A threshold transcranial Doppler mean flow-velocity value that would accurately diagnose ≥ 50% angiographic vasospasm remains elusive.
Abstract
BACKGROUND AND PURPOSE:
After aneurysmal SAH, transcranial Doppler is commonly used to monitor cerebral vasospasm. The diagnostic accuracy of transcranial Doppler flow velocity values in detecting angiographic vasospasm in patients requiring urgent endovascular intervention has not been established.
MATERIALS AND METHODS:
We performed a retrospective analysis of a consecutive series of patients with aneurysmal SAH who underwent transcranial Doppler (index test) within 24 hours of conventional angiography (reference test). The judgment of 33%, 50%, and 66% degree of vessel narrowing on angiography was independently established by multiple neuroendovascular clinicians. Vessel-specific per-segment and per-patient transcranial Doppler velocities were studied using receiver operating characteristic curves, the Youden index, and minimal acceptable sensitivity models. Optimal mean flow-velocity thresholds were explored to calculate sensitivity and specificity using a per-patient judgment of vasospasm of at least 50% angiographic narrowing in any large arterial segment except A1.
RESULTS:
In 221 patients, vasospasm was found in 15%, 8%, and 4% of arteries when the degree of reference angiographic luminal narrowing was 33%, 50%, and 66%, respectively. Mean flow velocities were significantly higher in vasospastic segments (P = . 001), but per-segment exploratory analyses yielded unsound mean flow velocity thresholds. The Youden and minimal acceptable sensitivity models proposed mean flow velocity thresholds of approximately 160 cm/s for the anterior circulation and 80 cm/s for the posterior circulation in the per-patient diagnosis of angiographic vasospasm (≥50%), yielding a sensitivity of 80%–90% (95% CI, 0.77–0.96), but with a corresponding specificity of 50% (95% CI, 0.40–0.56).
CONCLUSIONS:
In this study, a threshold transcranial Doppler mean flow-velocity value that would accurately diagnose ≥50% angiographic vasospasm remained elusive.
The use of transcranial Doppler (TCD) ultrasonography in the detection and management of patients at risk of vasospasm after intracranial aneurysm rupture has been studied by many groups since its introduction in 1982.1-11 The appeal of this noninvasive test is that unlike conventional angiography, it can be performed daily at the bedside in the intensive care unit, alerting clinicians that medical or endovascular interventions may be indicated to prevent delayed cerebral infarction. The use of TCD monitoring has been recommended by expert societies.12,13 The diagnostic accuracy of TCD in the diagnosis of vasospasm is important to verify because false-negative studies could result in vasospasm-related infarctions only found on CT when it is too late to intervene, and false-positives could lead to inappropriate interventions, including unnecessary premature or repeat catheter angiographies or even unnecessary angioplasty. Yet the diagnostic accuracy of TCD remains uncertain despite multiple previous studies, including 2 meta-analyses.5,14
There are a number of fundamental problems that became evident as the literature was reviewed. These problems have remained unsolved since the very early days of vasospasm research and include the following: 1) Although some boundaries are commonly mentioned, such as <120 (absent vasospasm) and >200 cm/s as indicative of severe vasospasm, no mean flow velocity (MFV) threshold value has been shown to accurately diagnose clinically significant angiographic vasospasm with a sensitivity and specificity at the arterial segment level; 2) there is no accepted definition of the degree of vessel narrowing sufficient to be considered a clinically significant angiographic vasospasm; and 3) there is no recognized method to assign a vasospasm score at the individual patient level. Other problems with previous studies include the following: limiting the analyses of performance to a particular arterial segment (often M1), failing to verify the reliability of the angiographic gold standard,15,16 failing to clearly define the intended role of the index test, and unclear methodology.14 The most important problem is that many reports used an angiographic threshold that was too low to define vasospasm (33% or 25% luminal diameter reduction). While the degree of luminal diameter reduction necessary for clinically relevant restrictions in blood flow is not known with certainty, it is at least 50%.17,18 Previous studies have commonly tried to determine the sensitivity and specificity of TCD in detecting any degree of angiographic vasospasm, even though some degree of vasospasm is almost always found after substantial subarachnoid bleeding.19 Such studies may have designated many patients with clinically irrelevant angiographic vasospasm as true-positives. A more pertinent role of TCD would be the identification of patients who may need urgent medical or endovascular intervention to reverse vasospasm or prevent infarction.
At our institution (University of Alberta Hospital), TCD is routinely used to monitor all patients with SAH during the vasospasm risk period (days 3–12 post-SAH). Furthermore, conventional angiography is also routinely performed 5–9 days after aneurysm treatment to confirm aneurysm occlusion and assess the presence of vasospasm. All patients are included in a prospective database. These data, covering 10 years of clinical practice, give us the opportunity to study anew the accuracy of TCD velocity measurement, this time not for the diagnosis of any degree of vasospasm but more specifically to detect patients who may require timely conventional angiography and possibly endovascular intervention.20
The main objective of this work was to study the diagnostic accuracy of various TCD flow-velocity values in the diagnosis of ≥50% angiographic vasospasm severe enough to consider conventional angiography and endovascular treatment when confirmed.
MATERIALS AND METHODS
Literature Review
The full-text English- and French-language articles included in the initial and updated meta-analyses of 20015 and 201814 on diagnostic accuracy of TCD were examined independently by 2 authors (A.M.C. and T.E.D.), and the reported degree of vessel narrowing used in each source article was extracted.
Diagnostic Accuracy Study
Institutional ethics approval was obtained for this study (Pro0080185). This work is reported according to the Standards for Reporting of Diagnostic Accuracy Studies.21
We attempted to address some of the shortcomings of previous studies by detailing the methodology we used, by clarifying the purported role of the index test (to detect vasospasm severe enough to justify angiography and endovascular rescue), by exploring 3 degrees of severity of vasospasm, by verifying the reliability of the reference test, by including all arterial segments (except A1) in the analysis, and by providing a definition of “vasospasm” at the individual patient level.
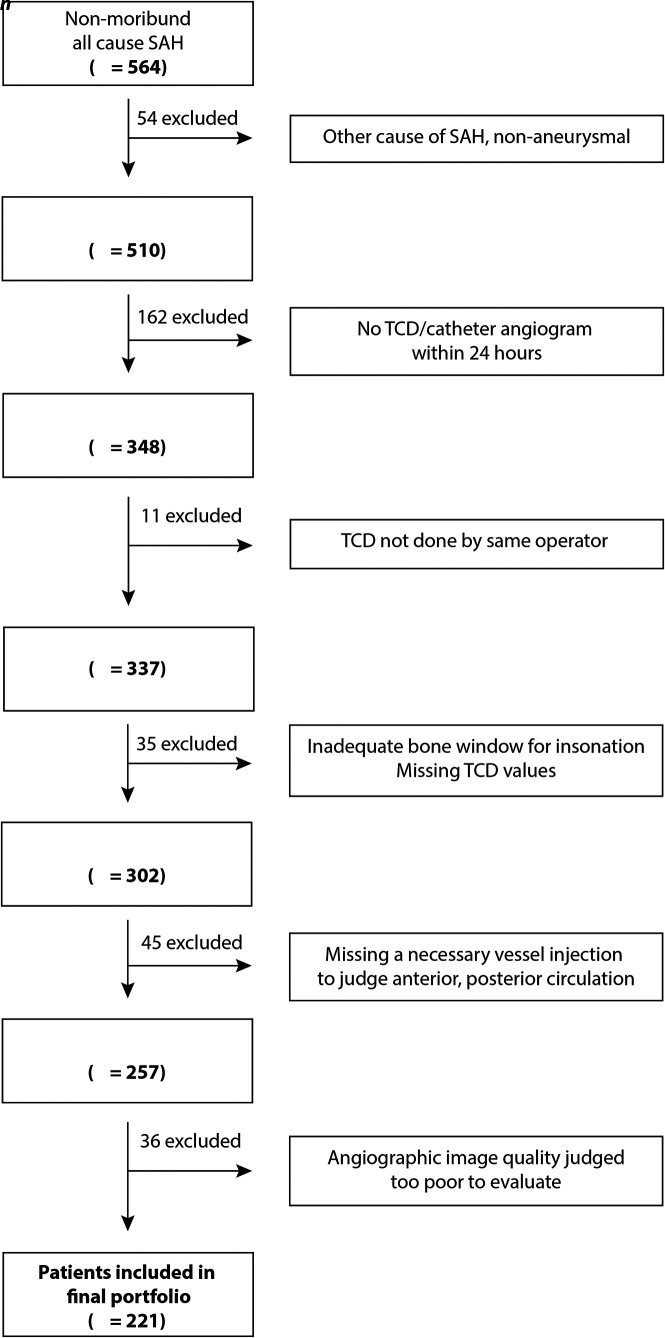
Patients
This retrospective study included consecutive adult (≥18 years of age) patients with nontraumatic SAH hospitalized at the University of Alberta Hospital from January 2007 to December 2017. Patients with SAH are monitored by TCD during the vasospasm risk period (days 3–12), and conventional angiography is routinely performed 5–9 days after aneurysm treatment. Conventional angiography may have been repeated in response to a concerning increase in TCD velocities or in the event of a new neurologic deficit. For the purpose of this study, each patient was represented by a single TCD-angiography pair of examinations: Patients were included when TCD MFV values were acquired on the same day as conventional angiography. Patients were excluded when the angiographic study did not include the left and right ICAs and at least 1 vertebral artery injection (see the flow chart, Fig 1). We did not record how patients were treated medically or study the effects of treatment on TCD velocities or angiographic determinations.
FIG 1.
Flow chart of patients included in the diagnostic accuracy study.
Transcranial Doppler (Index Test)
TCD readings (MFVs) were obtained daily or every other day starting on post-SAH day 3 and continuing until day 12 by the same dedicated sonographer (14 years of TCD experience at the beginning of this study interval) using a PMD 100 or 150 TCD system (Spencer Technologies) from 2007 to 2012, and a ST3 system (Spencer Technologies) from 2013 to 2017. Cases were not excluded when only 1 or 2 vessel segments could not be insonated (n = 25). One MFV value was acquired per vessel segment (ICA, M1, A1, and basilar artery), with results reviewed by a vascular neurologist and recorded in a prospectively maintained case log.
Angiographic Vasospasm (Reference Test)
Severe vasospasm was defined as ≥50% reduction in the diameter of the proximal intracranial arteries, as adjudicated by at least 14/17 (80%) interventionists independently rating catheter angiograms without knowledge of the TCD values. In a consensus session, 2 senior authors (T.E.D. and J.R.) reviewed the additionally identified cases of severe vasospasm as the number of raters agreeing on a verdict was lowered 1 reader at a time from 17/17 to 11/17 (65% of raters), while remaining blinded to TCD values. The result of this consensus session was that 14/17 readers provided the optimal collection of cases, and it was determined that only those cases would be considered correctly flagged by the triage TCD test. The reliability of the gold standard angiographic diagnosis of severe vasospasm in the same series of patients has previously been reported.15 Briefly, for each patient, clinicians were provided with anterior-posterior projections for 3 injections (right ICA, left ICA, and vertebral artery) and asked to visually judge the degree of vessel narrowing for each arterial segment at the level of the supraclinoid ICA, M1, A1, and basilar artery (7 arterial segments). The thresholds were as follows: none/mild vasospasm: <33% vessel narrowing; moderate vasospasm: 33%–50% vessel narrowing; severe vasospasm: >50% vessel narrowing, according to a modified scheme used at our institution.8 Raters were not provided with any clinical information concerning the case and were blinded to the scores given by other participants. Severe vasospasm was predefined as a 50% reduction in luminal diameter, but the effects of reducing this threshold to 33% were also examined. The scores provided were based on simple visual inspection of the degree of luminal narrowing. Four clinicians were also asked to evaluate the same permuted portfolio using a 66% vessel narrowing threshold. This time 66% severe vasospasm was adjudicated when 3 of 4 raters independently agreed.15
Definition of TCD MFV Thresholds
MFV thresholds were studied in 3 different ways: 1) by performing receiver operating characteristic (ROC) curves and exploring the velocities corresponding to the Youden index (maximizing sensitivity and specificity); 2) by examining the sensitivity and specificity of fixed-threshold MFV values (110, 120, 130, 140, 150, 160 cm/s); and 3) by exploring the threshold velocities corresponding to a minimal sensitivity of 80%, 85%, 90%, and 95% in diagnosing 50% angiographic vasospasm.
Per-Arterial Segment and Per-Patient Analyses
The distribution and diagnostic accuracy of TCD MFV values were first studied at the level of arterial segments (each patient contributed 7 arterial segments) using means and ROC curves.
A per-patient diagnosis of vasospasm was then defined when any 1 segment (or more) excluding A1 was adjudicated at a 50% degree of narrowing. Here, TCD was conceived as a “triage test” to detect patients who might benefit from conventional angiography and endovascular interventions: A true-positive TCD verdict was then adjudicated at the patient level when any segment (except A1) reached a certain velocity, even if that velocity concerned a different segment than the one judged to be 50% narrowed by angiography. A true-negative TCD verdict was adjudicated when no segment reached that velocity and no segment on angiography was perceived to have <50% narrowing in diameter. Analyses were repeated using 2 other angiographic thresholds (33% and 66%).
Statistical Analysis
All analyses were performed by statisticians (M.C., J.Z.) using STATA, Version 16.0 (StataCorp) and SPSS, Version 26 (IBM) with a significance level set at .05.
This study used all patients who fit the selection criteria without formally calculating the sample size necessary to test a prespecified hypothesis. Mean TCD velocities for each segment in nonsevere and severe vasospasm at the 3 different thresholds were compared using the Student t test. For each of the 7 segments, ROC curves were generated and the MFV at which sensitivity and specificity were optimized using the Youden index was determined. Corresponding sensitivities and specificities with 95% CIs were reported. We constructed Gaussian curves for patients with and without vasospasm at each of the 3 thresholds for visual comparison of the overlap of the curves, looking for fixed sensitivity values of 80%, 85%, 90%, and 95%. To evaluate the diagnostic accuracy of various TCD flow-velocity values, we selected 4 predefined levels of sensitivity (80%, 85%, 90%, and 95%). ROC curves were generated to define threshold MFVs for the diagnosis of severe vasospasm for each of the anterior circulation (bilateral ICA and M1 segments) and posterior circulation (basilar segment) arteries. The corresponding specificities were then determined. Actual sensitivities and specificities with 95% CIs are reported. Per-patient determinations (yes/no) of a correct TCD judgment of severe vasospasm was considered when these MFV thresholds of either the anterior or posterior circulation were exceeded.
RESULTS
Literature Review
The meta-analyses on the diagnostic accuracy of TCD identified 36 source articles published between 1984 and 2013.5,14 Two articles in Japanese were excluded, leaving 34 English- or French-language reports for full-text analysis (Online Supplemental Data). Thirty-one of the 34 (91%) articles included in the systematic reviews were diagnostic accuracy studies that compared the TCD index test with a reference test. Twenty-four of 31 (77%) diagnostic accuracy studies reported on anterior circulation velocities only, while 2 (6%) focused exclusively on posterior circulation vasospasm, with 5 (16%) reporting both.
The percentage of luminal narrowing on conventional angiography used as the reference test was ≥50% in 8/31 or 26% of articles [no threshold: 13 articles (42%); 20%–25% luminal narrowing: 8 articles (26%); 30%–33%: 2 articles (6%); 50%: 7 articles (23%); 75%: 1 article (3%)]. Reported MFV thresholds to diagnose vasospasm in the anterior circulation ranged from 90 to 180 cm/s and 60 to 95 cm/s for the posterior circulation. There were no reports regarding a per-patient judgment of vasospasm using a luminal narrowing threshold of at least 50%.
Diagnostic Accuracy Study
The flow chart of patients included in the diagnostic accuracy study is shown in Fig 1, and the characteristics of the 221 patients with SAH are available in the Online Supplemental Data.
Per-Segment Analyses
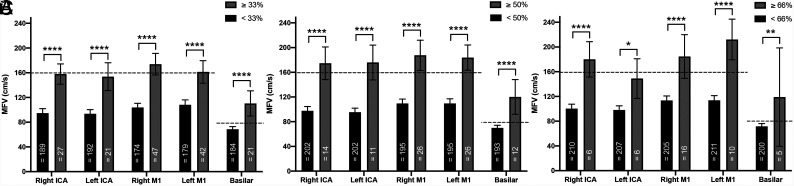
We first compared the MFVs of arterial segments with or without angiographic vasospasm, defined according to 33%, 50%, and 66% narrowing thresholds (Fig 2).
FIG 2.
Mean flow velocities for patients with and without vasospasm, defined as 33% (A), 50% (B), and 66% (C) vessel narrowing. MFV thresholds for anterior and posterior circulation vessels are shown (dashed lines). Note that for all individual arterial segments, MFVs were significantly higher for spastic vessels. Four asterisks indicate P < .0001; 2 asterisks, P < .01; 1 asterisk, P < .05. Error bars represent 95% confidence intervals.
The proportion of arterial segments judged to be vasospastic decreased with increasing angiographic thresholds (15%, 8%, and 4% of segments for >33%, 50%, and 66% vessel narrowing, respectively). The MFVs of vasospastic segments were significantly higher for all segments (P = .001). MFVs of basilar segments were significantly lower than those of anterior circulation arterial segments (P = .001).
The ROC curves of the TCD MFV values in the diagnosis of 50% vasospasm for each arterial segment (without prespecifying a threshold velocity) are available in the Online Supplemental Data. The areas under the curve and the MFVs corresponding to the Youden index (which optimizes sensitivity and specificity) are summarized in Table 1.
Table 1:
Per-arterial segment TCD MFVs corresponding to Youden index optimizing sensitivity and specificity in detecting 50% vasospasm
| MFV Threshold (cm/s) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95%CI) | |
|---|---|---|---|---|
| Right ICA | 154 | 0.86 (0.56–0.98) | 0.86 (0.81–0.90) | 0.88 (0.81–0.95) |
| Left ICA | 109 | 1.0 (0.68–1.0) | 0.69 (0.63–0.76) | 0.90 (0.84–0.96) |
| Right M1 | 157 | 0.73 (0.56–0.90) | 0.82 (0.76–0.87) | 0.84 (0.75–0.92) |
| Left M1 | 124 | 0.92 (0.73–0.99) | 0.65 (0.58–0.72) | 0.85 (0.78–0.91) |
| Basilar artery | 98 | 0.75 (0.51–0.81) | 0.81 (0.76–0.87) | 0.84 (0.73–0.95) |
Note:—AUC indicates area under the curve.
This data-dependent method of exploring optimal velocities to diagnose angiographic vasospasm provided clinically unsound results (ie, widely discrepant threshold velocity values for the right and left ICAs [154 and 109 cm/s], and also for right and left M1 segments [157–124 cm/s]), whereas similar results would be expected.
Per-Patient Analyses
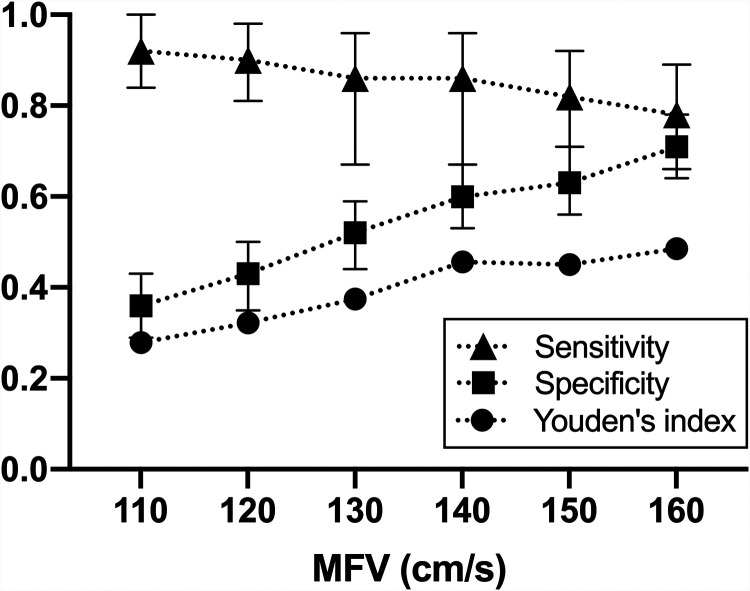
We then examined ROC curves and the sensitivity and specificity of various predefined threshold velocity values (when reached in any anterior circulation segment in the same patient) in the diagnosis of severe vasospasm at the patient level (defined as 50% narrowing in any segment). Figure 3 shows that low-velocity thresholds are sensitive but poorly specific; when velocities reach 160 cm/s (the velocities with the highest Youden number), the specificity reaches 70%, but sensitivity decreases below 80%.
FIG 3.
Sensitivity, specificity, and the Youden number at various possible mean flow velocity cutoffs for anterior circulation arterial segments. This data-driven method of analysis shows that the best MFV cutoff is close to 160 cm/s. Data for posterior circulation segments are not shown.
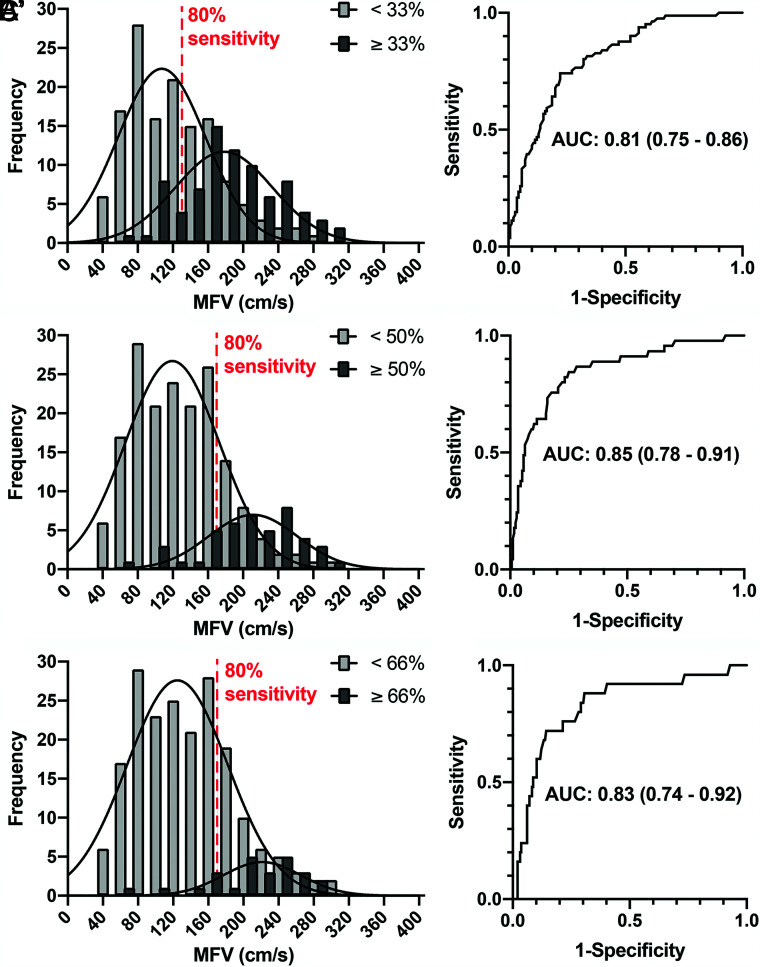
The distributions of the maximal velocity found in any segment of the anterior circulation in patients categorized with or without vasospasm (any segment) according to the 3 angiographic thresholds are shown in Fig 4, along with corresponding ROC curves and areas under the curve (results for basilar segments are not shown). The overlap between patients with and without vasospasm increases with sensitivity. Optimal velocities corresponding to a minimal sensitivity of 80% (along with the corresponding specificity and 95% confidence intervals) are summarized in Table 2. Optimal MFV threshold values for a minimal sensitivity of 80% in the diagnosis of 50% angiographic vasospasm were 164 cm/s (anterior circulation segments) and 80 cm/s (basilar artery). Yet, the specificity remained low (56%–71%).
FIG 4.
Per-patient analysis of the diagnostic accuracy of TCD. Distributions of the maximal mean flow velocity found in any segment of the anterior circulation with or without vasospasm according to thresholds of 33% (A), 50% (B), and C) 66%. The 80% sensitivity line is labeled. Corresponding ROC curves are presented in A’, B’, and C’. Note the large amount of overlap of the curves despite acceptable areas under the curve (AUCs).
Table 2:
Per-patient TCD MFV thresholds for various minimal sensitivities in detecting 50% vasospasm
| Minimum Sensitivity | Predefined MFV Threshold (cm/s)a |
|||
|---|---|---|---|---|
| ICA or M1 | Basilar Artery | Sensitivity (95% CI) | Specificity (95% CI) | |
| 80% | >164 | >80 | 0.86 (0.72–0.94) | 0.64 (0.56–0.71) |
| 85% | >160 | >61 | 0.90 (0.77–0.96) | 0.48 (0.40–0.56) |
| 90% | >126 | >60 | 0.94 (0.82–0.98) | 0.37 (0.30–0.45) |
| 95% | >95 | >56 | 0.96 (0.85–0.99) | 0.24 (0.18–0.31) |
Severe vasospasm was determined when 1 threshold was exceeded.
DISCUSSION
This study shows that high MFVs are closely associated with severe angiographic vasospasm. Nevertheless, TCD MFVs did not perform well when tested at the individual patient level to triage patients who showed angiographic vasospasm that might require urgent treatment: Using relatively high-threshold MFV values (such as 160 cm/s) would still unnecessarily send patients for cerebral angiography 50% of the time yet would still risk missing 10%–20% of patients with vasospasm (≥50%) sufficient enough to be at risk of cerebral infarction.
Our results differ from those of many of the studies collected in the most recent systematic review (2018), which showed TCD to be specific (90%) but not sensitive (67%).14
However, many of the included studies examined the performance of TCD when TCD played a different role; most used low MFV values (in the range of 120 cm/s) to identify patients with much lower gold standard angiographic thresholds for the diagnosis of vasospasm (≤25% narrowing). As revealed by the reported expected prevalence of 70% of vasospasm, these studies were designed to assess the accuracy of TCD in diagnosing any degree of vasospasm, no matter the clinical relevance.14 We are unsure of the added value of a diagnostic test that identifies any degree of vasospasm in patients with a high pretest probability of any vasospasm (70%). The more important role for TCD in this context should rather be to accurately identify patients who could eventually be rescued with induced hypertension or endovascular treatment. Unfortunately, the clinical benefit of medical or endovascular interventions has not been rigorously verified.22,23
One fundamental problem is that angiographic vasospasm itself is not a well-defined disease. It is rather a test finding that is vaguely associated with delayed cerebral ischemia, but the relationship between angiographic vasospasm and clinical outcomes is uncertain. Furthermore, there is no well-accepted definition of angiographic vasospasm at the arterial segment or per-patient level, and the reliability and clinical significance of the gold standard itself is, at best, questionable.15,16 In such circumstances, the diagnostic accuracy methodology we have used may not be the best way to assess the value of TCD monitoring in the prevention of delayed cerebral ischemia.24 With so much uncertainty at so many levels, we need to seek a scientific way to make progress.
The problem may call for an entirely different approach regarding how diagnoses are determined. Other medical specialties confronted with uncertain threshold values have recently addressed decades-old diagnostic controversies using pragmatic trial methodology.25 We believe progress in this field would also be possible by designing randomized trials that test the value of TCD monitoring, angiography, and endovascular intervention in the prevention of delayed cerebral ischemia after SAH.
This study has several limitations. The patients and the TCD values were prospectively collected, but angiograms were retrospectively analyzed for the purpose of this study. TCD was performed by a single expert technician (patients examined by other technicians or neurologists were excluded). While TCD is used to monitor patients during a period at risk of vasospasm, for each patient a single examination was retained for comparison with the same-day angiogram. We did not examine various other TCD indices (such as Lindegaard ratios26) nor attempt to identify patients with rising MFVs across time, which could have led to different results.8 We also did not examine the effects of diagnoses on patient outcomes.
This study assessed a snapshot comparison of TCD values and an angiographic verdict within 24 hours; it does not consider the way TCD results of each patient were judged in real-time or how they were used for clinical decisions and subsequent management.
In this analysis, we considered TCD a triage test to identify whether individual patients should undergo angiography. The gold standard angiographic criterion itself was previously shown to be poorly repeatable, and we had to arbitrarily fix a threshold (14/17 raters) to provide a final verdict necessary to proceed with a diagnostic-accuracy study.15 We chose to call true-positive per-patient TCD verdicts whenever the TCD threshold was exceeded in any segment in the same patient—even if it was not the correct segment found narrowed on catheter angiography. This evaluation of the diagnostic accuracy of TCD could be considered too generous. Finally, the methods we used to explore the threshold velocities that would maximize sensitivity and specificity are known to overestimate the diagnostic accuracy of the index test.21
CONCLUSIONS
There is a general correlation between blood flow velocity increases and angiographic vasospasm. Thus, TCD findings can alert clinicians to the possibility of vasospasm and to the need for careful patient assessment and examination for the development of neurologic findings. However, a threshold MFV value that can accurately distinguish patients with or without ≥50% angiographic narrowing remains elusive.
ABBREVIATIONS:
- MFV
mean flow velocity
- ROC
receiver operating characteristic
- TCD
transcranial Doppler
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982;57:769–74 10.3171/jns.1982.57.6.0769 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez NR, Boscardin WJ, Glenn T, et al. Vasospasm probability index: a combination of transcranial Doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 2007;107:1101–12 10.3171/JNS-07/12/1101 [DOI] [PubMed] [Google Scholar]
- 3.Krejza J, Kochanowicz J, Mariak Z, et al. Middle cerebral artery spasm after subarachnoid hemorrhage: detection with transcranial color-coded duplex US. Radiology 2005;236:621–29 10.1148/radiol.2362031662 [DOI] [PubMed] [Google Scholar]
- 4.Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl (Wien) 1988;42:81–84 [DOI] [PubMed] [Google Scholar]
- 5.Lysakowski C, Walder B, Costanza MC, et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke 2001;32:2292–98 10.1161/hs1001.097108 [DOI] [PubMed] [Google Scholar]
- 6.Okada Y, Shima T, Nishida M, et al. Comparison of transcranial Doppler investigation of aneurysmal vasospasm with digital subtraction angiographic and clinical findings. Neurosurg 1999;45:443–49; discussion 449–50 10.1097/00006123-199909000-00005 [DOI] [PubMed] [Google Scholar]
- 7.Proust F, Hannequin D, Langlois O, et al. Causes of morbidity and mortality after ruptured aneurysm surgery in a series of 230 patients: the importance of control angiography. Stroke 1995;26:1553–57 10.1161/01.str.26.9.1553 [DOI] [PubMed] [Google Scholar]
- 8.Vora YY, Suarez-Almazor M, Steinke DE, et al. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurg 1999;44:1237–47; discussion 1247–38 10.1227/00006123-199906000-00039 [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Offin R, Teasdale GM, et al. Is routine transcranial Doppler ultrasound monitoring useful in the management of subarachnoid hemorrhage? J Neurosurg 1998;88:272–76 10.3171/jns.1998.88.2.0272 [DOI] [PubMed] [Google Scholar]
- 10.Weir B, Grace M, Hansen J, et al. Time course of vasospasm in man. J Neurosurg 1978;48:173–78 10.3171/jns.1978.48.2.0173 [DOI] [PubMed] [Google Scholar]
- 11.Sebastian J, Derksen C, Khan K, et al. Derivation of transcranial Doppler criteria for angiographically proven middle cerebral artery vasospasm after aneurysmal subarachnoid hemorrhage. J Neuroimaging 2013;23:489–94 10.1111/j.1552-6569.2012.00771.x [DOI] [PubMed] [Google Scholar]
- 12.Diringer MN, Bleck TP, Hemphill JC 3rd, et al. ; Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care 2011;15:211–40 10.1007/s12028-011-9605-9 [DOI] [PubMed] [Google Scholar]
- 13.Edmonds HL Jr, Isley MR, Sloan TB, et al. American Society of Neurophysiologic Monitoring and American Society of Neuroimaging joint guidelines for transcranial Doppler ultrasonic monitoring. J Neuroimaging 2011;21:177–83 10.1111/j.1552-6569.2010.00471.x [DOI] [PubMed] [Google Scholar]
- 14.Mastantuono JM, Combescure C, Elia N, et al. Transcranial Doppler in the diagnosis of cerebral vasospasm: an updated meta-analysis. Crit Care Med 2018;46:1665–72 10.1097/CCM.0000000000003297 [DOI] [PubMed] [Google Scholar]
- 15.Darsaut TE, Derksen C, Farzin B, et al. Reliability of the diagnosis of cerebral vasospasm using catheter cerebral angiography: a systematic review and inter- and intraobserver study. AJNR Am J Neuroradiol 2021;42:501–07 10.3174/ajnr.A7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letourneau-Guillon L, Farzin B, Darsaut TE, et al. Reliability of CT angiography in cerebral vasospasm: a systematic review of the literature and an inter- and intraobserver study. AJNR Am J Neuroradiol 2020;41:612–18 10.3174/ajnr.A6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weir B. Vasospasm: does it cause infarction and poor outcome? J Neurosurg 2020;134:1006–11 10.3171/2020.7.JNS202551 [DOI] [PubMed] [Google Scholar]
- 18.Young DF. Fluid mechanics of arterial stenoses. J Biomech Eng 1979;101:157–75 10.1115/1.3426241 [DOI] [Google Scholar]
- 19.Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage, Part I: incidence and effects. J Clin Neurosci 1994;1:19–26 10.1016/0967-5868(94)90005-1 [DOI] [PubMed] [Google Scholar]
- 20.Rosenwasser RH, Armonda RA, Thomas JE, et al. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurg 1999;44:975–79; discussion 979–80 10.1097/00006123-199905000-00022 [DOI] [PubMed] [Google Scholar]
- 21.Bossuyt PM, Reitsma JB, Bruns DE, et al. ; STARD Group. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527 10.1136/bmj.h5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 2009;40:1963–68 10.1161/STROKEAHA.108.544700 [DOI] [PubMed] [Google Scholar]
- 23.Wang LS, He W, Zhang HQ, et al. Comparison of transcranial color Doppler sonography without and with contrast enhancement for detection and characterization of intracranial aneurysms. J Clin Ultrasound 2012;40:535–39 10.1002/jcu.21911 [DOI] [PubMed] [Google Scholar]
- 24.Rutjes AW, Reitsma JB, Coomarasamy A, et al. Evaluation of diagnostic tests when there is no gold standard: a review of methods. Health Technol Assess 2007;11:iii, ix–51 10.3310/hta11500 [DOI] [PubMed] [Google Scholar]
- 25.Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med 2021;384:895–904 10.1056/NEJMoa2026028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindegaard KF, Bakke SJ, Sorteberg W, et al. A non-invasive Doppler ultrasound method for the evaluation of patients with subarachnoid hemorrhage. Acta Radiol Suppl 1986;369:96–98 [PubMed] [Google Scholar]