To the Editor: Residents of long-term care facilities are particularly vulnerable to severe and fatal coronavirus disease 2019 (Covid-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 To protect this population, in April 2020, the Israeli government launched a national-level task force, “Senior Shield,” that aimed to support long-term care facilities in managing the Covid-19 crisis. The main efforts included supplying personal protective equipment, initiating weekly screening of health care workers with polymerase-chain-reaction (PCR) assays along with other outbreak-containment measures, and assigning responsibility for administering two doses of the BNT162b2 (Pfizer–BioNTech) vaccine.2
In June and July of 2021, a surge in Covid-19 cases occurred in Israel, including among vaccinated persons and with increased outbreaks in long-term care facilities.3 The surge was attributed to waning vaccine-induced immunity and the rapid spread of the B.1.617.2 (delta) variant.3,4
Consequently, on July 30, 2021, the Ministry of Health approved the administration of a BNT162b2 booster vaccine (third dose) for persons 60 years of age or older who had received the second vaccine dose at least 5 months earlier5; the approval was later extended to persons under 60 years of age. This approval prompted an immediate nationwide 3-week campaign of administering the BNT162b2 booster to residents in long-term care facilities between August 1 and August 22, 2021.
We evaluated the changes in the incidence of Covid-19 among such residents during the 5 weeks before and 6 weeks after the initiation of this campaign and compared the results with those in the general population. The surveillance included data for 41,623 residents of long-term care facilities who were 60 years of age or older, 1,521,340 persons in the same age group in the general population, 4,515,314 persons between the ages of 20 and 59 years, and 3,299,121 persons under 20 years of age. In this analysis, we calculated the weekly incidence of PCR-confirmed SARS-CoV-2 infection, hospitalization for severe Covid-19, and Covid-19–related death. Changes in incidence were analyzed with the use of Poisson regression models, as evaluated separately for each group and time period (weeks 26 to 30 before the booster campaign and weeks 31 to 36 after the booster campaign). We compared the rates during a calendar week of interest with the rates during the first week in each period. The relative reduction was calculated as 1 minus the incidence rate ratio times 100. In these analyses, a P value of less than 0.05 was considered to indicate statistical significance; P values were adjusted for multiplicity with the use of the Benjamini–Hochberg method. The study was approved by the ethics committee at Soroka University Medical Center.
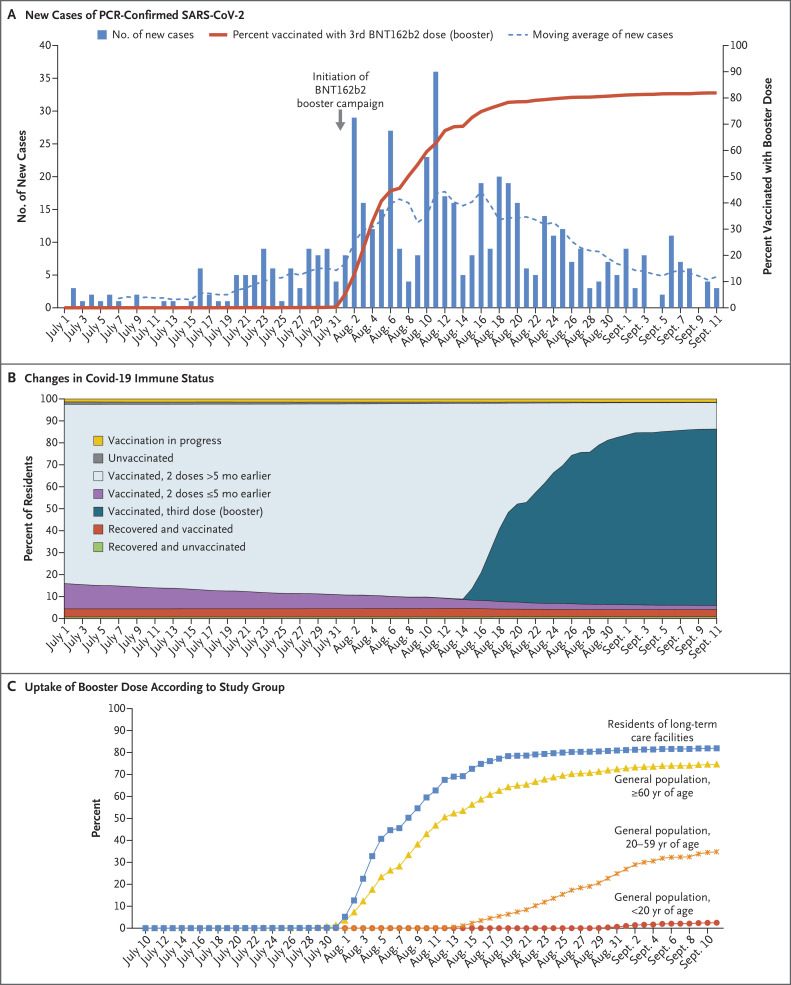
The mean age of the residents of long-term care facilities was 81.8 years; 70.6% of the residents were women (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). During the prebooster period, the incidences of SARS-CoV-2 infection (Table S2) and hospitalization for severe Covid-19 (Table S3) increased in all the study groups (Fig. S1A and S1B). The BNT162b2 booster campaign in the long-term care facilities was characterized by a rapid implementation (Figure 1A). Figure 1B shows the proportion of all residents of long-term care facilities according to their immune-status category and the time period. The uptake of the booster dose among persons who were 60 years of age or older in the general population was lower than that among the residents of long-term care facilities, and the uptake was lowest among persons in the younger age groups (Figure 1C).
Figure 1. Cases of SARS-CoV-2 Infection, Changes in Immune Status, and Uptake of BNT162b2 Booster (July–September 2021).
Panel A shows the daily number of new cases of SARS-CoV-2 infection, as confirmed on polymerase-chain-reaction (PCR) assay, among 41,623 residents of Israeli long-term care facilities, along with the cumulative uptake of the BNT162b2 booster dose, from July 1 through September 11, 2021. The dashed line represents the moving average of infections. Panel B shows the changes in Covid-19 immune status among residents of long-term care facilities, according to the following categories: vaccination in process, unvaccinated and no previous Covid-19, vaccination with the second dose more than 5 months earlier, vaccination with the second dose within the past 5 months, booster vaccination 14 or more days earlier, recovered from Covid-19 and vaccinated (any number of doses), and recovered from Covid-19 and unvaccinated. Panel C shows the cumulative uptake of the BNT162b2 booster dose according to study group.
During the booster period, the dynamics of Covid-19 incidence differed among the groups. Among the residents of long-term care facilities, significantly lower rates of SARS-CoV-2 infection and hospitalization for severe Covid-19 were observed starting at week 34 as compared with week 31 (the first week of the booster program). By week 36, the incidence rate ratio had reached 0.29 for overall infection and 0.20 for hospitalization, which corresponded to a relative rate reduction of 71% and 80%, respectively. Among persons who were 60 years of age or older in the general population, the decline after booster vaccination was of lower magnitude and was observed for all SARS-CoV-2 infections during weeks 35 and 36 only, with no significant decrease in the risk of hospitalization for severe disease. Among persons who were younger than 60 years of age, no significant decreases were observed in the incidence of either infection or hospitalization during the study period. Generally, mortality was higher among residents of long-term care facilities than among other groups. Rates of death varied widely during the booster period among the residents of long-term care facilities, with a decrease from 0.3 per 1000 population in week 34 to 0.1 per 1000 population in week 36. The rate of death continuously increased in the general population in the same age group, from 0.05 per 1000 population in week 31 to 0.1 per 1000 population in week 36 (Fig. S1C).
In a previous study involving participants who were 60 years of age or older and had received two doses of the BNT162b2 vaccine at least 5 months earlier, the rates of confirmed Covid-19 and severe illness were substantially lower among those who had received a booster dose.5 In the current study, after the initiation of an intensive BNT162b2 booster campaign with high vaccine uptake, we found a significant, rapid, and consistent reduction in the Covid-19 burden among persons in the same age group who were living in long-term care facilities. The reduction in the incidence of Covid-19 infection was delayed and of a lower magnitude among persons in the same age group in the general population during the booster period; among the younger age groups, no significant decreases were noted. Our results suggest the important real-life effects of the nationwide BNT162b2 vaccine booster program among residents in long-term care facilities.
Supplementary Appendix
Disclosure Forms
This letter was published on December 22, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Crotty F, Watson R, Lim WK. Nursing homes: the titanic of cruise ships — will residential aged care facilities survive the COVID-19 pandemic? Intern Med J 2020;50:1033-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muhsen K, Maimon N, Mizrahi A, et al. Effectiveness of BNT162b2 mRNA COVID-19 vaccine against acquisitions of SARS-CoV-2 among health care workers in long-term care facilities: a prospective cohort study. Clin Infect Dis 2021. October 26 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Israeli Ministry of Health. Israel COVID-19 data tracker: weekly surveillance reports. 2021. (https://www.gov.il/BlobFolder/reports/vaccine-efficacy-safety-follow-up-committee/he/files_publications_corona_ve-data-25072021.pdf).
- 4.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385(24):e84-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.