimäenpää M, Holopainen T, Holopainen JK. 2018. Understorey Rhododendron tomentosum and leaf trichome density affect mountain birch VOC emissions in the subarctic. Scientific Reports 8, 13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell K, Kessler A. 2017. Plant communication in a widespread goldenrod: keeping herbivores on the move. Functional Ecology 31, 1049–1061. [Google Scholar]
- Muroi A, Ramadan A, Nishihara M, Yamamoto M, Ozawa R, Takabayashi J, Arimura G. 2011. The composite effect of transgenic plant volatiles for acquired immunity to herbivory caused by inter-plant communications. PLoS One 6, e24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Fares S, Harley P, Jardine KJ. 2014. Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition. Plant, Cell & Environment 37, 1790–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic V. 2003. Volatile communication between barley plants affects biomass allocation. Journal of Experimental Botany 54, 1931–1939. [DOI] [PubMed] [Google Scholar]
- Noe SM, Copolovici L, Niinemets Ü, Vaino E. 2008. Foliar limonene uptake scales positively with leaf lipid content: “non-emitting” species adsorb and release monoterpenes. Plant Biology 10, 129–137. [DOI] [PubMed] [Google Scholar]
- Oki K, Masimbula R, Miyawaki K, Takata Y, Takahashi K, Matsuura H. 2019. 12OHJA, 12OGlcJA, and JA-L-Val as airborne MeJA metabolites. Bioscience, Biotechnology, and Biochemistry 83, 1709–1712. [DOI] [PubMed] [Google Scholar]
- Oluwafemi S, Dewhirst SY, Veyrat N, Powers S, Bruce TJ, Caulfield JC, Pickett JA, Birkett MA. 2013. Priming of production in maize of volatile organic defence compounds by the natural plant activator cis-jasmone. PLoS One 8, e62299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA. 2001. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. The Plant Cell 13, 179–191. [PMC free article] [PubMed] [Google Scholar]
- Orrock JL, Connolly BM, Choi WG, Guiden PW, Swanson SJ, Gilroy S. 2018. Plants eavesdrop on cues produced by snails and induce costly defenses that affect insect herbivores. Oecologia 186, 703–710. [DOI] [PubMed] [Google Scholar]
- Pashalidou FG, Eyman L, Sims J, Buckley J, Fatouros NE, De Moraes CM, Mescher MC. 2020. Plant volatiles induced by herbivore eggs prime defences and mediate shifts in the reproductive strategy of receiving plants. Ecology Letters 23, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Paul-Victor C, Züst T, Rees M, Kliebenstein DJ, Turnbull LA. 2010. A new method for measuring relative growth rate can uncover the costs of defensive compounds in Arabidopsis thaliana. New Phytologist 187, 1102–1111. [DOI] [PubMed] [Google Scholar]
- Pearse IS, Porensky LM, Yang LH, et al. 2012. Complex consequences of herbivory and interplant cues in three annual plants. PLoS One 7, e38105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Van Loon JJA, Zheng S, Dicke M. 2011. Herbivore-induced volatiles of cabbage (Brassica oleracea) prime defence responses in neighbouring intact plants. Plant Biology 13, 276–284. [DOI] [PubMed] [Google Scholar]
- Pettersson J, Ninkovic V, Ahmed E. 1999. Volatiles from different barley cultivars affect aphid acceptance of neighbouring plants. Acta Agriculturae Scandinavica Section B: Soil and Plant Science 49, 152–157. [Google Scholar]
- Quintana-Rodriguez E, Duran-Flores D, Heil M, Camacho-Coronel X. 2018. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Scientia Horticulturae 237, 207–220. [Google Scholar]
- Rahnamaie-Tajadod R, Loke KK, Goh HH, Noor NM. 2017. Differential gene expression analysis in Polygonum minus leaf upon 24h of methyl jasmonate elicitation. Frontiers in Plant Science 8, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades DF. 1983. Responses of alder and willow to attack by tent caterpillars and webworms: evidence for pheromonal sensitivity of willows. ACS Symposium Series 208, 55–68. [Google Scholar]
- Ribeiro LdP, Santana Klock AL, Wordell Filho JA, Tramontin MA, Trapp MA, Mithöfer A, Nansen C. 2018. Hyperspectral imaging to characterize plant-plant communication in response to insect herbivory. Plant Methods 14, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedlmeier M, Ghirardo A, Wenig M, Knappe C, Koch K, Georgii E, Dey S, Parker JE, Schnitzler JP, Vlot AC. 2017. Monoterpenes support systemic acquired resistance within and between plants. The Plant Cell 29, 1440–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Crafts-Brandner SJ, Paré PW, Henneberry TJ. 2001. Exogenous methyl jasmonate induces volatile emissions in cotton plants. Journal of Chemical Ecology 27, 679–695. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ. 2009. Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. Journal of Chemical Ecology 35, 163–175. [DOI] [PubMed] [Google Scholar]
- Ruther J, Kleier S. 2005. Plant–plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. Journal of Chemical Ecology 31, 2217–2222. [DOI] [PubMed] [Google Scholar]
- Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A. 2010. Real-time monitoring of herbivore induced volatile emissions in the field. Physiologia Plantarum 138, 123–133. [DOI] [PubMed] [Google Scholar]
- Schuman MC, Baldwin IT. 2018. Field studies reveal functions of chemical mediators in plant interactions. Chemical Society Reviews 47, 5338–5353. [DOI] [PubMed] [Google Scholar]
- Schuman MC, Barthel K, Baldwin IT. 2012. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. eLife 1, e00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri K, Ishizaki S, Ozawa R, Karban R. 2015. Airborne signals of communication in sagebrush: a pharmacological approach. Plant Signaling & Behavior 10, e1095416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Arnaud D. 2019. Hydrogen peroxide metabolism and functions in plants. New Phytologist 221, 1197–1214. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Matsui K, Iijima Y, et al. 2014. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proceedings of the National Academy of Sciences, USA 111, 7144–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamogami S, Rakwal R, Agrawal GK. 2008. Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochemical and Biophysical Research Communications 376, 723–727. [DOI] [PubMed] [Google Scholar]
- Tamogami S, Takahashi Y, Abe M, Noge K, Rakwal R, Agrawal GK. 2011. Conversion of airborne nerolidol to DMNT emission requires additional signals in Achyranthes bidentata. FEBS Letters 585, 1807–1813. [DOI] [PubMed] [Google Scholar]
- Tissier A, Morgan JA, Dudareva N. 2017. Plant volatiles: going ‘in’ but not ‘out’ of trichome cavities. Trends in Plant Science 22, 930–938. [DOI] [PubMed] [Google Scholar]
- Tscharntke T, Thiessen S, Dolch R, Boland W. 2001. Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa. Biochemical Systematics and Ecology 29, 1025–1047. [Google Scholar]
- Turlings TC, Ton J. 2006. Exploiting scents of distress: the prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Current Opinion in Plant Biology 9, 421–427. [DOI] [PubMed] [Google Scholar]
- Van Doan C, Zust T, Maurer C, Zhang X, Machado RAR, Mateo P, Ye M, Schimmel BCJ, Glauser G, Robert CAM. 2021. Volatile-mediated defence regulation occurs in maize leaves but not in maize root. Plant Cell and Environment 44, 2672–2686. [DOI] [PubMed] [Google Scholar]
- Van Meulebroek L, Meuninck B, Vanhaecke L, Smagghe G, Haesaert G, Audenaert K. 2020. Metabolomics reveal induction of ROS production and glycosylation events in wheat upon exposure to the green leaf volatile (Z)-3-hexenyl acetate. Frontiers in Plant Science 11, 596271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang NQ, Kong CH, Wang P, Meiners SJ. 2021. Root exudate signals in plant–plant interactions. Plant, Cell & Environment 44, 1044–1058. [DOI] [PubMed] [Google Scholar]
- Wasternack C. 2007. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany 100, 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HX, Qian LX, Wang XW, Shao RX, Hong Y, Liu SS, Wang XW. 2019. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proceedings of the National Academy of Sciences, USA 116, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan ZG, Wang CZ. 2006. Wound-induced green leaf volatiles cause the release of acetylated derivatives and a terpenoid in maize. Phytochemistry 67, 34–42. [DOI] [PubMed] [Google Scholar]
- Ye M, Glauser G, Lou Y, Erb M, Hu L. 2019. Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in rice. The Plant Cell 31, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Liu M, Erb M, Glauser G, Zhang J, Li X, Sun X. 2021. Indole primes defence signalling and increases herbivore resistance in tea plants. Plant, Cell & Environment 44, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Yi HS, Heil M, Adame-Alvarez RM, Ballhorn DJ, Ryu CM. 2009. Airborne induction and priming of plant defenses against a bacterial pathogen. Plant Physiology 151, 2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip EC, Tooker JF, Mescher MC, De Moraes CM. 2019. Costs of plant defense priming: exposure to volatile cues from a specialist herbivore increases short-term growth but reduces rhizome production in tall goldenrod (Solidago altissima). BMC Plant Biology 19, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebelo SA, Maffei ME. 2015. Role of early signalling events in plant–insect interactions. Journal of Experimental Botany 66, 435–448. [DOI] [PubMed] [Google Scholar]
- Zebelo SA, Maffei ME. 2016. Plant electrophysiology: early stages of the plant response to chemical signals. In: Blande JD, Glinwood R, eds. Deciphering the chemical language of plant communication, Vol. 1. Cham: Springer International Publishing, 153–174. [Google Scholar]
- Zebelo SA, Matsui K, Ozawa R, Maffei ME. 2012. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Science 196, 93–100. [DOI] [PubMed] [Google Scholar]
- Zhang P, Miska J, Lee-Chang C, et al. 2019. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proceedings of the National Academy of Sciences, USA 116, 23714–23723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PJ, Zhao C, Ye ZH, Yu XP. 2020. Trade-off between defense priming by herbivore-induced plant volatiles and constitutive defense in tomato. Pest Management Science 76, 1893–1901. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Turner JG. 2008. Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS One 3, e3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Davis LC, Verpoorte R. 2005. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advances 23, 283–333. [DOI] [PubMed] [Google Scholar]
- Zhao M, Wang L, Wang J, et al. 2020. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. Journal of Integrative Plant Biology 62, 1461–1468. [DOI] [PubMed] [Google Scholar]