Abstract
BACKGROUND AND PURPOSE:
Intracranial stents for the treatment of aneurysms can be responsible for parent artery straightening, a phenomenon with potential consequences for aneurysmal occlusion. We aimed to evaluate parent artery straightening following flow-diverter stent placement in patients with intracranial aneurysms and explored the association between parent artery straightening and subsequent aneurysm occlusion.
MATERIALS AND METHODS:
All patients treated with flow-diverter stents for anterior circulation aneurysms located downstream from the carotid siphon between January 2009 and January 2018 were screened for inclusion. Parent artery straightening was defined as the difference (α–β) in the parent artery angle at the neck level before (α angle) and after flow-diverter stent deployment (β angle). We analyzed the procedural and imaging factors associated with parent artery straightening and the associations between parent artery straightening and aneurysmal occlusion.
RESULTS:
Ninety-five patients met the inclusion criteria (n = 64/95 women, 67.4%; mean age, 54.1 [SD, 11.2] years) with 97 flow-diverter stents deployed for 99 aneurysms. Aneurysms were predominantly located at the MCA bifurcation (n = 44/95, 44.4%). Parent artery straightening was found to be more pronounced in patients treated with cobalt chromium stents than with nitinol stents (P = .02). In multivariate analysis, parent artery straightening (P = .04) was independently associated with aneurysm occlusion after flow-diverter stent deployment.
CONCLUSIONS:
The use of flow-diverter stents for distal aneurysms induces a measurable parent artery straightening, which is associated with higher occlusion rates. Parent artery straightening, in our sample, appeared to be more prominent with cobalt chromium stents than with nitinol stents. This work highlights the necessary trade-off between navigability and parent artery straightening and may help tailor the selection of flow-diverter stents to aneurysms and parent artery characteristics.
Flow-diverter stents are an interesting treatment option for selected patients with intracranial aneurysms.1,2 Their efficacy to obtain aneurysm occlusion depends notably on the incident angle of blood flow through the flow-diverter stents into the aneurysm sac,3 which directly correlates with parent artery anatomy. In patients with aneurysms located in the carotid siphon,4 flow-diverter stents have been shown to straighten the parent artery after deployment, and the straightening may independently be associated with higher aneurysm occlusion rates. Yet, this effect has not, to date, been explored in patients with more distal aneurysms, their parent vessels being less subjected to local constraints and, in turn, a likely more important susceptibility to deformation. Indeed, vessel anatomy is constrained by the local osseous environment in the siphon, whereas vessels located in the Sylvian cisterns or more distally at the surface of the brain are more prone to anatomic changes under external or internal constraint.
In a retrospective study, we aimed to measure parent artery straightening after deployment of flow-diverter stents in patients with intracranial aneurysms located beyond the carotid siphon and to assess whether parent artery straightening is associated with aneurysm occlusion.
MATERIALS AND METHODS
Ethics
The study protocol was approved by the local ethics committee at Rothschild Foundation Hospital. In line with regulations in France where the study was conducted, the institutional review board waived the need for patients’ signed consent. Patients were informed that they could refuse the use of their data.
Data Sharing
Data will be made available on reasonable request by a qualified investigator after institutional review board approval.
Population
All patients treated with flow-diverter stents for intracranial aneurysms located beyond the carotid siphon between January 2009 and January 2018 were included. At our institution, flow-diverter stents are used as an alternative to coiling or an operation for the treatment of complex aneurysms, including giant, fusiform, or wide-neck lesions. Flow-diverter stents are not used in the context of suspected mycotic lesions. Posterior circulation aneurysms, fusiform aneurysms, ruptured aneurysms, aneurysms without available images, and those treated with >1 flow-diverter stent or previously treated by stent placement were excluded.
Treatment
Endovascular treatment was performed with the patient under general anesthesia. All patients received systemic heparinization during the procedure. Four different flow-diverter stent devices were available: 2 cobalt chromium stents, the Pipeline Embolization Device (PED; Medtronic) and the Surpass FD (Stryker Neurovascular) and 2 nitinol stents, the Silk (Balt Extrusion) and the Flow-Redirection Endoluminal Device (FRED; MicroVention). Concomitant coiling was sometimes performed during the same procedure at the discretion of the operators.
Antiplatelet Therapy
In the postoperative period, all treated patients received dual-antiplatelet therapy by aspirin and clopidogrel for 3 months (except for one who received aspirin combined with ticagrelor) followed by monotherapy using aspirin alone.
Clinical and Imaging Follow-up
Patients were assessed by a first cerebral angiogram at 6–12 months and a second cerebral angiogram at 36 months, then by brain MR imaging.
Data Collection and Analysis
All clinical and imaging data were retrieved from electronic medical records. The parent artery angle was measured at the intersection of the central lines of the proximal and distal portions of the parent vessel to the center point of the parent artery facing the aneurysm neck, before and after stent deployment and at first follow-up (Fig 1). Parent artery straightening was defined as the difference between the postdeployment angle (β) and the initial angle (α). Two independent physicians blinded to the angiographic outcome of the aneurysm performed these measurements on DSA working projections. The postdeployment angle used for analyses was the mean of both raters’ measurements. Discrepancies of >10° of parent artery straightening were resolved by consensus. Aneurysm occlusion was evaluated using the O’Kelly-Marotta grading scale based on the DSA images.5
FIG 1.
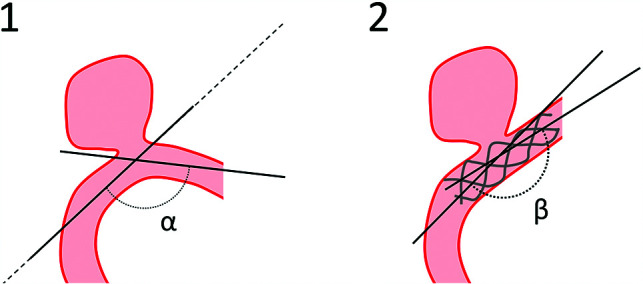
Measurement of parent artery straightening. Angles were measured at the intersection of 2 lines drawn from the neck center in the axis of the proximal and distal segments of the parent artery. is the angle measured before stent placement, and is the angle measured after stent deployment. Preoperative (1) and postoperative (2) drawings.
Statistical Analysis
Continuous variables were reported as mean (SD), with extreme values and categoric variables as number and percentage. Agreement between readers for angle measurements was determined using the interclass correlation coefficient and a 95% confidence interval. For complete aneurysm occlusion, univariate, logistic regression models were used to test the association, whereas a univariate linear regression model was used for factors associated with parent artery straightening. Variables with P < .20 in univariable analysis were included in a multiple logistic regression model for occlusion and a multiple linear regression model for the parent artery straightening angle. Backward variable selection was used to select the independent predictors of complete occlusion and postdeployment angle. P < .05 was considered significant. The interaction term “parent artery straightening × stent type” may allow us to understand whether the effect of parent artery straightening varies within stent type. All statistical analyses were performed using JMP Pro 14 (SAS Institute, 1989–2019).
RESULTS
Study Cohort Characteristics
Ninety-five patients were included in the present study (64 women, 67.4%; mean age, 54.1 [SD, 11.2] years) for a total of 99 aneurysms located downstream from the carotid siphon treated with 97 flow-diverter stents, including 45 aneurysms (45.5%) previously treated with coils. All aneurysms were saccular and measured 6.1 (SD, 5.0) mm in maximal diameter and 3.9 (SD, 2.1) mm at the neck. Fifty-two aneurysms were treated using a cobalt chromium stent (PED, Surpass) (52.5%) and 47, using a nitinol stent (Silk, FRED) (47.5%). Detailed cohort characteristics are shown in Table 1.
Table 1:
Comparison of demographic, angiographic, and treatment characteristics between complete occluded and incomplete occluded aneurysmsa
| All Aneurysms (n = 99) | Complete Occlusion (n = 64) | Incomplete Occlusion (n = 20) | P Value | |
|---|---|---|---|---|
| Age (yr) (mean) | 54.1 (SD, 11.2) | 53.8 (SD, 10.8) | 54.3 (SD, 10.4) | .76 |
| Sex, M/F | 31 (31.3%)/68 (68.7%) | 18 (28.1%)/46 (71.9%) | 9 (45%)/11 (55%) | .26 |
| Follow-up (mo) (n = 84) | 23.9 (SD, 16.3) | 24.2 (SD, 15.0) | 22.9 (SD, 20.2) | .27 |
| Previous treatment | 45 (45.5%) | 28 (43.8%) | 13 (65%) | .16 |
| Aneurysm location | .62 | |||
| MCA | 44 (44.4%) | 25 (39.1%) | 12 (60%) | |
| AcomA | 27 (27.3%) | 20 (31.3%) | 5 (25%) | |
| A1 | 2 (2.0%) | 2 (3.1%) | 0 | |
| A2 | 2 (2.0%) | 2 (3.1%) | 0 | |
| Pericallosal | 24 (24.2%) | 15 (23.4%) | 3 (15%) | |
| Aneurysm size (mm) | ||||
| Neck | 3.9 (SD, 2.1) | 3.6 (SD, 2.1) | 4.3 (SD, 2.0) | .06 |
| Diameter | 6.1 (SD, 5.0) | 6.1 (SD, 5.7) | 6.5 (SD, 3.5) | .14 |
| Concomitant coiling | 15 (15.2%) | 9 (14.1%) | 2 (10%) | 1 |
| Flow-diverter stent | ||||
| Length (mm) | 16.9 (SD, 3.9) | 16.5 (SD, 3.8) | 18.2 (SD, 4.3) | .42 |
| Type | .004 | |||
| Nitinol | 47 (47.5%) | 26 (40.6%) | 16 (80%) | |
| Cobalt chromium | 52 (52.5%) | 38 (59.4%) | 4 (20%) | |
| Angle of parent artery | ||||
| Initial angle | 124.1° (SD, 64.0°) | 118.3° (SD, 29.8°) | 122.7° (SD, 28.1°) | .58 |
| Postdeployment angle | 141.6° (SD, 24.8°) | 146.0° (SD, 21.8°) | 135.9° (SD, 24°) | .12 |
| Parent artery straightening | 3.8° (SD, 21.6°) | 27.7 (SD, 22.6) | 13.3° (SD, 12.3°) | <.001 |
Note:—AcomA indicates anterior communicating artery; A1, proximal segment of the anterior cerebral artery; A2, distal segment of the anterior cerebral artery.
Data are presented as mean (standard deviation, SD) for continuous variables and absolute number (percentage of column total) for discrete variables.
Parent Artery Straightening
The mean postdeployment angle (β) was significantly superior to the initial angle (α) (141.6° [SD, 24.8°] versus 124.1° [SD, 64]°; P < .001).
The mean follow-up angle was further significantly increased compared with the postdeployment angle β (148.7°[SD, 20.3°]; P < .001), with a mean first DSA follow-up time of 8.5 (SD, 4.3) months. According to the criteria of Cicchetti et al,6 interobserver agreement was considered good for all 3 measurements, with interclass correlation coefficient values of 0.64 (0.40–0.75) for the initial angle (α), 0.65 (0.46–0.78) for the postdeployment angle (β), and 0.70 (0.53–0.83) for the follow-up angle, respectively.
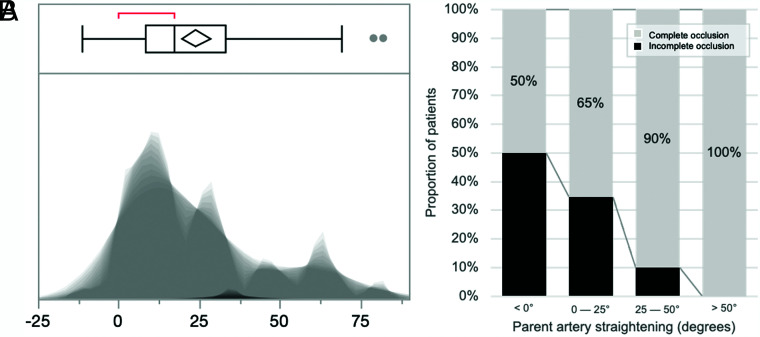
Parent artery straightening was common, with a median parent artery angle reduction of 17° (interquartile range, 8.25°–33°) (see Fig 2 for a complete distribution).
FIG 2.
Distribution of angles of parent artery straightening and association with aneurysmal occlusion. A, Shadowgram distribution of parent artery straightening. B, Stacked histograms of the proportion of patients with complete occlusion in bins of increasing parent artery straightening (offset = 0, width = 25°).
In univariate analysis, stent type (P = .02) and initial angle (α) (P < .001) were significantly associated with parent artery straightening. There was no association with patient sex, age, previous embolization, aneurysm location, aneurysm size, and associated coiling. The initial angle (α) (P = .01) and stent type (P < .001) remained associated independently with parent artery straightening (Online Supplemental Data). Figure 3 and the Online Supplemental Data show illustrations of parent artery straightening following flow-diverter stent placement.
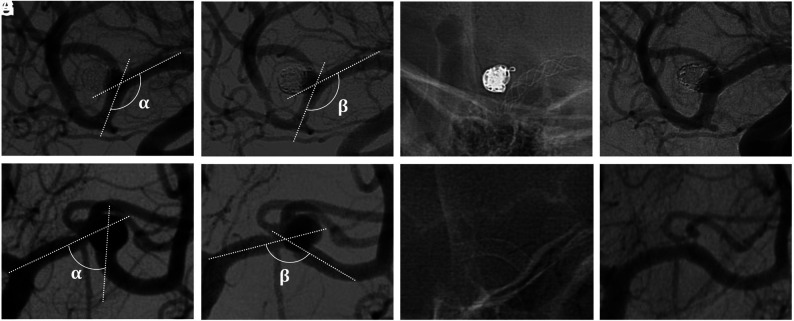
FIG 3.
Comparison of vascular geometry modifications in 2 treatments of MCA aneurysms. The second flow-diverter stent deployment appears to modify the vascular anatomy (E–G) more than the first flow-diverter stent (A–C). Angiogram controls show complete aneurysm occlusion with the second flow-diverter stent (H) compared with the first-flow diverter (D) stent. A and E, Preoperative angiogram. B and F, Angiogram after stent placement. C and G, Unsubtracted view after stent deployment. D and H, First angiogram follow-up. indicates the angle measured before stent placement; , the angle measured after stent placement.
Factors Associated with Aneurysm Occlusion
Fifteen patients with 15 aneurysms (15.2%) were lost during follow-up, leaving 84 aneurysms for this analysis. Complete occlusion (O’Kelly-Marotta score D) was observed in 64 aneurysms (76.2%) at follow-up, with a mean follow-up duration of 23.9 (SD, 16.3) months. In univariate analysis, cobalt chromium stents (P = .004) and higher parent vessel straightening (P < .001) were associated with complete aneurysm occlusion (Table 1). After adjustment, parent artery straightening (P = .036), stent type (P = .02), and aneurysm neck (P < .001) were independently associated with complete aneurysm occlusion. The interaction term between stent type and parent artery straightening was not significant (P = .055), meaning that there was no varying influence of parent artery straightening according to stent type. Detailed results are shown in Table 2.
Table 2:
Multivariable analysis of aneurysm occlusion determinants
| Variables | Multivariate Analysis, aOR (95% CI) | P Value |
|---|---|---|
| Aneurysm neck (mm) | 0.57 (0.38–0.87) | <.001 |
| Stent type: chromium cobalt | 2.13(1.26–11.93) | .020 |
| Parent artery straightening | 1.04° (1.01°–1.17°) | .036 |
| Initial angle | 0.95°(0.91°–1.02° | .401 |
| Parent artery straightening × stent typea | 0.99 (0.94–1.02) | .055 |
Note:—aOR indicates adjusted odds ratio.
Interaction term.
DISCUSSION
These results suggest that flow-diverter stents deployed beyond the carotid siphon modify the anatomy by inducing straightening of the parent artery, and parent artery straightening, in turn, favors higher rates of aneurysm occlusion. This result brings additional insight into the mechanisms at play after flow-diverter stent placement during aneurysm healing. It also suggests that while the distal navigability of a flow-diverter stent is highly desired for procedural success, it may come at the cost of lower parent artery straightening and lower odds of aneurysmal occlusion in aneurysms located beyond the carotid siphon.
Previous studies have reported failure rates as high as 17% after flow-diverter treatment in distal aneurysm locations,2 and mechanisms of occlusion failure still remain poorly understood.
Some animal studies have suggested that flow-diverter stent treatment was more effective when deployed in a straight artery than in a curved artery.7-9 Our study also suggests that this straightening phenomenon goes on after deployment, because follow-up angles were significantly superior to immediate postdeployment angles.4 In addition to its effects on the incident angle of blood flow into the aneurysm sac, this straightening effect could improve aneurysm occlusion through other mechanisms: It might influence the wall apposition of the flow-diverter stents,10 and it could also improve the “scaffolding” effect for reconstruction of the aneurysmal neck11 and promote arterial remodeling in a more favorable geometric configuration. It is, nonetheless, hypothesized that despite the flow-diverting effect, flow-diverter stents inducing important parent artery straightening may, in part, correct the geometric configuration that was involved in aneurysm development in the first place.
Hemodynamic factors indeed play an important role in the pathogenesis of cerebral aneurysms.12 Gao et al13 reported vascular geometric consequences of single conventional stent placement: Angular remodeling was more pronounced using the stiffer closed-cell-like flow-diverter stents. Our study demonstrates that flow-diverter stent placement in the distal artery straightens parent artery geometry like conventional stents, which has already been theoretically described in a computational fluid dynamics study.14 The hemodynamic efficiency of a flow-diverter stent is related to several parameters, including the porosity and metal coverage of the stent, but intra-aneurysmal hemodynamic changes are also affected by the curvature of the parent artery.14-16 When the aneurysm is developed on the convex wall of a curvature, cells of flow-diverter stents are more widely opened than when the artery is straightened, with a higher mesh density and increased flow diversion.17
Ishii et al18 reported that in stent-assisted coiled aneurysms, angular change induced by stent placement may affect aneurysm recanalization rates during follow-up more than coil packing density. Also, Funakoshi et al19,20 showed, in a cohort of 255 aneurysms, that distal aneurysms located in the ICA bifurcation, MCA, or anterior communicating artery develop in parent vessels that are not fixed by osseous structures, have characteristics such as small diameters and thin vessel walls, and can be more mobile. Progressive thrombosis is more often observed in these distal aneurysms treated by stent-assisted coiling; aggressive coiling may appear futile in these cases.
Our results suggest that parent artery straightening could be more important with cobalt chromium flow-diverter stents than with nitinol flow-diverter stents, probably due to the mechanical properties of their respective components. Nitinol is an alloy composed of near-equal parts of nickel and titanium, which exhibit unique properties: superelasticity and shape memory. Cobalt chromium is stronger than stainless steel, so a cobalt chromium stent can have similar strength with thinner braids.21,22 In the multivariable model, we indeed showed that cobalt chromium stents were associated with a 2-fold increase in subsequent aneurysm occlusion, and the interaction term between stent type and parent artery straightening attained near-significance (P = .055), implying that the degree of parent artery straightening might differ between both stents, which is substantiated by the analysis of parent artery straightening determinants. Most important, there are many other mechanical characteristics that differentiate these types of stents (such as navigability); therefore, our findings do not imply that one type of flow diverter stent is superior to the other. Indeed, our analysis was focused on aneurysms that are not constrained by osseous structures due to their distal situation. In these anatomic locations, increased navigability is a highly desirable characteristic of flow-diverter stents, and we cannot exclude a confounding by indication, which led to more distal/complex aneurysms being treated with nitinol stents in our sample.
Of interest, while software is currently being used to simulate the behavior of a stent after its deployment to help in planning treatment and choosing stent size,23 these tools assume that the vessel is a rigid, not deformable structure. Our results suggest that this approach might be misleading, at least for arteries beyond the circle of Willis, and there is room for incorporating parent artery straightening in this software to facilitate placement and postdeployment management in more complex cases.
Our study has several limitations due to its retrospective nature and the small number of patients included. Also, the angle measurements were made on 2D angiographic images, and measurement performed on 3D acquisitions might be more precise and could have shown different results.
CONCLUSIONS
In patients with intracranial aneurysms located beyond the carotid siphon, flow-diverter stent placement induces parent artery straightening, a phenomenon found in our sample to be associated with higher rates of subsequent aneurysmal occlusion. This feature may be of importance for device-selection planning in patients scheduled for elective flow-diverter stent placement and may need to be considered in future studies investigating the efficacy of flow-diverter stents to better comprehend the healing process of aneurysms.
Footnotes
The data used for this work have been presented at the 2019 annual conference of the French Neuroradiology Society, as an oral communication.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Pistocchi S, Blanc R, Bartolini B, et al. Flow diverters at and beyond the level of the circle of Willis for the treatment of intracranial aneurysms. Stroke 2012;43:1032–38 10.1161/STROKEAHA.111.636019 [DOI] [PubMed] [Google Scholar]
- 2.Primiani CT, Ren Z, Kan P, et al. A2, M2, P2 aneurysms and beyond: results of treatment with Pipeline embolization device in 65 patients. J Neuronterv Surg 2019;11:903–07 10.1136/neurintsurg-2018-014631 [DOI] [PubMed] [Google Scholar]
- 3.Meng H, Wang Z, Kim M, et al. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: implications of intravascular stenting. AJNR Am J Neuroradiol 2006;27:1861–65 [PMC free article] [PubMed] [Google Scholar]
- 4.Waihrich E, Clavel P, Mendes G, et al. Influence of anatomic changes on the outcomes of carotid siphon aneurysms after deployment of flow-diverter stents. Neurosurgery 2018;83:1226–33 10.1093/neuros/nyx618 [DOI] [PubMed] [Google Scholar]
- 5.O’Kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010;16:133–37 10.1177/159101991001600204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicchetti DV, Shoinralter D, Tyrer PJ. The effect of number of rating scale categories on levels of interrater reliability: a Monte Carlo investigation. Appl Psychol Meas 1985;9:31–36 10.1177/014662168500900103 [DOI] [Google Scholar]
- 7.Darsaut TE, Bing F, Salazkin I, et al. Flow diverters failing to occlude experimental bifurcation or curved sidewall aneurysms: an in vivo study in canines. J Neurosurg 2012;117:37–44 10.3171/2012.4.JNS111916 [DOI] [PubMed] [Google Scholar]
- 8.Fahed R, Raymond J, Ducroux C, et al. Testing flow diversion in animal models: a systematic review. Neuroradiology 2016;58:375–82 10.1007/s00234-015-1635-0 [DOI] [PubMed] [Google Scholar]
- 9.Fahed R, Darsaut TE, Gentric JC, et al. Flow diversion: what can clinicians learn from animal models? Neuroradiology 2017;59:255–61 10.1007/s00234-016-1781-z [DOI] [PubMed] [Google Scholar]
- 10.Rouchaud A, Ramana C, Brinjikji W, et al. Wall apposition is a key factor for aneurysm occlusion after flow diversion: a histologic evaluation in 41 rabbits. AJNR Am J Neuroradiol 2016;37:2087–91 10.3174/ajnr.A4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y, Zhang S, Du H, et al. Impact of vessel curvature on neointimal healing after stent implantation as assessed by optical coherence tomography. Medicine (Baltimore) 2018;97:e0518 10.1097/MD.0000000000010518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Can A, Du R. Association of hemodynamic factors with intracranial aneurysm formation and rupture: systematic review and meta-analysis. Neurosurgery 2016;78:510–20 10.1227/NEU.0000000000001083 [DOI] [PubMed] [Google Scholar]
- 13.Gao B, Baharoglu MI, Malek AM. Angular remodeling in single stent-assisted coiling displaces and attenuates the flow impingement zone at the neck of intracranial bifurcation aneurysms. Neurosurgery 2013;72:739–48 10.1227/NEU.0b013e318286fab3 [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Wu Z, Yu Y, et al. Combined effects of flow diverting strategies and parent artery curvature on aneurysmal hemodynamics: a CFD study. PLoS One 2015;10:e0138648 10.1371/journal.pone.0138648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King RM, Chueh JY, van der Bom IM, et al. The effect of intracranial stent implantation on the curvature of the cerebrovasculature. AJNR Am J Neuroradiol 2012;33:1657–62 10.3174/ajnr.A3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang QH, Wu YF, Xu Y, et al. Vascular geometry change because of endovascular stent placement for anterior communicating artery aneurysms. AJNR Am J Neuroradiol 2011;32:1721–25 10.3174/ajnr.A2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro M, Raz E, Becske T, et al. Variable porosity of the Pipeline embolization device in straight and curved vessels: a guide for optimal deployment strategy. AJNR Am J Neuroradiol 2014;35:727–33 10.3174/ajnr.A3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii A, Chihara H, Kikuchi T, et al. Contribution of the straightening effect of the parent artery to decreased recanalization in stent-assisted coiling of large aneurysms. J Neurosurg 2017;127:1063–69 10.3171/2016.9.JNS16501 [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi Y, Imamura H, Tani S, et al. Progressive thrombosis of unruptured aneurysms after coil embolization: analysis of 255 consecutive aneurysms. J Neurointerv Surg 2019;11:1113–17 10.1136/neurintsurg-2019-014775 [DOI] [PubMed] [Google Scholar]
- 20.Funakoshi Y, Imamura H, Tani S, et al. Effect of straightening the parent vessels in stent-assisted coil embolization for anterior communicating artery aneurysms. World Neurosurg 2019;126:e410–16 10.1016/j.wneu.2019.02.066 [DOI] [PubMed] [Google Scholar]
- 21.Rajah G, Narayanan S, Rangel-Castilla L. Update on flow diverters for the endovascular management of cerebral aneurysms. Neurosurg Focus 2017;42:E2 10.3171/2017.3.FOCUS16427 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Takao H, Fujimura S, et al. Selection of helical braided flow diverter stents based on hemodynamic performance and mechanical properties. J Neurointerv Surg 2017;9:999–1005 10.1136/neurintsurg-2016-012561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piergallini L, Cagnazzo F, Conte G, et al. Virtual simulation with Sim&Size software for Pipeline Flex Embolization: evaluation of the technical and clinical impact. J Neurointerv Surg 2020;12:968–73 10.1136/neurintsurg-2020-015813 [DOI] [PubMed] [Google Scholar]