Piwi-interacting RNAs (piRNAs) are a class of small regulatory RNAs that safeguard the genome and control gene expression in the germline [1]. Guiding associated PIWI proteins, piRNAs play conserved roles in silencing transposable elements (TEs) and ensuring animal fertility in both sexes [1]. In mammals, however, it has been enigmatic why the piRNA pathway lacks an apparent function in female reproduction. This is because genetic studies have been exclusively conducted in mouse models and mutations of various piRNA pathway genes in mice cause only male-specific sterility. Even female mice with the ablation of all three Piwi genes are fertile [2]. Interestingly, emerging evidence suggests mice could be an outlier as a mammalian model. PIWI proteins and piRNAs are abundantly expressed in the female germline of all mammals examined [3–6]. Most mammals including farm animals, monkeys, humans, and even non-murine rodents have four Piwi genes (Piwil1, Piwil2, Piwil3, and Piwil4). Mice by contrast only possess three, lacking Piwil3, a gene with female germ cell-specific expression (Figure 1A). In addition, the presence of a robust oocyte-specific endo-siRNA pathway in mice might mask the dependence on piRNAs in the female germline [7–9]. Do mammalian piRNAs have any function in females? Using golden hamsters as an alternative mammalian model, three recent studies published in Nature Cell Biology by Hasuwa et al., Loubalova et al., and Zhang et al. have solved this long-lasting puzzle by reporting a critical function of the piRNA pathway in mammalian female fertility and embryogenesis [10–12].
Figure 1.
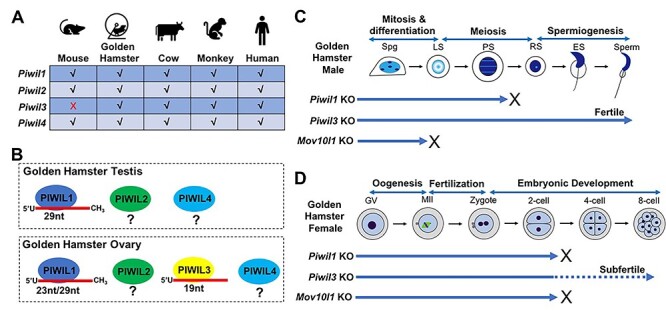
The PIWI/piRNA system in golden hamsters. (A) The lack of Piwil3 gene in the mouse genome. (B) The presence of PIWI proteins and associated piRNAs in the golden hamster testis and ovary. (C) Effect of disruption of piRNA pathway genes Piwil1, Piwill3, or Mov10l1 on male germ cell development in golden hamsters. Spg: spermatogonium; LS: leptotene spermatocyte; PS: pachytene spermatocyte; RS: round spermatid; ES: elongating spermatid. (D) Effect of disruption of piRNA pathway genes Piwil1, Piwll3, or Mov10l1 on female germ cell development and embryogenesis in golden hamsters. GV: germinal vesicle oocyte; MII: metaphase II oocyte.
To interrogate the function of the piRNA pathway in golden hamsters, three groups exploited CRISPR-Cas9 genome editing to disrupt hamster Piwi genes or a piRNA biogenesis factor. Hasuwa et al. deleted Piwil1 and Piwil3; Loubalova et al. mutated piRNA biogenesis factor Mov10l1; and Zhang et al. disrupted Piwil1 and Mov10l1 in golden hamsters [10–12]. Similar to male knockout mice [13–15], male hamsters deficient in MOV10L1 or PIWIL1 are sterile due to spermatogenic arrest [10–12] (Figure 1C). Particularly, stages of germ cell arrest show degree of differences between mice and hamsters [10–12]. As expected, PIWIL3-deficient male hamsters are fertile, consistent with its lack of expression in the male gonad [10] (Figure 1C). Strikingly, female hamsters lacking MOV10L1 or PIWIL1 are completely sterile, and PIWIL3-deficient females exhibit subfertility [10–12] (Figure 1D). This is in stark contrast to the dispensability of the piRNA pathway genes in mouse models and reveals for the first time the disruption of the piRNA pathway indeed affects female fertility in mammals. Notably, in all three mutants, ovary development and oogenesis appear normal. Oocytes produced are fertilizable by wild-type sperm, but embryo developmental arrest occurs at distinct stages (Figure 1D).
Three major populations of piRNAs (19, 23, and 29 nt) are dynamically expressed in golden hamster oocytes and embryos, corresponding to PIWIL1-bound 23 nt/29 nt and PIWIL3-bound 19-nt piRNAs, respectively (Figure 1B). Disruption of Mov10l1 severely ablates all three populations of piRNAs in hamster oocytes and unleashes LINE1 and LTR retrotransposon overexpression [11, 12]. This suggests that at least some TE-related piRNAs play an active role in transposon silencing in hamster oocytes. PIWIL1 and PIWIL3 have distinct functions in transposon regulation in oocytes [10, 12]. Like MOV10L1, PIWIL1 is required for transposon suppression in hamster oocytes, which coincides with PIWIL1-bound TE piRNAs targeting complementary young retrotransposons [10, 12]. Surprisingly, PIWIL3 deficiency does not overly de-repress retrotransposons despite the presence of PIWIL3-bound TE piRNAs in the wild-type oocytes [10]. These findings together uncover an essential role of oocyte piRNAs and distinctive PIWI proteins in TE regulation in golden hamsters.
The functions of PIWIL1 and PIWIL3 also differ in the regulation of mRNA transcriptome in hamster oocytes. RNA sequencing reveals numerous differentially expressed genes (DEGs) in PIWIL1-deficient oocytes but little change of transcriptome in PIWIL3-deficient oocytes [10, 12]. Ovary-specific PIWIL3 associates with short 19-nt oocyte-specific piRNAs that are also found in humans [16]. One remarkable feature of PIWIL3-piRNAs is the lack 2′-O methylation at piRNA 3′ ends, which differs from all other known PIWI proteins [5]. In addition, hamster PIWIL3 is heavily phosphorylated during oocyte maturation [5]. These unique features suggest PIWIL3 could differ from conventional PIWI proteins in piRNA processing and function. Indeed, PIWIL3 is largely not involved in TE silencing and mRNA transcriptome regulation, but instead is required for global DNA methylation in oocytes [10]. Despite the biological significance of its role in DNA methylation remains unclear, this finding opens a new direction for studying the unique function of PIWIL3 in non-murine mammals including humans.
A novel role of mammalian piRNAs in embryogenesis has been uncovered for the first time by these three studies. Each demonstrates that maternal deficiency in PIWI proteins or piRNAs has a detrimental effect on hamster early embryogenesis [10–12]. piRNAs are maternally deposited into embryos and their expression persists beyond the two-cell stage. PIWIL1-deficient embryos exhibit reduced maternal mRNA degradation and failure in zygotic genome activation. The arrest at the two-cell stage could result from TE derepression and/or an abnormal transcriptome [10, 12]. Embryos derived from MOV10L1-deficient oocytes also consistently arrest at the two-cell stage, although piRNA populations are affected differently in PIWIL1- and MOV10L1-deficient oocytes [11, 12]. Embryos derived from PIWIL3-deficient oocytes suffer from developmental delay with some embryos arrested at two- to eight-cell stages. Some abnormal embryos contain three or five cells apparently resulting from abnormal embryo cleavage [10]. Since PIWIL3 deficiency does not apparently affect TE silencing and mRNA transcriptome, global DNA methylation defect in PIWIL3-deficient oocyte might be a link to the embryonic defects [10]. Collectively, these results clearly demonstrate distinct roles of maternal PIWI proteins and piRNAs in early embryonic development, but the exact mechanism awaits further investigation.
It remains unknown the function of PIWIL2 and PIWIL4, if any, in hamster female germ cells and embryos. PIWIL2 is expressed in golden hamster oocytes and human fetal ovaries [4, 5]. Piwil4 mRNA is detectable in hamster MII oocytes [11, 12]. Understanding the function of all individual Piwi genes and their inter-relationships is key to piecing together how the entire piRNA pathway operates to regulate female germline function.
Together, three elegant studies by Hasuwa et al., Loubalova et al., and Zhang et al. have unveiled a crucial function of PIWI proteins and piRNAs in female fertility and embryogenesis in golden hamsters. This opens an exciting new chapter for studying piRNA biology in mammalian female reproduction. Many outstanding questions remain: How does the piRNA biogenesis machinery operate in female germ cells and how does it differ from its male counterpart? What do oocyte and embryonic piRNAs target to enable oocyte competency and embryogenesis? How does the piRNA pathway regulate female reproduction in other mammals such as agricultural species and humans? This ground-breaking discovery will undoubtedly stimulate a new wave of research on the piRNA pathway in various mammalian species to understand the basic and clinical aspects of female fertility, infertility and embryonic development.
References
- 1. Ozata DM, Gainetdinov I, Zoch A, O’Carroll D, Zamore PD. PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet 2019; 20:89–108. [DOI] [PubMed] [Google Scholar]
- 2. Cheng EC, Kang D, Wang Z, Lin H. PIWI proteins are dispensable for mouse somatic development and reprogramming of fibroblasts into pluripotent stem cells. PLoS One 2014; 9:e97821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roovers EF, Rosenkranz D, Mahdipour M, Han CT, He N, Chuva de Sousa Lopes SM, van der Westerlaken LA, Zischler H, Butter F, Roelen BA, Ketting RF. Piwi proteins and piRNAs in mammalian oocytes and early embryos. Cell Rep 2015; 10:2069–2082. [DOI] [PubMed] [Google Scholar]
- 4. Williams Z, Morozov P, Mihailovic A, Lin C, Puvvula PK, Juranek S, Rosenwaks Z, Tuschl T. Discovery and characterization of piRNAs in the human fetal ovary. Cell Rep 2015; 13:854–863. [DOI] [PubMed] [Google Scholar]
- 5. Ishino K, Hasuwa H, Yoshimura J, Iwasaki YW, Nishihara H, Seki NM, Hirano T, Tsuchiya M, Ishizaki H, Masuda H, Kuramoto T, Saito K et al. Hamster PIWI proteins bind to piRNAs with stage-specific size variations during oocyte maturation. Nucleic Acids Res 2021; 49:2700–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kabayama Y, Toh H, Katanaya A, Sakurai T, Chuma S, Kuramochi-Miyagawa S, Saga Y, Nakano T, Sasaki H. Roles of MIWI, MILI and PLD6 in small RNA regulation in mouse growing oocytes. Nucleic Acids Res 2017; 45:5387–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flemr M, Malik R, Franke V, Nejepinska J, Sedlacek R, Vlahovicek K, Svoboda P. A retrotransposon-driven dicer isoform directs endogenous small interfering RNA production in mouse oocytes. Cell 2013; 155:807–816. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T, Surani MA, Sakaki Y et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 2008; 453:539–543. [DOI] [PubMed] [Google Scholar]
- 9. Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, Hannon GJ. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 2008; 453:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hasuwa H, Iwasaki YW, Au Yeung WK, Ishino K, Masuda H, Sasaki H, Siomi H. Production of functional oocytes requires maternally expressed PIWI genes and piRNAs in golden hamsters. Nat Cell Biol 2021; 23:1002–1012. [DOI] [PubMed] [Google Scholar]
- 11. Loubalova Z, Fulka H, Horvat F, Pasulka J, Malik R, Hirose M, Ogura A, Svoboda P. Formation of spermatogonia and fertile oocytes in golden hamsters requires piRNAs. Nat Cell Biol 2021; 23:992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang H, Zhang F, Chen Q, Li M, Lv X, Xiao Y, Zhang Z, Hou L, Lai Y, Zhang Y, Zhang A, Gao S et al. The piRNA pathway is essential for generating functional oocytes in golden hamsters. Nat Cell Biol 2021; 23:1013–1022. [DOI] [PubMed] [Google Scholar]
- 13. Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, Stark A, Sachidanandam R, Pillai RS, Wang PJ. Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci U S A 2010; 107:11841–11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2002; 2:819–830. [DOI] [PubMed] [Google Scholar]
- 15. Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, Olson EN. MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci U S A 2010; 107:11847–11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Q, Li R, Lyu Q, Hou L, Liu Z, Sun Q, Liu M, Shi H, Xu B, Yin M, Yan Z, Huang Y et al. Single-cell CAS-seq reveals a class of short PIWI-interacting RNAs in human oocytes. Nat Commun 2019; 10:3389. [DOI] [PMC free article] [PubMed] [Google Scholar]


