Abstract
Background
Before the emergence of the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), vaccination reduced transmission of SARS-CoV-2 from vaccinated persons who became infected, potentially by reducing viral loads. Although vaccination still lowers the risk of infection, similar viral loads in vaccinated and unvaccinated persons who are infected with the delta variant call into question the degree to which vaccination prevents transmission.
Methods
We used contact-testing data from England to perform a retrospective observational cohort study involving adult contacts of SARS-CoV-2–infected adult index patients. We used multivariable Poisson regression to investigate associations between transmission and the vaccination status of index patients and contacts and to determine how these associations varied with the B.1.1.7 (alpha) and delta variants and time since the second vaccination.
Results
Among 146,243 tested contacts of 108,498 index patients, 54,667 (37%) had positive SARS-CoV-2 polymerase-chain-reaction (PCR) tests. In index patients who became infected with the alpha variant, two vaccinations with either BNT162b2 or ChAdOx1 nCoV-19 (also known as AZD1222), as compared with no vaccination, were independently associated with reduced PCR positivity in contacts (adjusted rate ratio with BNT162b2, 0.32; 95% confidence interval [CI], 0.21 to 0.48; and with ChAdOx1 nCoV-19, 0.48; 95% CI, 0.30 to 0.78). Vaccine-associated reductions in transmission of the delta variant were smaller than those with the alpha variant, and reductions in transmission of the delta variant after two BNT162b2 vaccinations were greater (adjusted rate ratio for the comparison with no vaccination, 0.50; 95% CI, 0.39 to 0.65) than after two ChAdOx1 nCoV-19 vaccinations (adjusted rate ratio, 0.76; 95% CI, 0.70 to 0.82). Variation in cycle-threshold (Ct) values (indicative of viral load) in index patients explained 7 to 23% of vaccine-associated reductions in transmission of the two variants. The reductions in transmission of the delta variant declined over time after the second vaccination, reaching levels that were similar to those in unvaccinated persons by 12 weeks in index patients who had received ChAdOx1 nCoV-19 and attenuating substantially in those who had received BNT162b2. Protection in contacts also declined in the 3-month period after the second vaccination.
Conclusions
Vaccination was associated with a smaller reduction in transmission of the delta variant than of the alpha variant, and the effects of vaccination decreased over time. PCR Ct values at diagnosis of the index patient only partially explained decreased transmission. (Funded by the U.K. Government Department of Health and Social Care and others.)
Randomized, controlled trials1-3 and real-world population studies4,5 have shown that vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (Covid-19), have prevented infection and adverse outcomes from several SARS-CoV-2 variants, including the B.1.1.7 (alpha) and B.1.617.2 (delta) variants.6-8 Vaccination may also prevent onward transmission both by reducing symptomatic infections and asymptomatic infections (and therefore the number of infectious persons) and by reducing onward spread from persons who have become infected despite vaccination. Household studies have shown that vaccination reduced onward transmission of the alpha variant from persons who became infected despite vaccination.9-12 One hypothesized mechanism is that viral loads observed in persons infected with the alpha variant after vaccination7,13 are lower than those among unvaccinated persons, and the viral load is associated with the likelihood of infection in contacts.14,15
However, in persons infected with the delta variant, viral loads are similar in vaccinated and unvaccinated persons,8,16 although the duration of viral shedding may be reduced.17,18 The absence of a reported difference in viral loads between vaccinated and unvaccinated infected persons calls into question whether vaccination controls the spread of the delta variant as effectively as it controls the spread of the alpha variant and whether, with increased transmissibility,19 the maintained viral load after vaccination explains the rapid global spread of the delta variant despite increasing vaccination coverage.
We used national contact-testing data from England to investigate the effect of vaccination on onward transmission of SARS-CoV-2. We also examined how this effect varies with the alpha and delta variants.
Methods
Index Patients, Contacts, and Variants
We performed a retrospective observational cohort study involving adult contacts (≥18 years of age) of symptomatic or asymptomatic SARS-CoV-2–infected adult index patients. Data were obtained from the National Health Service (NHS) Test and Trace, a contact-tracing and testing service. Contacts (persons living in the same household or in face-to-face distance from an index patient, within <1 m for ≥1 minute or within <2 m for ≥15 minutes) were eligible for inclusion in the study if they had undergone polymerase-chain-reaction (PCR) testing 1 to 10 days after the index patient had a positive PCR test (typically after the development of symptoms of Covid-19, but also after positive asymptomatic antigen screening). The 1- to 10-day period was chosen to enrich for contacts for whom the index patient was the most likely source of any infection15 (details about alternative periods that were tested in a sensitivity analysis are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org).
We included only index patients with PCR tests performed by one of three national “lighthouse” laboratories (Milton Keynes, Alderley Park, and Glasgow) that used the same standardized workflow and TaqPath PCR assay (Thermo Fisher Scientific) to test for three gene targets: spike (S), nucleocapsid (N), and open reading frame 1ab (ORF1ab). Contacts could undergo testing at any community or hospital laboratory that reported results to Test and Trace. The vaccination status of patients and contacts was obtained from the National Immunisation Management Service (details are provided in the Supplementary Appendix).
Index patients who had undergone testing between January 1 and July 31, 2021, were included. Cases were classified as alpha variant infections on the basis of S-gene target failure while this was a reliable proxy (until June 6, after which <6% of the patients had S-gene target failure). After May 10, 2021, spread of the delta variant throughout the United Kingdom meant that more than 98% of sequenced SARS-CoV-2 samples were classified as the alpha or delta variants,19 so S-gene detection after May 10 was used as a proxy for the delta variant (details are provided in the Supplementary Appendix). In order to control as much as possible for biases related to health-seeking behavior (including differences in behavior before and after vaccination), access to testing, and case ascertainment, we restricted our study to tested contacts.20
Study Oversight
The study was performed as part of public health surveillance and NHS Test and Trace program quality assurance, under Section 251 of the NHS Act 2006, with approvals from Public Health England and the Department of Health and Social Care. The Research Ethics and Governance Group of Public Health England (the research ethics committee of that organization) reviewed the study protocol and confirmed compliance with all regulatory requirements. Given that no regulatory or ethical issues were identified, it was decided that full ethical review was not a requirement for this study, and the protocol was approved. The authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Statistical Analysis
We used multivariable Poisson regression to investigate associations between onward transmission (i.e., to contacts with PCR tests positive for SARS-CoV-2) and the vaccination status of index patients (unvaccinated, partially vaccinated [from the date of the first vaccination to 13 days after the second vaccination], or vaccinated twice [≥14 days after the second vaccination]) and the vaccine type (ChAdOx1 nCoV-19 [also known as AZD1222; AstraZeneca] or BNT162b2 [Pfizer–BioNTech]). We investigated differences between transmission from index patients infected with the alpha variant and transmission from those infected with the delta variant, and we used prespecified interaction terms to assess whether vaccine associations differed according to variant. We also included model terms for the time since the second BNT162b2 or ChAdOx1 nCoV-19 vaccination.
Adjustment was made for the following covariates: the type of exposure between index patients and contacts (living in the same household or residence, visiting a household, at activities or events, or at the workplace or an educational facility); index-patient characteristics (age, sex, and symptom status); contact characteristics (age, sex, vaccination status, and time since vaccination, as described above); socioeconomic disadvantage as assessed with an index of multiple deprivation (a national indication of the level of social, health-related, and economic deprivation according to local geographic area of residence); local weekly incidence of SARS-CoV-2 infection as determined from national testing data; and calendar time (reflecting temporal changes in behavior and social distancing, the likelihood of acquisition of SARS-CoV-2 from a third party, population-wide vaccine uptake, and the percentage of unvaccinated persons who were previously infected) (Table S1 in the Supplementary Appendix). We accounted for nonlinearity, interactions, and multiple testing. Heterogeneity rate ratios and 95% confidence intervals were calculated with the use of interaction terms and contrasts between levels of categorical variables. Additional details of all statistical methods used are provided in the Supplementary Methods section in the Supplementary Appendix.
We refitted models to include cycle-threshold (Ct) values (indicative of viral load21) in the index patient to investigate the relationship between Ct values and transmission. We used these models to perform a mediation analysis to assess whether the effect of the vaccination status of the index patient was explained by Ct values at diagnosis.
Results
Patients and Contacts
We obtained data on 661,315 adult contacts of 374,115 adult index patients; 173,460 of these contacts (26%) had undergone PCR testing between January 2 and August 2, 2021. The demographic characteristics of the patients and contacts were broadly representative of persons with Covid-19 in England (Table S2) and were similar in the contacts who had undergone testing and those who had not undergone testing (Table S3).
A total of 27,217 of the contacts who had undergone testing (16%) and had incomplete data were excluded (see the Results section in the Supplementary Appendix). Of the remaining 146,243 tested contacts of 108,498 index patients, 54,667 (37%) had positive SARS-CoV-2 PCR tests. The median age of the index patients was 34 years (interquartile range, 24 to 49; range, 18 to 102), and the median age of the contacts was 43 years (interquartile range, 29 to 54; range, 18 to 107). A total of 55,354 of the index patients (51%) and 83,206 of the contacts (57%) were female (Tables S4 and S5). Among the 147,279 exposures between index patients and contacts, 97,204 occurred within households and residences (66%), 16,505 during visits to households (11%), 16,114 at events and activities (11%), and 16,420 at the workplace or an educational facility (11%).
Index-Patient Vaccination and Onward Transmission
A total of 35,459 of 76,401 contacts of unvaccinated index patients (46%) had positive PCR tests, as did 3878 of 11,236 (35%) contacts of index patients who were partially vaccinated with ChAdOx1 nCoV-19, 7947 of 31,039 (26%) contacts of index patients who were partially vaccinated with BNT162b2, 6067 of 21,421 (28%) contacts of patients vaccinated twice with ChAdOx1 nCoV-19, and 1316 of 6146 (21%) contacts of patients vaccinated twice with BNT162b2. Among the index patients who were vaccinated twice, the median time from the second vaccination to a positive PCR test for the alpha variant was 27 days (interquartile range, 18 to 43) with the ChAdOx1 nCoV-19 vaccine and 42 days (interquartile range, 26 to 63) with the BNT162b2 vaccine; the median time from the second vaccination to a positive PCR test for the delta variant was 51 days (interquartile range, 35 to 70) and 90 days (interquartile range, 69 to 110), respectively. Among twice-vaccinated index patients, dosing intervals were more than 6 weeks in 14,811 of 15,083 patients (98%) who received ChAdOx1 nCoV-19 and in 3759 of 4233 patients (89%) who received BNT162b2.
In a multivariable model (Table 1 and Table S6), vaccination with BNT162b2 in index patients infected with the alpha variant was independently associated with less PCR positivity in contacts than no vaccination; two vaccinations (adjusted rate ratio at 14 days after the second vaccination as compared with no vaccination, 0.32; 95% CI, 0.21 to 0.48) were associated with greater decreases in transmission than partial vaccination (adjusted rate ratio, 0.88; 95% CI, 0.85 to 0.91). Similarly, two ChAdOx1 nCoV-19 vaccinations were associated with less transmission (adjusted rate ratio, 0.48; 95% CI, 0.30 to 0.78) than partial vaccination (adjusted rate ratio, 0.90; 95% CI, 0.86 to 0.94). A difference between BNT162b2 and ChAdOx1 nCoV-19 with respect to decreases in transmission of the alpha variant after two vaccinations was not observed (heterogeneity rate ratio, 1.51; 95% CI, 0.81 to 2.85).
Table 1. Relationship between Positive PCR Tests in Contacts and the Vaccination Status of Index Patients and Contacts.*.
| Characteristic | Transmission of Alpha Variant | Transmission of Delta Variant | Delta Variant vs. Alpha Variant | ||
|---|---|---|---|---|---|
| Index Patient– Contact Pairs |
Adjusted Rate Ratio (95% CI) |
Index Patient– Contact Pairs |
Adjusted Rate Ratio (95% CI) |
Rate Ratio for Interaction (95% CI) |
|
| number | number | ||||
| Vaccination status of index patient | |||||
| Unvaccinated | 52,566 | — | 23,835 | — | — |
| Partially vaccinated† | |||||
| ChAdOx1 nCoV-19 | 3,619 | 0.90 (0.86–0.94) | 7,617 | 0.95 (0.91–0.99) | 1.06 (1.00–1.12) |
| BNT162b2 | 3,917 | 0.88 (0.85–0.91) | 27,122 | 0.83 (0.81–0.86) | 0.94 (0.90–0.99) |
| Vaccinated twice‡ | |||||
| ChAdOx1 nCoV-19 | 99 | 0.48 (0.30–0.78) | 21,322 | 0.76 (0.70–0.82) | 1.58 (0.97–2.56) |
| BNT162b2 | 176 | 0.32 (0.21–0.48) | 5,970 | 0.50 (0.39–0.65) | 1.59 (1.07–2.35) |
| Vaccination status of contact | |||||
| Unvaccinated | 52,321 | — | 12,796 | — | — |
| Partially vaccinated† | |||||
| ChAdOx1 nCoV-19 | 3,739 | 0.94 (0.91–0.98) | 8,568 | 0.69 (0.66–0.72) | 0.73 (0.69–0.77) |
| BNT162b2 | 3,829 | 0.85 (0.82–0.88) | 17,170 | 0.67 (0.65–0.69) | 0.79 (0.76–0.83) |
| Vaccinated twice‡ | |||||
| ChAdOx1 nCoV-19 | 151 | 0.40 (0.27–0.59) | 32,212 | 0.42 (0.38–0.45) | 1.04 (0.70–1.53) |
| BNT162b2 | 337 | 0.15 (0.11–0.21) | 15,120 | 0.19 (0.16–0.23) | 1.28 (0.92–1.78) |
Results for index patients and contacts who received two vaccinations were estimated 14 days after the second vaccination. Adjustment was made for the type of exposure between patients and contacts, index-patient characteristics (age, sex, and symptom status), contact characteristics (age and sex), local deprivation, local incidence of severe acute respiratory syndrome coronavirus 2 infection, and calendar time. There was no evidence that adding an interaction between the index patient and contact vaccination status improved the model fit. There was evidence of greater associated reductions in transmission of the delta variant after the second vaccination in the index patient with BNT162b2 than with ChAdOx1 nCoV-19 (heterogeneity rate ratio, 1.51; 95% confidence interval [CI], 1.15 to 1.97) but no evidence of a difference between the vaccines with respect to transmission of the alpha variant (heterogeneity rate ratio, 1.51; 95% CI, 0.81 to 2.85). Two BNT162b2 vaccinations in contacts were associated with greater reductions in the incidence of positive PCR tests than two ChAdOx1 nCoV-19 vaccinations for both the alpha variant (heterogeneity rate ratio, 2.68; 95% CI, 1.61 to 4.47) and the delta variant (heterogeneity rate ratio, 2.17; 95% CI, 1.78 to 2.65).
Partial vaccination encompasses the period from the date of the first vaccination to 13 days after the second vaccination.
Persons were considered to be vaccinated twice 14 or more days after the second vaccination.
The delta variant was associated with more onward transmission from symptomatic index patients than the alpha variant, in a contact age–dependent manner (e.g., adjusted rate ratio with a contact age of 18 years, 1.24; 95% CI, 1.12 to 1.38) and with more onward transmission from asymptomatic index patients than the alpha variant (e.g., adjusted rate ratio with a contact age of 18 years, 1.40; 95% CI, 1.22 to 1.59), independent of patient and contact vaccination status. Associations were attenuated as the contact age increased (Fig. S2).
Decreases in transmission of the delta variant were greater after two BNT162b2 vaccinations (adjusted rate ratio for the comparison with no vaccination, 0.50; 95% CI, 0.39 to 0.65) than after two ChAdOx1 nCoV-19 vaccinations (adjusted rate ratio, 0.76; 95% CI, 0.70 to 0.82) (heterogeneity rate ratio, 1.51; 95% CI, 1.15 to 1.97). Partial vaccination was associated with limited reductions in transmission (adjusted rate ratio with BNT162b2 for the comparison with no vaccination, 0.83; 95% CI, 0.81 to 0.86; and with ChAdOx1 nCoV-19, 0.95; 95% CI, 0.91 to 0.99). After the second BNT162b2 vaccination, decreases in transmission of the delta variant were smaller than decreases in transmission of the alpha variant by a factor of 1.6 (adjusted rate ratio, 1.59; 95% CI, 1.07 to 2.35), and this difference between decreases in transmission of the two variants was similar after the second ChAdOx1 nCoV-19 vaccination (adjusted rate ratio, 1.58; 95% CI, 0.97 to 2.56).
Vaccination in Contacts
The estimated effect of the vaccination status of contacts did not necessarily reflect overall vaccine effectiveness because contacts were included in the study only if they had undergone testing. However, PCR positivity was highest in unvaccinated contacts (in 34,041 of 65,117 contacts [52%]), followed by those who were partially vaccinated with ChAdOx1 nCoV-19 (3987 of 12,307 contacts [32%]) or BNT162b2 (6756 of 20,999 contacts [32%]). PCR positivity was lowest in contacts who had been vaccinated twice with ChAdOx1 nCoV-19 (7241 of 32,363 contacts [22%]) or BNT162b2 (2642 of 15,457 contacts [17%]).
Independent of the effects of vaccination in index patients, the incidence of positive PCR tests for the alpha variant was lower among contacts who were vaccinated twice with BNT162b2 (adjusted rate ratio 14 days after the second vaccination as compared with no vaccination, 0.15; 95% CI, 0.11 to 0.21) than among contacts who received ChAdOx1 nCoV-19 (adjusted rate ratio, 0.40; 95% CI, 0.27 to 0.59) (heterogeneity rate ratio, 2.68; 95% CI, 1.61 to 4.47) (Table 1). Vaccinated contacts were more likely to have positive PCR tests for the delta variant than for the alpha variant because of increases in the transmissibility of the delta variant, independent of vaccination status. However, there was no strong evidence of a difference between the alpha and delta variants with respect to the effectiveness of two vaccinations with BNT162b2 or ChAdOx1 nCoV-19, as compared with no vaccination (heterogeneity rate ratio for BNT162b2 [delta variant as compared with alpha variant], 1.26; 95% CI, 0.91 to 1.75; and heterogeneity rate ratio for ChAdOx1 nCoV-19, 0.99; 95% CI, 0.67 to 1.45). Two BNT162b2 vaccinations remained more effective against the delta variant (adjusted rate ratio as compared with no vaccination, 0.19; 95% CI, 0.16 to 0.23) than two ChAdOx1 nCoV-19 vaccinations (adjusted rate ratio, 0.42; 95% CI, 0.38 to 0.45) (heterogeneity rate ratio, 2.17; 95% CI, 1.78 to 2.65).
Duration of Protection and Reductions in Transmission
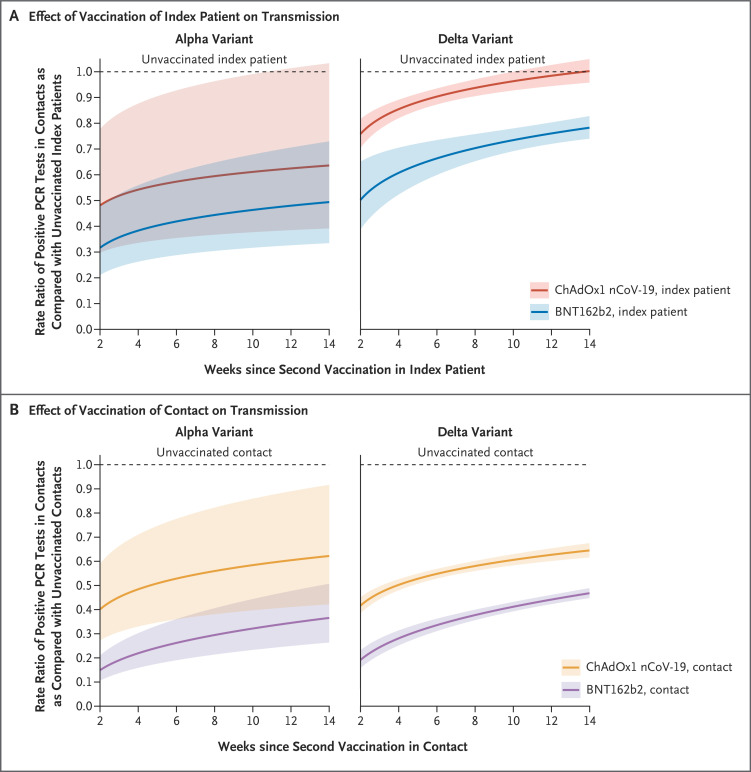
Vaccine-associated reductions in onward transmission of the alpha and delta variants declined over time after the second vaccination in index patients (Figure 1A). Independent of the vaccination status of contacts, for each doubling of weeks since 14 days after the second vaccination in index patients, the percentage of persons with positive PCR tests increased by a factor of 1.08 (95% CI, 1.05 to 1.11) among contacts of patients vaccinated with ChAdOx1 nCoV-19 and by a factor of 1.13 (95% CI, 1.05 to 1.21) among contacts of those vaccinated with BNT162b2, with no evidence of a difference between vaccines (heterogeneity rate ratio, 0.96; 95% CI, 0.87 to 1.03).
Figure 1. Rate Ratios of Positive PCR Tests in Contacts, According to Time since the Second Vaccination in Index Patients and Contacts, SARS-CoV-2 Variant, and Vaccine Type.
The rate ratios of positive polymerase-chain-reaction (PCR) tests in contacts according to index-patient vaccination status (Panel A) and contact vaccination status (Panel B) are shown. The shaded areas indicate 95% confidence intervals. There was no evidence that fitting different rates according to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant for the change in protection over weeks since the second vaccination improved the model fit. The broad confidence intervals for the alpha variant show that relatively few persons who were vaccinated twice were infected before the delta variant became the dominant lineage.
Two weeks after the second vaccination with BNT162b2 in index patients, transmission of the alpha variant was 68% (95% CI, 52 to 79) lower than transmission of this variant from unvaccinated index patients; this decrease was 52% (95% CI, 29 to 67) by 12 weeks, with reductions of 52% (95% CI, 22 to 70) 2 weeks after the second vaccination with ChAdOx1 nCoV-19 and 38% (95% CI, −1 to 62) 12 weeks after the second vaccination with ChAdOx1 nCoV-19. Two weeks after the second BNT162b2 vaccination, transmission of the delta variant was reduced by 50% (95% CI, 35 to 61), and 12 weeks after the second BNT162b2 vaccination, transmission of the delta variant was reduced by 24% (95% CI, 20 to 28); the corresponding reductions after the second vaccination with ChAdOx1 nCoV-19 were 24% (95% CI, 18 to 30) and 2% (95% CI, −2 to 6), respectively. Figure S5 shows probabilities according to the vaccine status of the patients and contacts. The findings were similar when the analysis was restricted to contacts who had undergone testing 2 to 7 days after testing in the index patient (Table S7 and Figs. S6 and S7).
Contacts who received BNT162b2 had a lower risk of testing positive throughout the 14 weeks after the second vaccination than those who received ChAdOx1 nCoV-19, even though the protective effect of BNT162b2 waned faster (adjusted rate ratio per doubling of weeks since 14 days after second vaccination, 1.27; 95% CI, 1.21 to 1.34) than that of ChAdOx1 nCoV-19 (adjusted rate ratio per doubling of weeks since 14 days after second vaccination, 1.13; 95% CI, 1.10 to 1.16) (heterogeneity rate ratio, 1.13; 95% CI, 1.07 to 1.20) (Figure 1B).
Other Risk Factors for Transmission
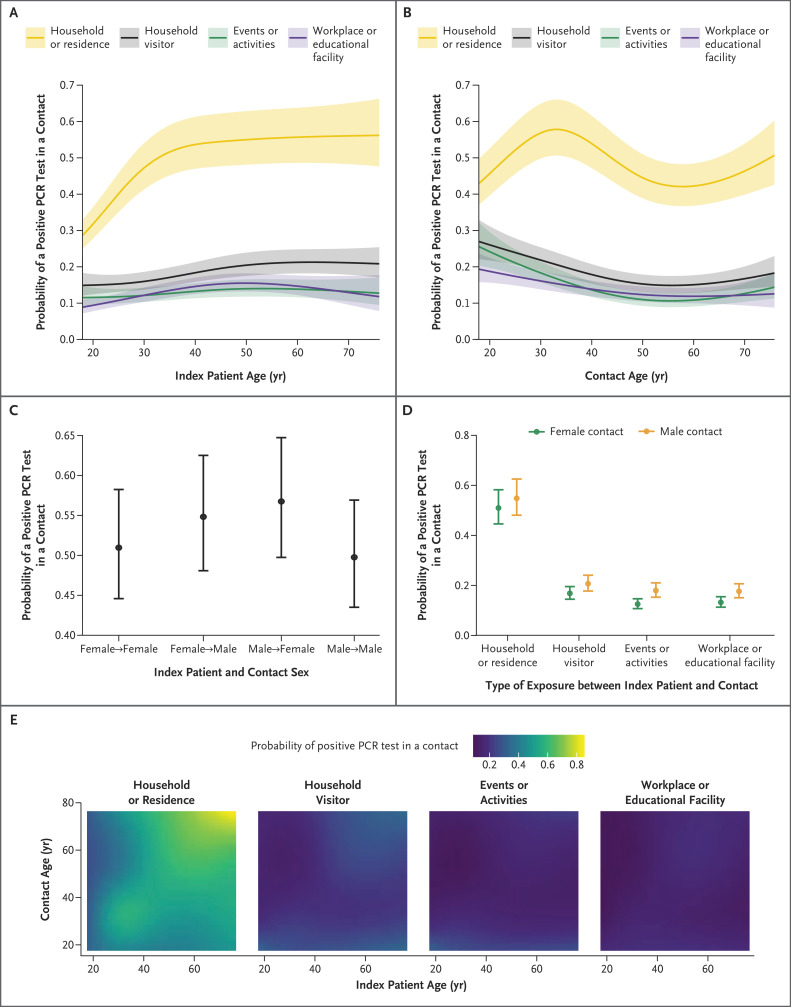
Multiple other factors were associated with positive PCR tests in contacts, including the type of exposure between patients and contacts and the age of the index patient, with the highest rates of PCR positivity after household exposure to index patients who were at least 40 years of age and lower rates after exposure at the workplace or educational facility or at events or activities (Figure 2A). Contacts in their 30s and 70s had the highest incidence of positive tests after exposure to an index patient in their household, whereas contacts in their 20s had the highest incidence after exposure to an index patient outside their own home (Figure 2B). Contacts of index patients of the opposite sex were more likely to test positive than contacts of index patients of the same sex (Figure 2C), and male contacts were more likely than female contacts to be infected outside the home (Figure 2D).
Figure 2. Estimated Probabilities of a Positive PCR Test among Contacts.
Shown are the estimated probabilities of a positive PCR test among contacts, according to the type of exposure between the index patient and contact and the age of the index patient (Panel A), the type of exposure and the age of the contact (Panel B), the sex of the index patient and contact (Panel C), the sex of the contact and the type of exposure (Panel D), and the type of exposure and age of the index patient and contact (Panel E). For each panel, all the other covariates are set to reference values for categorical values and to median values for continuous variables (i.e., the type of exposure is set to household or residence; for index-patient characteristics, age is set to the median, sex to female, vaccination status to unvaccinated, and symptom status to symptomatic; for contact characteristics, age is set to the median, sex to female, and vaccination status to unvaccinated). Local deprivation rank (socioeconomic disadvantage according to geographic area of residence) is adjusted for in the model along with the other covariates listed; local deprivation rank and the local incidence of SARS-CoV-2 infection and calendar time are set to the median. Shaded areas in Panels A and B and 𝙸 bars in Panels C and D indicate 95% confidence intervals.
Contacts of asymptomatic index patients were less likely to test positive for the alpha variant than those who were contacts of symptomatic index patients (adjusted rate ratio, 0.53; 95% CI, 0.50 to 0.55); contacts of asymptomatic index patients were also less likely to test positive for the delta variant than those who were contacts of symptomatic index patients (adjusted rate ratio, 0.59; 95% CI, 0.55 to 0.63). Contacts who lived in more deprived areas and areas with a higher incidence of SARS-CoV-2 infection (Fig. S3) were more likely to test positive. Positivity varied according to calendar time (Fig. S4).
Ct Values and the Effect of Vaccination on Transmission
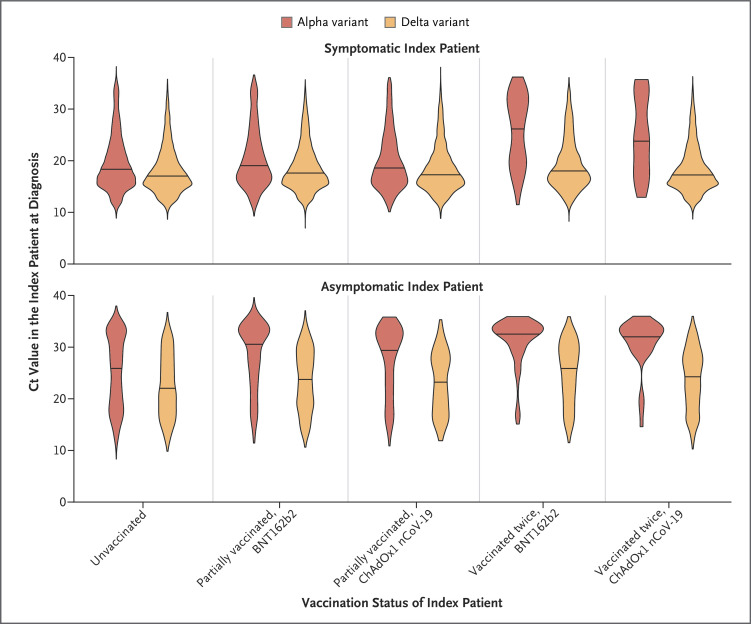
Index patients who were infected with the alpha variant had higher PCR Ct values (lower viral loads) at diagnosis if they had received two vaccinations with BNT162b2 (e.g., in symptomatic index patients, median Ct value, 27.4; interquartile range, 19.7 to 32.1) or ChAdOx1 nCoV-19 (in symptomatic index patients, median Ct value, 23.9; interquartile range, 18.1 to 32.5) than if they were unvaccinated (in symptomatic index patients, median Ct value, 18.4; interquartile range, 15.7 to 22.5). Both symptomatic index patients and asymptomatic index patients who were infected with the delta variant had lower Ct values than those who were infected with the alpha variant (Figure 3). Increases in Ct values after vaccination were smaller in index patients who were infected with the delta variant than those in index patients who were infected with the alpha variant. For example, in symptomatic index patients infected with the delta variant who had received two BNT162b2 or ChAdOx1 nCoV-19 vaccinations, the median Ct values were 18.0 (interquartile range, 15.8 to 21.8) and 17.3 (interquartile range, 15.3 to 20.6), respectively, as compared with 17.0 (interquartile range, 15.1 to 20.3) in symptomatic index patients who were unvaccinated. Covariate-adjusted estimates for Ct changes with vaccination are shown in Table S8.
Figure 3. Distribution of Ct Values, According to Vaccination Status of the Index Patient, SARS-CoV-2 Variant, and Symptoms.
The violin plots show the observed frequency density of patients with a given result, and the solid line in each plot indicates the median. Cycle-threshold (Ct) values are indicative of viral load. Lee et al.15 describe details of equivalent viral loads in copies per milliliter (log10 viral load=12.0−0.328×Ct).
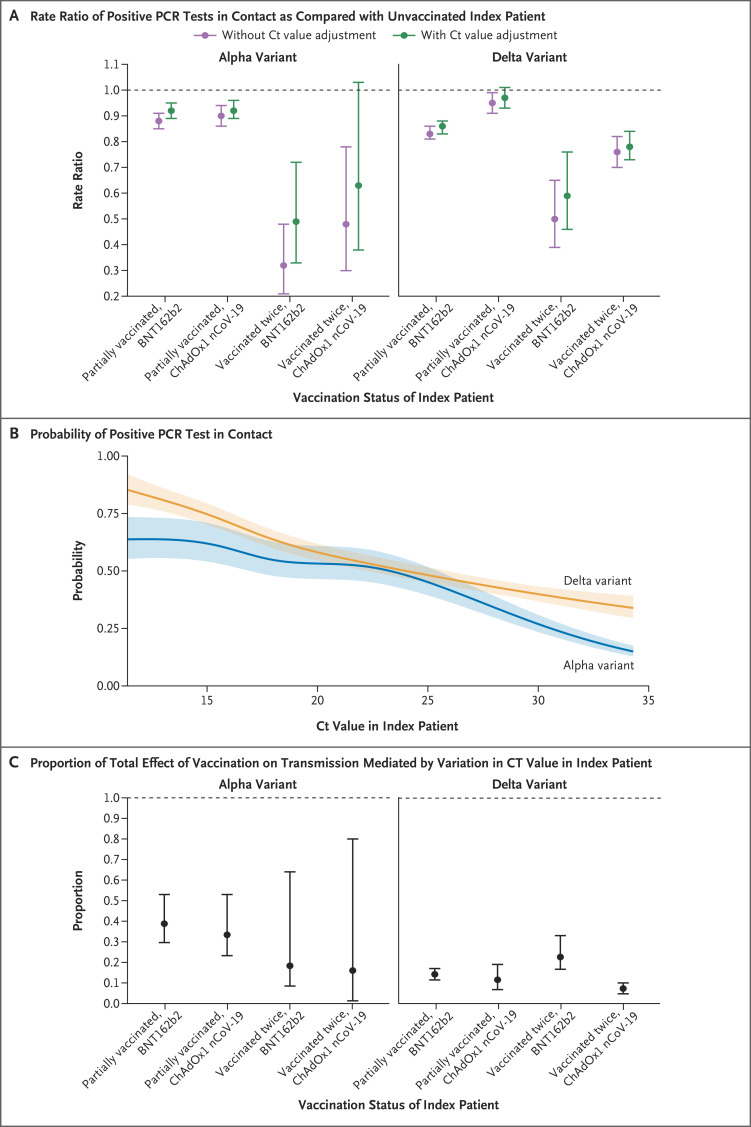
When we refitted our model for transmission to include Ct values (Figure 4A), lower Ct values (higher viral loads) were independently associated with increased transmission of both the alpha variant and the delta variant, but with a greater reduction in transmission as the Ct increased (i.e., the viral load decreased) with the alpha variant than with the delta variant (Figure 4B). A small proportion of the effect of two vaccinations with BNT162b2 or ChAdOx1 nCoV-19 on transmission was mediated through variation in Ct values at diagnosis in the index patient (Figure 4C and Table S9). The proportion of the total effect (mediated by Ct values) of two vaccinations on transmission of the alpha variant was 18% (95% CI, 9 to 64) with the BNT162b2 vaccine and 16% (95% CI, 1 to 80) with the ChAdOx1 nCoV-19 vaccine; the proportion of the total effect mediated by Ct values of two vaccinations on transmission of the delta variant was 23% (95% CI, 17 to 33) and 7% (95% CI, 5 to 10), respectively.
Figure 4. Extent of Vaccine-Associated Reductions in Transmission That Were Explained by Variation in Ct Values at Diagnosis in the Index Patient.
Panel A shows the effect of vaccination of the index patient on onward transmission in models with and without adjustment for the Ct value in the index patient. Panel B shows the relationship between the Ct value in the index patient and onward transmission in a model with adjustment for the Ct value in the index patient at the time of diagnosis. Panel C shows the proportion of the total effect of vaccination of the index patient mediated by variations in the Ct value. 𝙸 bars in Panels A and C and shaded areas in Panel B indicate 95% confidence intervals. Apart from the SARS-CoV-2 variant, there was no evidence that interactions between the Ct value and any other main effect of the model improved the model fit.
Discussion
We found that both the BNT162b2 and ChAdOx1 nCoV-19 vaccines were associated with reduced onward transmission of SARS-CoV-2 from index patients who became infected despite vaccination. However, in index patients who were vaccinated with BNT162b2 and probably in those who were vaccinated with ChAdOx1 nCoV-19, reductions in transmission of the delta variant were smaller than reductions in transmission of the alpha variant. In population-based studies, vaccines have continued to provide protection against infection with the delta variant, but to a lesser degree than against infection with the alpha variant.8 Therefore, the delta variant eroded vaccine-associated protection against transmission both by making infection more common and by increasing transmission from infected vaccinated persons.
Vaccines have been hypothesized to reduce onward transmission by reducing viral loads.14,15 In our study, vaccination was associated with higher Ct values (lower viral loads) of the alpha variant and, to a smaller extent, with higher Ct values of the delta variant. Higher Ct values were associated with less transmission (Figure 4B). However, we found that differences in Ct values at diagnosis in the index patient accounted for only 7 to 23% of the effect of vaccination, with most of the effect of vaccination probably occurring through other mechanisms. This finding indicates that Ct values measured in diagnostic testing are not necessarily a surrogate for the effect of vaccination on transmission. Ct values at diagnosis are probably imperfectly representative of viral loads at transmission, despite the relationship observed between Ct values and transmission, because viral loads are dynamic over time.22 Vaccination may also act by facilitating faster clearance of viable infectious virions,17,18 but they may leave damaged ineffective virions behind that still contain PCR-detectable RNA. Studies of this possibility and of how antigen assays perform after vaccination could lead to improvement in diagnostic tests after vaccination.
We found differences between vaccines that may have reflected their differing mechanisms of action. Index patients who were vaccinated with BNT162b2 had contacts who were less likely to have positive PCR tests for the delta variant than those of index patients who had received ChAdOx1 nCoV-19. There was potentially insufficient power to resolve differences between the vaccines with respect to the alpha variant because relatively few persons who were vaccinated twice became infected before the delta variant became the dominant lineage. The incidences of infections with the alpha variant and those with the delta variant were also lower among contacts vaccinated twice with BNT162b2 than among those vaccinated twice with ChAdOx1 nCoV-19.
Protection against onward transmission waned during the 3-month period after the second vaccination. Some protection against the alpha variant remained, but much of the protection against onward transmission of the delta variant was lost, particularly with ChAdOx1 nCoV-19. Waning of protective behaviors may explain some of the change, because the use of measures such as social distancing and mask wearing in vaccinated persons may have decreased. However, reductions in antibody levels23 and vaccine effectiveness8 over time provide support for the importance of biologic explanations. In addition, some of the observed decline in protection may be attributed to a longer period since vaccination in persons who were vaccinated early; these persons may have been clinically vulnerable, with immune systems that were weaker than those of persons who were vaccinated more recently.
Contacts were also more likely to test positive as the time since their second vaccination increased. Although contacts who received BNT162b2 had increased protection throughout the 3-month period after the second vaccination, this protection waned faster with BNT162b2 than with ChAdOx1 nCoV-19, as was also seen with new infections in a representative survey in the United Kingdom.8
Our study has several limitations. In order to minimize bias introduced by differences in testing behavior arising for multiple reasons, including the vaccination status of contacts, we included only contacts who had undergone PCR testing. Therefore, we cannot estimate secondary attack rates according to the vaccination status of patients and contacts, and the absolute protective effects of vaccination on transmission may be underestimated because vaccine-protected, uninfected contacts may not have sought testing. Our approach is also unlikely to eliminate bias, particularly if test-seeking behavior is related to perceived vaccine efficacy, given the nonspecificity of many symptoms of Covid-19.24
Some contacts may have been infected by a source other than the identified “index patient”; this would attenuate associations between index-patient–related variables, including vaccination status, and the outcome. To minimize this effect, we restricted our study to contacts who had undergone testing 1 to 10 days after testing in an index patient, with very similar findings when the analysis was restricted to 2 to 7 days. Better data on symptom onset and the timing of exposures between patients and contacts could improve estimates.
In addition, we did not have sufficient data to account for previous infection status, which is also imperfectly ascertained in national testing programs. Increasing immunity arising from previous infection in the unvaccinated comparator group potentially reduces estimates of vaccine effectiveness over time; however, with adjustment for calendar time, previous infection can be allowed for at a population level, along with changes in test-seeking behavior and the incidence of other infections that cause symptoms that are similar to those of Covid-19.25
We used S-gene target failure and time, rather than sequencing, as a proxy to distinguish infection with the alpha variant from that with the delta variant; thus, some low-viral-load delta variant infections with S-gene target failure may have been misclassified as alpha variant infections. However, we restricted the time period of our data set to minimize this effect. We considered all PCR tests in contacts, including results of assays without an S-gene target, so we could not assess the concordance of patient–contact S-gene target failure as evidence supporting transmission.
Finally, we did not have data to adjust for coexisting conditions in clinically vulnerable persons or for health care workers. Both of these groups were vaccinated earlier in the Covid-19 pandemic and were more likely to have had shorter dosing intervals than those who were vaccinated later. This lack of adjustment may have affected the findings, particularly on waning of vaccine protection over time and differences according to vaccine type; it also precluded analysis of the effect of the dosing interval.8
The delta variant has spread globally and caused resurgences of infection even in areas with high vaccination coverage. Increased onward transmission from persons who become infected despite vaccination is probably an important reason for this spread. Booster vaccination campaigns that are being considered and implemented26 may help to control transmission as well as prevent infections.
Supplementary Appendix
Disclosure Forms
The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, the Department of Health, or Public Health England.
This article was published on January 5, 2022, at NEJM.org.
Applications to use the data in this study can be made to the Data Access Request Service of NHS Digital (https://digital.nhs.uk/services/data-access-request-service-dars).
Footnotes
Supported by the U.K. Government Department of Health and Social Care; the National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, Oxford University, in partnership with Public Health England (NIHR200915); and the NIHR Biomedical Research Centre, Oxford. Dr. Eyre is a Robertson Foundation Fellow and an NIHR Oxford Biomedical Research Centre Senior Fellow; and Dr. Walker is an NIHR Senior Investigator.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088-n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 2021;27:1370-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouwels KB, Pritchard E, Matthews PC, et al. Impact of delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. August 24, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.18.21262237v1). preprint. [DOI] [PMC free article] [PubMed]
- 9.Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med 2021;385:759-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Layan M, Gilboa M, Gonen T, et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. July 16, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.12.21260377v1). preprint. [DOI] [PMC free article] [PubMed]
- 11.Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. July 16, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.13.21260393v1). preprint. [DOI] [PMC free article] [PubMed]
- 12.Salo J, Hägg M, Kortelainen M, et al. The indirect effect of mRNA-based Covid-19 vaccination on unvaccinated household members. July 10, 2021. (https://www.medrxiv.org/content/10.1101/2021.05.27.21257896v2). preprint. [DOI] [PMC free article] [PubMed]
- 13.Levine-Tiefenbrun M, Yelin I, Katz R, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med 2021;27:790-792. [DOI] [PubMed] [Google Scholar]
- 14.Marks M, Millat-Martinez P, Ouchi D, et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis 2021;21:629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee LYW, Rozmanowski S, Pang M, et al. SARS-CoV-2 infectivity by viral load, S gene variants and demographic factors and the utility of lateral flow devices to prevent transmission. Clin Infect Dis 2021. May 11 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1059-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2021. October 29 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 18.Chia PY, Ong SWX, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 delta variant vaccine-breakthrough infections: a multi-center cohort study. July 31, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.28.21261295v1). preprint. [DOI] [PMC free article] [PubMed]
- 19.Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 21. August 20, 2021. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1012644/Technical_Briefing_21.pdf).
- 20.Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine 2017;35:4796-4800. [DOI] [PubMed] [Google Scholar]
- 21.Public Health England. Understanding cycle threshold (Ct) in SARS-CoV-2 RT-PCR: a guide for health protection teams. 2020. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926410/Understanding_Cycle_Threshold__Ct__in_SARS-CoV-2_RT-PCR_.pdf).
- 22.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672-675. [DOI] [PubMed] [Google Scholar]
- 23.Wei J, Stoesser N, Matthews PC, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol 2021;6:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewnard JA, Patel MM, Jewell NP, et al. Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines. Epidemiology 2021;32:508-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vihta K-D, Pouwels KB, Peto T, et al. Symptoms and SARS-CoV-2 positivity in the general population in the UK. August 24, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.19.21262231v1). preprint.
- 26.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.