To The Editor: The newly emerged B.1.1.159 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1 has a large number of changes — 32 — in its spike protein relative to that of the original virus (Wuhan-hu-1), particularly in the receptor-binding domain and the N-terminal domain, the primary targets of neutralizing antibodies. Previously, we showed that approximately 20 changes introduced into a synthetic polymutant spike protein (PMS20) are sufficient for substantial evasion of the polyclonal neutralizing antibodies elicited in the majority of persons who have recovered from coronavirus disease 2019 (Covid-19) or have received two doses of an mRNA vaccine.2 Of note, several changes in the PMS20 spike protein are the same as or similar to changes in the omicron variant (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
We measured neutralizing antibody titers against Wuhan-hu-1, PMS20, and omicron spike pseudotypes in 169 plasma specimens from 47 persons with diverse exposures to SARS-CoV-2 antigens through infection, vaccination, or both (see Supplementary Methods and Tables S1, S2, and S3).3-5 In plasma specimens obtained at approximately 1 month and 6 months after infection from persons who had recovered from Covid-19, the 50% neutralization titer (NT50) values were a mean (±SD) of 60±47 and 37±27 times lower for PMS20 than for Wuhan-hu-1, respectively, and 58±51 and 32±23 times lower for omicron than for Wuhan-hu-1 (Fig. S2A and S2B). Similarly, plasma specimens obtained from different persons in the same cohort 1 year after infection had NT50 values that were 34±24 times lower for PMS20 and 43±23 times lower for omicron than for Wuhan-hu-1 (Fig. S2C).
In plasma specimens from persons who had received two doses of an mRNA vaccine (BNT162b2 [Pfizer–BioNTech] or mRNA-1273 [Moderna]) 1.3 months before sampling, the NT50 values were 187±24 times lower for PMS20 and 127±66 times lower for omicron than for Wuhan-hu-1 (Fig. S3A). At 5 months after vaccination, the neutralization potency was 58±23 times lower for PMS20 and 27±17 times lower for omicron (Fig. S3B). Many plasma specimens from recipients of the single-dose Ad26.COV2.S vaccine (Johnson & Johnson–Janssen), obtained 1 or 5 months after vaccination, lacked detectable neutralizing activity against PMS20 or omicron (Fig. S3C and S3D), which precluded a meaningful quantitative assessment of variant-specific differences.
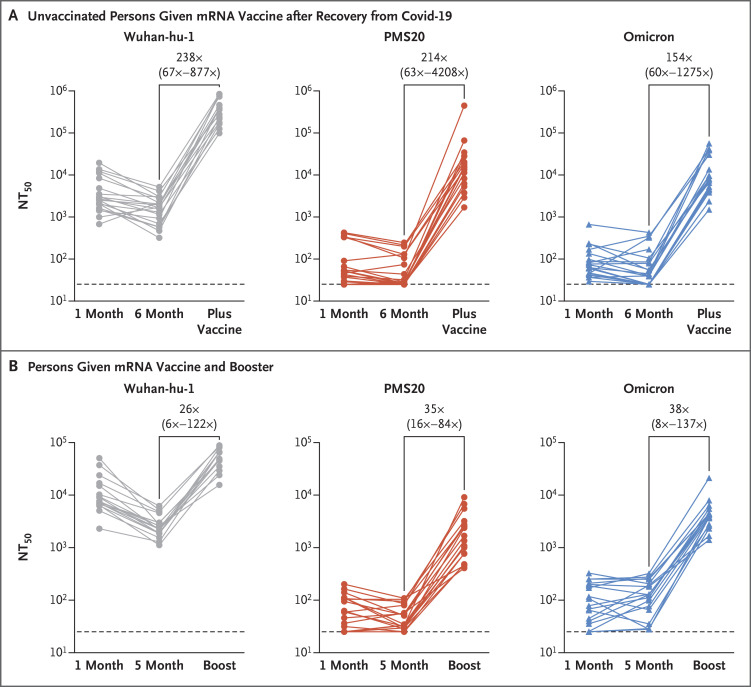
Of note, however, vaccination of persons who had recovered from Covid-19 or administration of a third dose of an mRNA vaccine to vaccinated persons at least 6 months after the second dose of an mRNA vaccine led to a substantial gain in neutralizing activity against PMS20 and omicron (Fig. S4). Specifically, after vaccination in persons who had previously been infected with SARS-CoV-2, the NT50 values were 238 times, 214 times, and 154 times greater for Wuhan-hu-1, PMS20, and omicron pseudotypes, respectively, than the prevaccination convalescent-phase titers in the same persons (Figure 1A). For those who had received two doses of an mRNA vaccine approximately 6 months earlier and then received a third dose of an mRNA vaccine approximately 1 month before sampling, the NT50 values after the booster dose were 26 times greater for Wuhan-hu-1, 35 times greater for PMS20, and 38 times greater for omicron (Figure 1B). Neutralizing titers against omicron were substantial (ranging from 1411 to 56,537) in all persons who had had Covid-19 and were then vaccinated and in those who had received three doses of an mRNA vaccine, but titers were low or undetectable in many unvaccinated persons who had had Covid-19 and in recipients of only two doses of an mRNA vaccine (Figure 1).
Figure 1. Wuhan-hu-1, PMS20, and Omicron Plasma Neutralizing Titers.
Panel A shows the trajectories of NT50 values against Wuhan-hu-1, polymutant spike protein (PMS20), and omicron pseudotypes in previously unvaccinated persons who had recovered from Covid-19, measured approximately 1 month (mean ±SD, 41±12 days) and 6 months (194±12 days) after infection and then at approximately 1 year (360±15 days) after infection, which corresponded to 41±21 days after vaccination (“plus vaccine”) (see Table S2). Panel B shows the trajectories of NT50 values against Wuhan-hu-1, PMS20, and omicron pseudotypes in persons who had received an mRNA vaccine, measured 1 month (42±19 days) and 5 months (165±33 days) after the second dose of an mRNA vaccine and at 30±18 days after the third dose (“boost”) that was administered at least 6 months after the second dose.
Although these findings indicate that the omicron variant shows an unprecedented degree of neutralizing antibody escape, they also suggest that boosting and promoting affinity maturation of antibodies in persons who have previously been infected or vaccinated,4,5 with the use of existing Wuhan-hu-1–based vaccine immunogens, will provide additional protection against infection with the omicron variant and subsequent disease.
Supplementary Appendix
Disclosure Forms
This letter was published on December 30, 2021, at NEJM.org.
Footnotes
Supported by grants from the National Institutes of Health (R37AI64003 and R01AI501111, to Dr. Bieniasz; R01AI78788, to Dr. Hatziioannou; and P01-AI138398-S1 and 2U19AI111825, to Dr. Nussenzweig). Dr. Gaebler’s work is supported by the Robert S. Wennett Post-Doctoral Fellowship, the National Center for Advancing Translational Sciences (National Institutes of Health Clinical and Translational Science Award program, grant UL1 TR001866), and the Shapiro–Silverberg Fund for the Advancement of Translational Research. Drs. Bieniasz and Nussenzweig are Howard Hughes Medical Institute Investigators.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021;398:2126-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt F, Weisblum Y, Rutkowska M, et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature 2021;600:512-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020;584:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021;591:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021;595:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.