Abstract
Significant innovations in the management of acute ischemic stroke have led to an increased incidence in the long-term complications of stroke. Therefore, there is an urgent need for improvements in and refinement of rehabilitation interventions that can lead to functional and neuropsychological recovery. The goal of this review is to summarize the current progress and challenges involved with preclinical stroke recovery research. Moving forward, stroke recovery research should be placing an increased emphasis on the incorporation of comorbid diseases and biological variables in preclinical models in order to overcome translational roadblocks to establishing successful clinical rehabilitation interventions.
Keywords: Cognition, enrichment, preclinical, recovery, rehabilitation, stroke, vascularization
Introduction
Acute ischemic stroke is a leading cause of adult disability worldwide with more than 60% of stroke survivors struggling to perform daily activities and to maintain their independence.[1] Stroke can lead to prolonged physical, cognitive, emotional, and social problems.[2] These can include deficits in memory, attention and concentration, perception, spatial awareness (neglect), apraxia, executive functioning as well as depression. As the mortality rate decreased for the 800,000 annual stroke patients due to advancements in acute ischemic stroke treatment,[3] unfortunately, the incidence of these long-term complications increased.
Stroke is not only associated with an acute decline in cognitive function but also accelerated and persistent poststroke cognitive impairment and poststroke anxiety/depression.[4] This phenomenon is of growing interest to stroke recovery and rehabilitation researchers. In this review, we will use the agreed definitions for recovery and rehabilitation that were described in great detail by the stroke recovery and rehabilitation roundtable task force.[5] Briefly, the term stroke recovery will be used here when considering the extent to which body structure, physiology, and activities have returned to their prestroke state. Whereas the term rehabilitation reflects the interventions or process of care designed to aid recovery.
Stroke recovery is influenced by many factors, including timing of treatment, severity of baseline impairments, comorbid conditions, age, and sex. The goal of this review is to briefly discuss rehabilitation strategies used in patients and then review how these approaches are applied to preclinical models with an emphasis on comorbid diseases and biological variables. Potential mechanisms contributing to the efficacy of some commonly used preclinical rehabilitation paradigms are also discussed.
Stroke Rehabilitation: long-term Functional, Cognitive, and Physical Outcomes
Stroke rehabilitation traditionally consists of some combination of physical, speech, and occupational therapies, depending on the patient's unique deficits. Unfortunately, few patients recover fully from stroke, and there is a need for new interventions that can improve motor, cognitive, and psychological impairments. Due in large part to spontaneous recovery, many patients make rapid gains in motor function over the first few weeks following stroke, then motor recovery appears to plateau around 3–4 months poststroke[6] with some modest, gradual improvements over the next few years.[7] However, the gains observed in motor function may also be mediated by compensatory strategies as both humans and animals are very adept at finding alternative means to accomplish the activities of daily living.
It is also not clear if these clinical poststroke rehabilitation interventions have a significant impact on true recovery (reduction of impairment).[8,9,10] The most efficacious form of recovery is spontaneous recovery from a clinical[8] and preclinical perspective.[11,12] The current standards of care most likely contribute to functional improvements through compensatory behaviors rather than true recovery. While compensatory behaviors can help survivors carry out daily tasks, the long-term neural and behavioral ramifications of compensation are not well understood. There is an opportunity for preclinical researchers to design studies to (1) delineate the impact of physical rehabilitation on cognitive function and vice versa, (2) identify pathways that lead to true recovery rather than compensatory behaviors, and (3) differentiate spontaneous recovery from rehabilitation-induced recovery. Although differentiating between true recovery and compensation is challenging, one potential strategy is to use a single pellet reaching task that factors in multiple behavioral measures.[13] For example, analyzing a variety of reach behavior measures, such as end-point measures (successes, first try successes, and total reaching attempts), observational measures, movement-notation based measures, and kinematic measures, can help provide insight into whether or not rodent responses in skilled reaching are compensatory or if they reflect true recovery.[13]
Figure 1 summarizes the broad scope of functional and neuropsychological deficits associated with the long-term complications of stroke and some of the nonpharmacological therapeutic modalities currently being investigated by stroke recovery researchers, which are discussed below.
Figure 1.
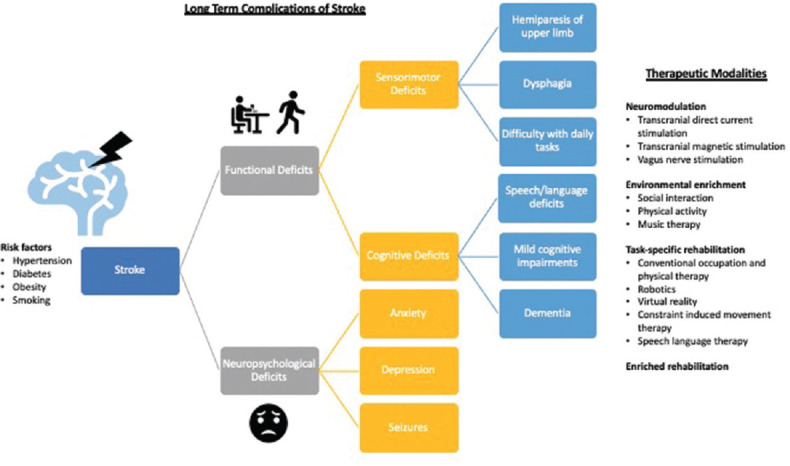
Long-term complications of and therapeutic modalities for stroke. Stroke can lead to numerous long-term functional and neuropsychological deficits. Functional deficits can result from both sensorimotor and cognitive deficits, which neuropsychological deficits, such as anxiety and depression, can severely impact quality of life and adversely affect functional recovery. There is a broad range of therapeutic modalities currently being investigated in stroke recovery research
Neuromodulation
Neuromodulation via central and peripheral nervous system stimulation devices is not currently standard-of-care but is considered by many experts in the field to be the next major frontier in stroke recovery research, although many unknowns remain. The therapeutic response depends on many factors, including the stimulation site and protocol (facilitatory vs. inhibitory) and the stroke phase and lesion location. We highlight three emerging approaches in the field of neuromodulation, including transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS), and vagus nerve stimulation (VNS), with a brief description of their clinical application and then discussion of preclinical applications of each approach, including potential mechanisms.
Brain modulation tools like tDCS can modulate cortical excitability and have lasting, dose-dependent effects.[14] Despite the potential of tDCS to modulate human behavior, little is known about the underlying mechanisms. Neurotrophic factors like brain-derived neurotrophic factor (BDNF) may play an important role in the long-term effects of tDCS.[15] The BDNF val66 met polymorphism that partially affects activity-dependent BDNF secretion[16] is associated with impaired motor skill acquisition in humans and mice. Additionally, tDCS did not induce long-lasting synaptic potentiation in BDNF and TrkB mutant mice, suggesting that tDCS improves skilled motor learning in a BDNF-dependent manner.[15] Identifying mechanistic pathways in preclinical models could help identify biomarkers that could be used to validate/refine the application of tDCS clinically.
Both clinical and preclinical studies have indicated that repetitive TMS (rTMS) has therapeutic potential in a variety of nervous system disorders, including stroke, trauma, depression, and Parkinson's disease.[17,18] TMS generates rapid changes in the magnetic field in order to deliver electrical currents through the brain that can modulate cortical excitability by initiating action potentials.[19] Preclinical studies involving TMS have primarily focused on the acute phase of stroke care and motor deficits; however, they have been promising. Interestingly, combination therapy with rTMS and human neural stem cell transplantation led to accelerated functional recovery after ischemic stroke in rats that was associated with enhanced neurogenesis and increased levels of BDNF.[20] Possible mechanisms of TMS include increased synaptic plasticity, increased neurotrophic factors, and enhanced neurogenesis.[21] The role of BDNF in TMS-induced plasticity is of particular interest to stroke recovery researchers because genetic variants of BDNF are associated with differential responses to TMS.[22] Studies in humans and animals have shown up-regulation of BDNF following high-intensity rTMS.[23,24,25,26]
Targeted VNS is another promising area in stroke neuromodulation research.[27] Key findings from preclinical studies have formed the basis for two multi-center, randomized controlled pilot trials to test the efficacy of VNS paired with rehabilitation in patients with moderate to severe upper limb weakness following ischemic stroke.[28] Preclinical studies have shown that VNS paired with rehabilitative training significantly improves forelimb motor functions compared to rehabilitative training alone.[29,30] Preclinical studies have also identified improvements in memory in response to VNS.[31] Repetitive bursts of VNS paired with movement have been shown to modify motor synaptic connectivity, reorganize the motor cortex, and facilitate recovery of the forelimb after ischemic stroke.[32] VNS most likely leads to improvements via activation of the cholinergic nucleus basalis and the noradrenergic locus coeruleus, which then engages the sensory-motor network during task-specific learning.[33] A recent review discussed in detail the potential mechanisms responsible for the neuroprotective and neuroplasticity-enhancing properties of VNS.[34] In summary, VNS stimulation may have anti-inflammatory, neurogenic, and angiogenic properties, while it also works to inhibit oxidative stress, reduce excitotoxicity, protect the blood-brain barrier, attenuate brain edema, suppress cell apoptosis, and ameliorate mitochondrial dysfunction.[34]
Task-specific rehabilitation
Task-specific rehabilitation can involve conventional occupational and physical therapy techniques or more elaborate, advanced technology via robotics or virtual reality. Conventional task-specific therapies in clinical practice for cognitive rehabilitation include training to specifically target a type of deficit and compensatory strategy training.[35,36] While compensatory strategies can improve the performance of activities of daily living, they can cloud our understanding of the degree to which true recovery processes are being activated in response to rehabilitation. In response to a motor disability, the natural response is to learn new ways of accomplishing tasks in the form of compensatory behaviors. This can manifest in stroke survivors with upper extremity impairments when they learn to rely on the nonparetic hand and arm for daily activities. Unfortunately, this can exacerbate impairments in the paretic side.[37]
Preclinical applications of task-specific rehabilitation include skilled reaching tasks. Skilled reaching tasks can be applied to experimental models to investigate motor behavior and sensorimotor integrations in stroke recovery. Skilled reaching is a learned, composite movement that involves reaching to grasp an object, such as a food pellet or dried pasta, inside a reaching box. Rodents rely on olfactory cues, proprioception, whiskers, and tactile nose sense to locate the food and to define a reaching path for the paw to target the pellet. Rats can readily learn to reach for food pellets by either single pellet or staircase tasks. These tests can be quantified and reveal both initial and chronic upper limb impairments.[38,39] These tests can be configured to promote the use of the impaired limb to discourage compensatory behavior. Additionally, constraint-induced movement therapy can be used to encourage the use of the paretic limb through restraint of the nonparetic limb during task-oriented training. Alternatively, bimanual task training that encourages the use of both the nonparetic and paretic limbs may also have some benefits, such as improved unimanual function of the paretic side, even with some compensatory reliance on the nonparetic limb.[40]
Environmental enrichment
Environmental enrichment (EE) has predominantly been studied in preclinical applications with little translation into the clinical setting.[41] There is no standardized form of EE for clinical application. EE paradigms engage participants in a variety of dynamic physical, sensory, cognitive, and social activities or experiences. No large-scale clinical trials to test the effectiveness of EE have been undertaken, but a few small (n = 14) to medium-sized studies (n = 52) have demonstrated that activity levels can be increased[42] and appear to remain sustained over time within units.[43] Evidence of improvements in terms of disability, function, or participation is limited.
Enrichment can be multifaceted and comprised of many elements including social interaction, exercise, and nonspecific sensory, motor, and cognitive stimulation, making it difficult to attribute specific components to the recovery process.[44] However, it is apparent that the interaction of these elements induces changes in the brain by altering neuronal activity, dendritic morphology, resting-state functional connectivity, and suppressing plasticity-inhibitory factors.[44] EE can recruit endogenous repair mechanisms to enhance recovery by reversing learning impairment in the Morris water maze[45] and by promoting improved forelimb motor function and enhanced dendritic growth after focal ischemic injury in rats.[46] A recent study suggests that EE can enhance angiogenesis in the ischemic brain.[47] Angiogenesis may be an important target for stroke recovery interventions because greater microvessel density in the ischemic border correlates with longer survival in stroke patients.[48] EE in preclinical models has also been associated with enhanced vascular repair,[49] induction of angiogenesis,[47,50] and upregulation of nitric oxide synthase.[51]
Preclinical EE conditions can vary widely, and concerns have been raised about how well it can mimic clinical EE conditions. Although hospitalized subacute patients can be significantly deprived of physical activity and social interaction, the least enriched human situation is unlikely to be equivalent to being in a cage all day with nesting materials and a couple of cage mates. In this case, EE may only normalize living conditions if standard rodent housing conditions are considered relatively impoverished compared to the human experience. Preclinical EE outcomes should include measures of anxiety/depression to compare to standard housing conditions. Behavioral mapping in preclinical settings using video shape recognition or radio-frequency identification tagging can also help to align preclinical and clinical EE methodologies tracked using wearable devices.
Enriched rehabilitation
Interestingly, EE alone does not promote recovery of skilled forelimb movements[52] that require fine motor dexterity, such as pellet retrieval, and it may not be an alternative for task-specific therapies that target the primary motor impairment. The combination of EE and task-specific training in the form of enriched rehabilitation (ER) is thought to capitalize on the neuroplastic milieu that EE stimulates so that task-specific training can induce neuroplastic changes with maximal efficiency. Additionally, the social interaction component of ER decreases anxiety and depression-like behaviors, which can also positively impact functional recovery.[53] Although the mechanisms are not clear, socialization can contribute to more rapid and extensive function recovery from stroke compared to social isolation.[54] Potential synergistic mechanisms of EE and task-specific rehabilitation in ER are summarized in Figure 2. ER likely contributes to motor, cognitive, and psychological recovery via vascular mechanisms. Brain microvascular endothelial cells in particular are a significant source of proangiogenic and neurotrophic BDNF,[55] which has known effects on recovery.[56] There is a need for more research in this area to understand how angiogenesis and vascular remodeling contribute to the EE-associated neuroplastic milieu and neuroplastic changes in response to task-specific training.
Figure 2.
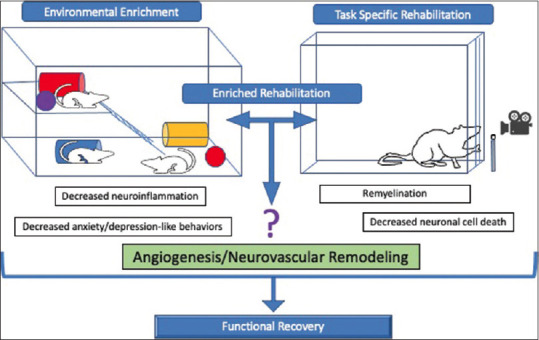
Potential mechanisms of enriched rehabilitation in stroke recovery. Enriched rehabilitation is a term used to describe a combination therapy that includes environmental enrichment and task-specific rehabilitation. Potential mechanisms of enriched rehabilitation include decreased neuroinflammation, increased angiogenesis, increased remyelination, decreased neuronal cell death, and decreased anxiety and depression-like behaviors
Translational Challenges and Gaps in Preclinical Stroke Recovery Research
Over the past few decades, researchers have identified many compounds that were effective in preclinical models of acute stroke that were found to be ineffective in clinical trials. These failures led to consortiums that identified differences in the overall achievements of preclinical and clinical stroke research (i.e. STAIR and CAMARADES).[57] These consortiums have noted (1) the experimental protective effect of pharmacological agents is typically determined by reduction of the size of the lesion, while clinically, functional recovery is expected, (2) a large majority of the preclinical studies have been conducted in the short term (a few days) while functional recovery in patients surviving stroke is expected over the long-term, and (3) preclinical models are often transient models of ischemia, which has become an important factor with an increasing number of patients receiving intravenous thrombolysis or endovascular treatment. However, interventions also need to be tested in permanent ischemia models because in clinical practice long, or even permanent, ischemia is still often observed. Importantly, these preclinical studies have been geared more towards neuroprotection and acute stroke treatment to halt ischemic injury rather than the subsequent recovery-promoting strategies. In this section, we will discuss in more detail some specific translational challenges and gaps in stroke recovery research, including dosing, experimental models and comparative hurdles, sex differences, and comorbid disease models.
Dose reporting across preclinical and clinical stroke recovery
From a stroke recovery perspective, the SRRR recently developed a framework for stroke recovery trial development, which identified key issues and translational challenges observed in clinical and preclinical stroke recovery treatment trials.[58] The SRRR emphasized the importance of clear, operational definitions of dose (i.e. number of repetitions, duration, and intensity) and dose schedule in both preclinical and clinical studies. While preclinical studies typically report the number of repetitions of a task, clinical studies generally report a threshold dose in units of time (minutes of training), and both preclinical and clinical studies commonly fail to include measures of training intensity. Experts in stroke recovery research also recently published a framework for transparent and accurate reporting of nonpharmacological dosing.[59] The optimal timing of treatments relative to stroke onset to promote upper limb recovery and cognitive improvements is also currently unknown. Preclinical evidence suggests that starting upper limb training and exposure to EE >/=5–14 days poststroke is feasible, safe, and has better recovery outcomes in comparison to later start points.[60] There is less confidence about when to initiate upper limb rehabilitation in human trials, and this is often determined by practicality rather than biological variables.[58]
An example of how to incorporate some of the SRRR can be found in a recent study published by McDonald et al. They compared early versus late delivery of remote ischemic conditioning following ischemic stroke using an endothelin-1 (ET-1) reperfusion model using both male and female animals.[61] Although delayed Remote ischemic conditioning (RIC) failed to enhance poststroke behavioral recovery, their findings did suggest that RIC may be effective in the hyperacute and early acute phases of stroke, when other interventions such as exercise would be contraindicated.[61]
Experimental animal models for stroke recovery research
There is a large variety of experimental stroke models that each has their own advantages and disadvantages for stroke recovery research. The four most common experimental models used in stroke recovery research are intraluminal middle cerebral artery (MCAO), distal MCAO, photothrombotic (PT) stroke, and ET-1-induced stroke. These models have been reviewed extensively elsewhere, and we would like to refer the reader to one of these for more details on the advantages and disadvantages of a specific model.[62,63,64] The most frequently used experimental model in preclinical stroke research is the intraluminal suture MCAO model. MCAO can be used to model permanent ischemia or transient focal cerebral ischemia with a variety of reperfusion time points. This model closely resembles numerous manifestations of human stroke since most thromboembolic infarcts in humans occur in the MCA territory;[65] however, intraluminal MCAO can lead to variable damage with large infarcts making it difficult to target therapies. Distal MCAO is easier to perform and is associated with higher survival than the intraluminal MCAO model, but it does not significantly impact motor function.[66] The PT stroke model is reproducible and ideal when the goal is to induce stroke for in vivo optical studies in mice or for targeting a specific cortical region, but it cannot be used to target subcortical brain structures. Similarly, ET-1 can also be used to target specific brain structures in rats, but it has reduced efficacy in mice.
There are also some notable species differences among rodent models used for experimental stroke recovery research. For example, rats frequently survive permanent filament MCAO, while mice have much higher mortality postfilament MCAO secondary to inadequate food and/or water intake.[67] Providing mice with nutritional support reduces the mortality bias significantly to allow for the long-term morphological and functional sequelae of stroke.[67] It would be wise, therefore, for preclinical researchers to pay careful attention to food and/or water intake and report any nutritional supplementation, considering the impact on mortality. Because food and water intakes are closely monitored in the clinical setting, preclinical researchers should consider nutritional supplementation in the postoperative period regardless of species.
Comparative anatomical and physiological hurdles
Experimental models of stroke have unique sensory pathways that do not always translate to the human condition. For example, rodents have facial whiskers that provide important spatial and textural information about their immediate surroundings. All other mammals besides humans have whiskers, but human fingertips may be represented by isolated regions of cortex like whisker sensory pathways. Furthermore, rodent reticulo- and vestibulospinal tracts are larger in size and play a bigger role in motor control (e.g. voluntary locomotion and grooming).[68] Furthermore, there is not enough evidence to conclude whether secondary motor areas in rodents are true homologs of the primate premotor cortex, supplementary motor cortex, or a combination of the two. Thus, identical appearing strokes in the rodent and human may have different clinical presentations. For example, many have documented motor deficits in humans with cortical lesions that have not been observed in rodents with similar lesions.[69]
Preclinical research should prioritize the study of potential mechanisms involved in recovery and responsiveness to training in models that better reflect lesion profiles that are common in the stroke population. Having preclinical stroke models that are not representative of the clinical population can also negatively affect translational outcomes, and lesion location can differentially affect functional outcomes following stroke.[70] Lesion location is a prognostic biomarker that has been relatively neglected in both preclinical and clinical studies, but a recent study showed a relationship between lesion location and functional impairment and recovery in reaching/grasping, spontaneous limb use, and hindlimb placement during walking.[71] This could explain some of the variability in differential responses to treatment between individuals in clinical studies. Standardizing lesion mapping practices across preclinical and clinical domains could help maximize the likelihood of translational success.[72]
Preclinical models have the potential to inform how noninvasive brain stimulation can be refined for clinical applications, especially promoting recovery of reach-to-grasp behavior following brain injury. One advantage of this behavioral outcome measure is that there is a high degree of homology in the circuitry involved with reach-to-grasp behavior in humans and rodents.[73] However, reach-to-grasp behavior is rarely assessed because it is extremely time intensive and has traditionally involved manual video analysis. Advances in automated/AI-based analysis of rodent behavior with tools such as Imetronic (Pessac, France, www.imetronic.com) may circumvent some of these time constraints, allowing more researchers to incorporate skilled reaching outcome measures. Reach-to-grasp task or reaching tasks are considered skilled learned tasks that involve the acquisition of a specific and fine-tuned sequence of movements.[74] Skilled motor learning is dependent on intra-cortical integration of sensory and planning information[75,76] in addition to cholinergic[77,78] and dopaminergic[79,80] afferent projections to the motor cortex. However, there are notable differences in morphology, laminar distribution, and density of cholinergic basal forebrain innervation among humans, nonhuman primates, and rodents.[81,82] The role of this system in stroke recovery is not clear, but the laminar distribution of cholinergic systems and control of synaptic plasticity by cholinergic inputs is highly conserved between rodents and humans.[83,84]
Sex differences in stroke recovery research
Significant sex differences in poststroke quality of life measures have been observed.[85,86] A recent study investigating factors contributing to sex differences in poststroke quality of life measures found that women's advanced age, stroke severity, prestroke dependency, and poststroke depression were all factors associated with poorer health-related quality of life.[87] Unfortunately, few preclinical studies include both male and female animals, and those that do have resulted in some conflicting data. Some studies have shown greater benefits of EE in females compared to males or vice versa,[88,89] while others suggest that there are no sex differences.[90] However, only ~17% of EE studies have included both male and female animals, and only a minority of these have been concerned with stroke recovery.[91]
Stroke severity has been linked to hormonal fluctuations that occur in a rodent's reproductive cycle (4–5 days).[92] For translational researchers who intend to model clinical stroke, reproductively senescent or aged female animals would be recommended.[93] If intact cycling females are used, researchers may encounter large within-group variations that could mask the effects of female sex hormone contributions in preclinical studies that are designed to directly address sex differences.[93] One approach to control for this would be to monitor the estrus cycle by daily vaginal smear and select for females in estrus or diestrus (when estrogen levels are low) when initiating experimental stroke. One study has shown that this can provide smaller within-group variations in infarct volume and functional outcomes.[94]
Another important consideration for sex-specific differences in stroke recovery is that preclinical outcomes in stroke recovery research have traditionally employed behavioral tests, such as novel object recognition, Y-maze, and passive avoidance, that were optimized for relatively young male animals with the assumption often being that the same conditions could be used to draw conclusions about females, when females happened to be included in the experimental design. Before conclusions can be drawn, researchers need to consider optimizing behavioral testing procedures for female animals.
Comorbid disease models: from hypertension and diabetes to autoimmune-rheumatic diseases
Comorbid diseases such as obesity, hypercholesterolemia, diabetes, hypertension, and autoimmune-rheumatic diseases are associated with increased risk for stroke and are often associated with worsened outcomes.[95] The use of predominantly young and healthy male animals in experimental stroke research has hindered progress in the translation of findings to clinically relevant patient populations. Predominantly young and healthy animals are suitable for pure mechanistic research and ideal for the more fundamental, basic science studies because they limit confounding factors. However, subsequent studies with comorbid models should be considered prior to translation in the clinical setting. By expanding the number of preclinical studies that utilized animal models with these comorbid conditions, stroke recovery researchers could identify novel mechanisms with the strategic potential to improve long-term functional, cognitive, and psychological outcomes in the alarming number of individuals affected by stroke with these underlying conditions. While the underlying reasons of worsened stroke recovery in comorbid diseases are multifactorial, vascular disease and inflammation appear to be common themes. Here we will briefly suggest some comorbid disease models that can be utilized for stroke research, but for more information about specific experimental models for common comorbidities, we would like to direct the reader to a related review on the topic from our laboratory.[96]
Hypertensive animals have larger infarct sizes compared to normotensive animals with a similar duration of experimental ischemia, while also being less responsive to many therapeutic interventions.[97] Chronic hypertension results in significant remodeling of the cerebrovasculature with reduction of the lumen diameter occurring in resistance vessels and an increase in wall thickness and wall-to-lumen ratios.[98] This cerebrovascular remodeling can lead to impaired blood flow autoregulation and increased infarct size. It is not yet clear how abnormal neurovascular remodeling, inflammation, and blood–brain barrier dysfunction in hypertension can impact neurorestorative pathways in response to specific rehabilitation paradigms. Male spontaneously hypertensive rats are by far the most common model for experimental hypertension and stroke.[96] The appropriate age for inducing experimental stroke in this strain, which gradually develops hypertension, is most likely 17–19 weeks, when they have a significant and sustained increased in blood pressure.[99]
Diabetes and its associated hyperglycemia also induce dysfunction and pathological remodeling of the cerebrovasculature.[100] Ischemic injury in the setting of diabetes is associated with exacerbation of deleterious microglial activation.[101] This microglial activation can adversely affect neurogenesis, angiogenesis, and restoration of the blood–brain barrier, which are key factors in functional recovery after stroke.[102] Our laboratory has shown that there is significant vasoregression after stroke in diabetes and this is associated with poor stroke recovery. The impact of rehabilitation on vascular and neuronal repair and restoration after stroke is unknown. The streptozotocin (STZ)-induced model of Type 1 diabetes is the most commonly used diabetes model in stroke research,[96] while low dose STZ (30 mg/kg) paired with high-fat diet has been used to model Type 2 diabetes.[103,104]
Although obesity is a known risk factor for stroke, the effect of obesity on functional recovery is less clear. In a recent systematic review, 3,070 participants from 7 studies and 5 countries undergoing inpatient stroke rehabilitation were evaluated.[105] Two of the studies found a positive association between obesity and functional outcomes measures, while two studies found no association, and the three remaining studies reported a negative association.[105] The clinical correlation between obesity and other comorbid conditions, like hypertension and diabetes, that contribute to metabolic syndrome in clinical stroke makes it challenging to attribute one specific risk factor to poor functional outcomes. Preclinical models that can mimic one or a combination of these risk factors could lead to more clear guidelines. It has been theorized that obesity can aid recovery by protecting against poststroke catabolism because weight loss after stroke is a common observation and important determinant of outcome.[106,107] The most commonly used model for studying obesity and the closely related pathologies of hyperlipidemia or dyslipidemia is high fat diet-induced.[96]
The female predominance of many autoimmune rheumatic diseases is also an important consideration for stroke recovery. Autoimmune-rheumatic diseases are associated with an increased risk for atherosclerosis and vascular-endothelial dysfunction, but there are few studies concerned with how autoimmunity can influence stroke recovery pathways. Functional recovery after stroke can be delayed or reduced when there are preexisting conditions associated with joint pain and swelling of the extremities.[108] There are limited studies concerning the impact of autoimmune-rheumatic diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) on functional outcomes following rehabilitation. Patients with RA from a retrospective cohort analysis were found to have lower functional status upon discharge from an inpatient rehabilitation unit compared to patients without RA or SLE when the data was adjusted for admission functional independence measure score.[108] There is a need for more autoimmune-rheumatic disease-focused stroke recovery research on both clinical and preclinical sides.
Looking beyond motor impairments in preclinical applications
There has been a larger focus on motor compared to nonmotor recovery in the preclinical stroke field by using sensorimotor tasks, such as rotarod, ladder crossing, limb placement, and adhesive strip removal.[41] Nonmotor deficits range from disabilities related to learning and memory to increased risk of depression, addictive behaviors, and epilepsy. Depression and anxiety are major clinical findings following stroke that affect approximately 40% of stroke survivors.[109] Poststroke anxiety/depression can greatly impact quality of life and adversely affect functional recovery. Therefore, psychological health should be considered in rehabilitation paradigms. Unfortunately, many clinical trials have excluded patients with poststroke depression and identifying depressive behaviors in animal models can be challenging. Moving forward, it will be important for preclinical and clinical stroke rehabilitation and recovery researchers to work together to refine methods to account for this wide range of nonmotor functional deficits that can lead to long-term consequences. Implementing these types of studies in preclinical studies will not be easy, as they will require long-term follow-up and require additional resources.
Preclinical stroke researchers have been gradually implementing anxiety-and depression-related behavioral tests, such as the elevated plus maze, shuttle box, open field test, forced swim test, and sucrose consumption, with some success.[110] Advantages and disadvantages of these tests have been recently reviewed.[111] The extensive amount of time it takes to habituate and test these animals and analyze the data for signs of anxiety or depression is a major hurdle for preclinical stroke researchers. This is another area where advances in automated/AI-based platforms can help advance the field.
In addition to these functional outcome measures, careful consideration should also be given to neuroimaging techniques that are commonly used to characterize pathophysiological changes in preclinical and clinical studies. Longitudinal, simultaneous mapping of neural activity and hemodynamic changes is recommended, although difficult to achieve. A recent study by He et al. employed a multimodal neural platform in a mouse model of stroke for long-term, spatially resolved tracking of intracortical neural activity and cerebral blood flow with speckle imaging in brain regions.[112] Disrupted neurovascular coupling was observed immediately after small-scale stroke, extended into chronic periods (8-week follow-up), and varied with the level of ischemia.[112]
Conclusions
Preclinical stroke recovery research would be more likely to lead to significant patient outcomes if more studies would include models that mimic the comorbidities and variability seen in the stroke patient population. Incorporation of more biological variables into preclinical stroke recovery research experimental design is needed to increase the translational impact of promising rehabilitation paradigms. Unfortunately, this will require more time and resources to complete studies that are powered sufficiently with well-designed inclusion and exclusion criteria. It also means that the field may need to be more open to and encouraging of the publication of negative data because chronic comorbid disease will likely lead to worsened recovery in response to rehabilitation paradigms compared to otherwise healthy animals. Preclinical researchers have traditionally felt pressured to focus on more short-term, but only partially clinically relevant, mechanistic studies because they have a better chance of being published in high-impact journals compared to potentially negative, although more clinically relevant, findings.
Financial support and sponsorship
Veterans Affairs (VA) Merit Review (BX000347), VA Senior Research Career Scientist Award (IK6 BX004471), National Institute of Health (NIH) RF1NS083559 and R01NS104573 (multi-PI, Susan C. Fagan as co-PI) to Adviye Ergul and NIH Postdoctoral Training Grants T32HL 007260 and F32HL158011 to Victoria Wolf.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank MUSC Stroke Recovery Research Center COBRE (GM109040) investigators for their support and guidance.
References
- 1.World Health Organization. The Atlas of Heart Disease and Stroke/Judith Mackay and George Mensah; with Shanthi Mendis and Kurt Greenland. Geneva: World Health Organization; 2004. The Atlas of Heart Disease and Stroke/Judith Mackay and George Mensah; with Shanthi Mendis and Kurt Greenland. [Google Scholar]
- 2.Balkaya M, Cho S. Optimizing functional outcome endpoints for stroke recovery studies. J Cereb Blood Flow Metab. 2019;39:2323–42. doi: 10.1177/0271678X19875212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: Advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8:319–29. doi: 10.1007/s13311-011-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314:41–51. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12:444–50. doi: 10.1177/1747493017711816. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. [PubMed] [Google Scholar]
- 7.Kwakkel G, Kollen BJ, Van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb. Stroke. 2003;34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 8.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 9.Veerbeek JM, Van Wegen E, Van Peppen R, Van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9:e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French B, Thomas L, Leathley M, Sutton C, McAdam J, Forster A, et al. Does repetitive task training improve functional activity after stroke. A Cochrane systematic review and meta-analysis. J Rehabil Med. 2010;42:9–14. doi: 10.2340/16501977-0473. [DOI] [PubMed] [Google Scholar]
- 11.Jeffers MS, Karthikeyan S, Corbett D. Does stroke rehabilitation really matter? Part A: Proportional stroke recovery in the rat. Neurorehabil Neural Repair. 2018;32:3–6. doi: 10.1177/1545968317751210. [DOI] [PubMed] [Google Scholar]
- 12.Jeffers MS, Karthikeyan S, Gomez-Smith M, Gasinzigwa S, Achenbach J, Feiten A, et al. Does stroke rehabilitation really matter. Part B: An algorithm for prescribing an effective intensity of rehabilitation. Neurorehabil Neural Repair. 2018;32:73–83. doi: 10.1177/1545968317753074. [DOI] [PubMed] [Google Scholar]
- 13.Alaverdashvili M, Whishaw IQ. A behavioral method for identifying recovery and compensation: Hand use in a preclinical stroke model using the single pellet reaching task. Neurosci Biobehav Rev. 2013;37:950–67. doi: 10.1016/j.neubiorev.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 15.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang A, Thickbroom G, Rodger J. Repetitive transcranial magnetic stimulation of the brain: Mechanisms from animal and experimental models. Neuroscientist. 2017;23:82–94. doi: 10.1177/1073858415618897. [DOI] [PubMed] [Google Scholar]
- 19.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Peng JJ, Sha R, Li MX, Chen LT, Han XH, Guo F, et al. Repetitive transcranial magnetic stimulation promotes functional recovery and differentiation of human neural stem cells in rats after ischemic stroke. Exp Neurol. 2019;313:1–9. doi: 10.1016/j.expneurol.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci. 2015;9:303. doi: 10.3389/fnhum.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–25. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanardini R, Gazzoli A, Ventriglia M, Perez J, Bignotti S, Rossini PM, et al. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. J Affect Disord. 2006;91:83–6. doi: 10.1016/j.jad.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Castillo-Padilla DV, Funke K. Effects of chronic iTBS-rTMS and enriched environment on visual cortex early critical period and visual pattern discrimination in dark-reared rats. Dev Neurobiol. 2016;76:19–33. doi: 10.1002/dneu.22296. [DOI] [PubMed] [Google Scholar]
- 25.Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–6. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller MB, Toschi N, Kresse AE, Post A, Keck ME. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology. 2000;23:205–15. doi: 10.1016/S0893-133X(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 27.Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted vagus nerve stimulation for rehabilitation after stroke. Front Neurosci. 2019;13:280. doi: 10.3389/fnins.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimberley TJ, Prudente CN, Engineer ND, Pierce D, Tarver B, Cramer SC, et al. Study protocol for a pivotal randomised study assessing vagus nerve stimulation during rehabilitation for improved upper limb motor function after stroke. Eur Stroke J. 2019;4:363–77. doi: 10.1177/2396987319855306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60:80–8. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, 2nd, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair. 2014;28:698–706. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–6. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- 32.Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, et al. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabil Neural Repair. 2016;30:676–84. doi: 10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, 2nd, et al. Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 2016;9:174–81. doi: 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, Qiao P, Li Q, Wang Y, Zhang L, Yan LJ, et al. Vagus nerve stimulation as a promising adjunctive treatment for ischemic stroke. Neurochem Int. 2019;131:104539. doi: 10.1016/j.neuint.2019.104539. [DOI] [PubMed] [Google Scholar]
- 35.Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81:1596–615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- 36.Desmond DW, Moroney JT, Sano M, Stern Y. Recovery of cognitive function after stroke. Stroke. 1996;27:1798–803. doi: 10.1161/01.str.27.10.1798. [DOI] [PubMed] [Google Scholar]
- 37.Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: Experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci. 2010;124:124–32. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: A proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- 39.Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: A measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–28. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- 40.Dutcher AM, Truong KV, Miller DD, Allred RP, Nudi E, Jones TA. Training in a cooperative bimanual skilled reaching task, the popcorn retrieval task, improves unimanual function after motor cortical infarcts in rats. Behav Brain Res. 2021;396:112900. doi: 10.1016/j.bbr.2020.112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald MW, Hayward KS, Rosbergen IC, Jeffers MS, Corbett D. Is environmental enrichment ready for clinical application in human post-stroke rehabilitation? Front Behav Neurosci. 2018;12:135. doi: 10.3389/fnbeh.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen H, Ada L, Bernhardt J, McElduff P, Pollack M, Nilsson M, et al. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: A pilot non-randomized controlled trial. Disabil Rehabil. 2014;36:255–62. doi: 10.3109/09638288.2013.788218. [DOI] [PubMed] [Google Scholar]
- 43.Rosbergen IC, Grimley RS, Hayward KS, Walker KC, Rowley D, Campbell AM, et al. Embedding an enriched environment in an acute stroke unit increases activity in people with stroke: A controlled before-after pilot study. Clin Rehabil. 2017;31:1516–28. doi: 10.1177/0269215517705181. [DOI] [PubMed] [Google Scholar]
- 44.Jeffers MS, Corbett D. Synergistic effects of enriched environment and task-specific reach training on poststroke recovery of motor function. Stroke. 2018;49:1496–503. doi: 10.1161/STROKEAHA.118.020814. [DOI] [PubMed] [Google Scholar]
- 45.Dahlqvist P, Rönnbäck A, Bergström SA, Söderström I, Olsson T. Environmental enrichment reverses learning impairment in the Morris water maze after focal cerebral ischemia in rats. Eur J Neurosci. 2004;19:2288–98. doi: 10.1111/j.0953-816X.2004.03248.x. [DOI] [PubMed] [Google Scholar]
- 46.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–80. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie H, Yu K, Zhou N, Shen X, Tian S, Zhang B, et al. Enriched environment elicits proangiogenic mechanisms after focal cerebral ischemia. Transl Stroke Res. 2019;10:150–9. doi: 10.1007/s12975-018-0629-8. [DOI] [PubMed] [Google Scholar]
- 48.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 49.Shilpa BM, Bhagya V, Harish G, Srinivas Bharath MM, Shankaranarayana Rao BS. Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:88–100. doi: 10.1016/j.pnpbp.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Xie Q, Cheng J, Pan G, Wu S, Hu Q, Jiang H, et al. Treadmill exercise ameliorates focal cerebral ischemia/reperfusion-induced neurological deficit by promoting dendritic modification and synaptic plasticity via upregulating caveolin-1/VEGF signaling pathways. Exp Neurol. 2019;313:60–78. doi: 10.1016/j.expneurol.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Koester-Hegmann C, Bengoetxea H, Kosenkov D, Thiersch M, Haider T, Gassmann M, et al. High-altitude cognitive impairment is prevented by enriched environment including exercise via VEGF signaling. Front Cell Neurosci. 2018;12:532. doi: 10.3389/fncel.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grabowski M, Brundin P, Johansson BB. Paw-reaching, sensorimotor, and rotational behavior after brain infarction in rats. Stroke. 1993;24:889–95. doi: 10.1161/01.str.24.6.889. [DOI] [PubMed] [Google Scholar]
- 53.O’Keefe LM, Doran SJ, Mwilambwe-Tshilobo L, Conti LH, Venna VR, McCullough LD. Social isolation after stroke leads to depressive-like behavior and decreased BDNF levels in mice. Behav Brain Res. 2014;260:162–70. doi: 10.1016/j.bbr.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64:1888–92. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 55.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–64. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 56.Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–5. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 57.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–50. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- 58.Bernhardt J, Hayward KS, Dancause N, Lannin NA, Ward NS, Nudo RJ, et al. A stroke recovery trial development framework: Consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2019;33:959–69. doi: 10.1177/1545968319888642. [DOI] [PubMed] [Google Scholar]
- 59.Hayward KS, Churilov L, Dalton EJ, Brodtmann A, Campbell BC, Copland D, et al. Advancing stroke recovery through improved articulation of nonpharmacological intervention dose. Stroke. 2021;52:761–9. doi: 10.1161/STROKEAHA.120.032496. [DOI] [PubMed] [Google Scholar]
- 60.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–54. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald MW, Dykes A, Jeffers MS, Carter A, Nevins R, Ripley A, et al. Remote ischemic conditioning and stroke recovery. Neurorehabil Neural Repair. 2021;35:545–9. doi: 10.1177/15459683211011224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sommer CJ. Ischemic stroke: Experimental models and reality. Acta Neuropathol. 2017;133:245–61. doi: 10.1007/s00401-017-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 2015;9:3445–54. doi: 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caleo M. Rehabilitation and plasticity following stroke: Insights from rodent models. Neuroscience. 2015;311:180–94. doi: 10.1016/j.neuroscience.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 65.Olsen TS, Skriver EB, Herning M. Cause of cerebral infarction in the carotid territory. Its relation to the size and the location of the infarct and to the underlying vascular lesion. Stroke. 1985;16:459–66. doi: 10.1161/01.str.16.3.459. [DOI] [PubMed] [Google Scholar]
- 66.Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, et al. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat Protoc. 2009;4:1560–4. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- 67.Lourbopoulos A, Mamrak U, Roth S, Balbi M, Shrouder J, Liesz A, et al. Inadequate food and water intake determine mortality following stroke in mice. J Cereb Blood Flow Metab. 2017;37:2084–97. doi: 10.1177/0271678X16660986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeiler SR. Should we care about early post-stroke rehabilitation? Not yet, but soon. Curr Neurol Neurosci Rep. 2019;19:13. doi: 10.1007/s11910-019-0927-x. [DOI] [PubMed] [Google Scholar]
- 69.Kawai R, Markman T, Poddar R, Ko R, Fantana AL, Dhawale AK, et al. Motor cortex is required for learning but not for executing a motor skill. Neuron. 2015;86:800–12. doi: 10.1016/j.neuron.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karthikeyan S, Jeffers MS, Carter A, Corbett D. Characterizing spontaneous motor recovery following cortical and subcortical stroke in the rat. Neurorehabil Neural Repair. 2019;33:27–37. doi: 10.1177/1545968318817823. [DOI] [PubMed] [Google Scholar]
- 71.Jeffers MS, Touvykine B, Ripley A, Lahey G, Carter A, Dancause N, et al. Poststroke impairment and recovery are predicted by task-specific regionalization of injury. J Neurosci. 2020;40:6082–97. doi: 10.1523/JNEUROSCI.0057-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corbett D, Carmichael ST, Murphy TH, Jones TA, Schwab ME, Jolkkonen J, et al. Enhancing the alignment of the preclinical and clinical stroke recovery research pipeline: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable translational working group. Int J Stroke. 2017;12:462–71. doi: 10.1177/1747493017711814. [DOI] [PubMed] [Google Scholar]
- 73.Latchoumane CV, Barany DA, Karumbaiah L, Singh T. Neurostimulation and reach-to-grasp function recovery following acquired brain injury: Insight from pre-clinical rodent models and human applications. Front Neurol. 2020;11:835. doi: 10.3389/fneur.2020.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buitrago MM, Ringer T, Schulz JB, Dichgans J, Luft AR. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behav Brain Res. 2004;155:249–56. doi: 10.1016/j.bbr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 75.Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–8. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heindorf M, Arber S, Keller GB. Mouse motor cortex coordinates the behavioral response to unpredicted sensory feedback. Neuron. 2018;99:1040–54.e5. doi: 10.1016/j.neuron.2018.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramanathan D, Tuszynski MH, Conner JM. The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. J Neurosci. 2009;29:5992–6000. doi: 10.1523/JNEUROSCI.0230-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conner JM, Kulczycki M, Tuszynski MH. Unique contributions of distinct cholinergic projections to motor cortical plasticity and learning. Cereb Cortex. 2010;20:2739–48. doi: 10.1093/cercor/bhq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hosp JA, Molina-Luna K, Hertler B, Atiemo CO, Luft AR. Dopaminergic modulation of motor maps in rat motor cortex: An in vivo study. Neuroscience. 2009;159:692–700. doi: 10.1016/j.neuroscience.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 80.Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31:2481–7. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Obermayer J, Verhoog MB, Luchicchi A, Mansvelder HD. Cholinergic modulation of cortical microcircuits is layer-specific: Evidence from rodent, monkey and human brain. Front Neural Circuits. 2017;11:100. doi: 10.3389/fncir.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coppola JJ, Disney AA. Is there a canonical cortical circuit for the cholinergic system? Anatomical differences across common model systems. Front Neural Circuits. 2018;12:8. doi: 10.3389/fncir.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verhoog MB, Obermayer J, Kortleven CA, Wilbers R, Wester J, Baayen JC, et al. Layer-specific cholinergic control of human and mouse cortical synaptic plasticity. Nat Commun. 2016;7:12826. doi: 10.1038/ncomms12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramanathan DS, Conner JM, Anilkumar AA, Tuszynski MH. Cholinergic systems are essential for late-stage maturation and refinement of motor cortical circuits. J Neurophysiol. 2015;113:1585–97. doi: 10.1152/jn.00408.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 86.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phan HT, Blizzard CL, Reeves MJ, Thrift AG, Cadilhac DA, Sturm J, et al. Sex differences in long-term quality of life among survivors after stroke in the INSTRUCT. Stroke. 2019;50:2299–306. doi: 10.1161/STROKEAHA.118.024437. [DOI] [PubMed] [Google Scholar]
- 88.Pereira LO, Strapasson AC, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but not in male, rats after neonatal hypoxia-ischemia. Brain Res. 2008;1218:257–66. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 89.Langdon KD, Granter-Button S, Harley CW, Moody-Corbett F, Peeling J, Corbett D. A cognitive rehabilitation paradigm effective in male rats lacks efficacy in female rats. J Cereb Blood Flow Metab. 2014;34:1673–80. doi: 10.1038/jcbfm.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saucier DM, Yager JY, Armstrong EA. Housing environment and sex affect behavioral recovery from ischemic brain damage. Behav Brain Res. 2010;214:48–54. doi: 10.1016/j.bbr.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 91.Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats-behavioural and neurochemical aspects. Behav Brain Res. 2011;222:246–64. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278:H290–4. doi: 10.1152/ajpheart.2000.278.1.H290. [DOI] [PubMed] [Google Scholar]
- 93.Ahnstedt H, McCullough LD, Cipolla MJ. The importance of considering sex differences in translational stroke research. Transl Stroke Res. 2016;7:261–73. doi: 10.1007/s12975-016-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahnstedt H, Mostajeran M, Blixt FW, Warfvinge K, Ansar S, Krause DN, et al. U0126 attenuates cerebral vasoconstriction and improves long-term neurologic outcome after stroke in female rats. J Cereb Blood Flow Metab. 2015;35:454–60. doi: 10.1038/jcbfm.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ergul A, Hafez S, Fouda A, Fagan SC. Impact of comorbidities on acute injury and recovery in preclinical stroke research: Focus on hypertension and diabetes. Transl Stroke Res. 2016;7:248–60. doi: 10.1007/s12975-016-0464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Collins VE, Donnan GA, Macleod MR, Howells DW. Hypertension and experimental stroke therapies. J Cereb Blood Flow Metab. 2013;33:1141–7. doi: 10.1038/jcbfm.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301:H87–97. doi: 10.1152/ajpheart.01206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reckelhoff JF, Yanes Cardozo LL, Fortepiani ML. Models of hypertension in aging. In: Ram JL, Conn PM, editors. Conn's Handbook of Models for Human Aging. 2nd ed. Ch. 52. London UK: Academic Press; 2018. pp. 703–20. [Google Scholar]
- 100.Coucha M, Abdelsaid M, Ward R, Abdul Y, Ergul A. Impact of metabolic diseases on cerebral circulation: Structural and functional consequences. Compr Physiol. 2018;8:773–99. doi: 10.1002/cphy.c170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eldahshan W, Fagan SC, Ergul A. Inflammation within the neurovascular unit: Focus on microglia for stroke injury and recovery. Pharmacol Res. 2019;147:104349. doi: 10.1016/j.phrs.2019.104349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rajkovic O, Potjewyd G, Pinteaux E. Regenerative medicine therapies for targeting neuroinflammation after stroke. Front Neurol. 2018;9:734. doi: 10.3389/fneur.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holmes A, Coppey LJ, Davidson EP, Yorek MA. Rat models of diet-induced obesity and high fat/low dose streptozotocin type 2 diabetes: Effect of reversal of high fat diet compared to treatment with enalapril or menhaden oil on glucose utilization and neuropathic endpoints. J Diabetes Res. 2015;2015:307285. doi: 10.1155/2015/307285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdul Y, Abdelsaid M, Li W, Webb RC, Sullivan JC, Dong G, et al. Inhibition of toll-like receptor-4 (TLR-4) improves neurobehavioral outcomes after acute ischemic stroke in diabetic rats: Possible role of vascular endothelial TLR-4. Mol Neurobiol. 2019;56:1607–17. doi: 10.1007/s12035-018-1184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.MacDonald SL, Journeay WS, Uleryk E. A systematic review of the impact of obesity on stroke inpatient rehabilitation functional outcomes. NeuroRehabilitation. 2020;46:403–15. doi: 10.3233/NRE-192979. [DOI] [PubMed] [Google Scholar]
- 106.Haley MJ, Mullard G, Hollywood KA, Cooper GJ, Dunn WB, Lawrence CB. Adipose tissue and metabolic and inflammatory responses to stroke are altered in obese mice. Dis Model Mech. 2017;10:1229–43. doi: 10.1242/dmm.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: Observational data from the FOOD trial. Stroke. 2003;34:1450–6. doi: 10.1161/01.STR.0000074037.49197.8C. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen-Oghalai TU, Wu H, McNearney TA, Granger CV, Ottenbacher KJ. Functional outcome after stroke in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2008;59:984–8. doi: 10.1002/art.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verma R, Friedler BD, Harris NM, McCullough LD. Pair housing reverses post-stroke depressive behavior in mice. Behav Brain Res. 2014;269:155–63. doi: 10.1016/j.bbr.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kronenberg G, Gertz K, Heinz A, Endres M. Of mice and men: Modelling post-stroke depression experimentally. Br J Pharmacol. 2014;171:4673–89. doi: 10.1111/bph.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen D, Wang J, Xing Y, Jia P, Zhang Y, Wang J, et al. Behavioral assessment of post-stroke depression and anxiety in rodents. Brain Hemorrhages. 2020;1:105–11. [Google Scholar]
- 112.He F, Sullender CT, Zhu H, Williamson MR, Li X, Zhao Z, et al. Multimodal mapping of neural activity and cerebral blood flow reveals long-lasting neurovascular dissociations after small-scale strokes. Sci Adv. 2020;6:eaba1933. doi: 10.1126/sciadv.aba1933. [DOI] [PMC free article] [PubMed] [Google Scholar]


