Abstract
Objective:
Duck virus enteritis is a severe viral disease that kills ducks and swans worldwide. The clinical manifestations, gross pathology, molecular detection, and characterization of the duck virus enteritis virus (DVEV) in Australian black swans at a safari park in Bangladesh were described in this case report.
Materials and Methods:
On a safari park in Bangladesh, an Australian black swan flock exhibited clinical signs of anorexia, greenish watery diarrhea, increased thirst, partial paralysis, and death. Postmortem examinations of deceased swans revealed extensive pathological abnormalities in the trachea, liver, and spleen. To isolate DVEV, a viral inoculum produced from the liver and spleen of dead swans was implanted into 9–13-day-old embryonated duck eggs via the chorioallantoic membrane (CAM) route. DVEV was confirmed using a polymerase chain reaction (PCR) assay. Phylogenetic analysis was used to determine the genetic relationship between the DVEV isolates from Australian black swans, and 16 DVEV isolates previously described in the GenBank.
Results:
Hemorrhage was noted in the annular ring of the trachea, as well as an enlarged and hemorrhagic liver and spleen. The PCR assay amplified a 446-bp fragment of the DVEV DNA polymerase gene in the liver, spleen, and CAM homogenates. The phylogenetic analysis found that the DVEV isolates from swans were comparable to those from Bangladesh, India, Vietnam, China, Germany, the USA, and Egypt.
Conclusion:
According to the findings of this study, the DVEV was the cause of illness and mortality in an Australian black swan flock.
Keywords: Australian black swan, Bangladesh, duck viral enteritis virus, phylogenetic analysis, safari park
Introduction
Duck virus enteritis (DVE), often known as duck plague, is caused by a double-stranded DNA virus under the family Herpesviridae [1]. Extreme thirst, watery greenish diarrhea, dehydration, paresis, trembling, and abrupt death of ducks are all symptoms of the disease [2]. Infected ducks exhibit extensive bleeding in the body cavities, gut, spleen, liver, heart, and pericardium [3]. DVE is diagnosed based on a history of illness outbreaks, clinical symptoms, and gross lesions in the ducks’ affected organs. Confirmatory diagnosis, on the other hand, requires virus isolation and identification. For the detection of duck virus enteritis virus (DVEV), polymerase chain reaction (PCR)-based molecular techniques are sensitive and accurate [4].
DVE has been detected in several countries that produce ducks, including Canada, England, Hungary, Denmark, Austria, Belgium, China, Vietnam, India, and Thailand [2]. It is a disease that affects both wild and domestic waterfowls, such as ducks, geese, and swans [2,5]. Domestic ducks are primarily affected by the epidemic in a location adjacent to aquatic settings co-habiting with wild waterfowl [6,7]. Migratory waterfowl aid in the transmission of information within and between continents. Although the consumption of infected water is the primary transmission mechanism, the virus can also be transferred via contact [6].
Wild ducks, swans, graylag geese, tundra bean geese, and grey herons have a high incidence of DVEV and may act as a carrier in neighboring water bodies [7]. Even recovered birds can serve as reservoirs for DVEV for up to a year, allowing the virus to propagate among susceptible waterfowl and contaminated settings [8,9]. Scavenging and decay of DVEV-infected carcasses can contaminate soil and water, hence spreading the virus throughout the environment. Numerous findings indicate that DVEV is transmitted via contaminated bird eggs [2,9].
Worldwide commerce, including international trade in live animals and animal products, is almost certainly the primary source of new disease outbreaks among wildlife inhabitants. Imported live animals are mostly used for commercial breeding, zoo and animal park operations, and laboratory research. The ongoing disintegration of barriers between livestock and wild animals facilitates the transmission of disease into livestock. Frequent interaction between diverse populations may result in the transmission of infections. Duck plague began in domestic birds in North America and quickly spread to wild ducks [10]. The DVE was initially identified in 1980 in Bangladesh [11]. The disease is common in Bangladesh, killing between 60% and 75% of the duck population [12]. DVE outbreaks have been documented in captive ducks and swans at zoos and recreational areas [13–15].
In 2018, a possible DVE epidemic was discovered in a flock of Australian black swans in a Bangladesh safari park. The current report discussed the clinical symptoms, gross pathology, isolation, and characterization of DVEV from DVE-infected Australian black swans.
Material and Methods
In March 2018, a suspected DVE outbreak was reported in an Australian black swan flock at a safari park in Bangladesh, located at latitude 24.1716663°N and longitude 90.3926611°E. The affected swan flock manifested clinical signs of anorexia, diarrhea, partial paralysis, and death, similar to infection caused by DVEV. The swan flock size was 20, which were reared in a lake of the safari park. The migratory waterfowls and other wild birds were frequently seen along with the swan flock. The swan flock was kept under a semi-intensive husbandry system with feed supplementation.
Dead swans were collected and packed in an icebox and transported to the laboratory for postmortem examination and virological analysis. Clinical signs of the affected swans were recorded. Spleen and liver samples were aseptically collected from dead Australian black swans. Gross pathological lesions of the visceral organ such as liver, spleen, and trachea were recorded.
To isolate DVEV from dead swans, a 10% tissue homogenate was prepared from the liver and spleen in PBS and centrifuged at 10,000 rpm for 10 min [16]. The sterile viral inoculum was inoculated into 9–13-day-old embryonated duck eggs through the chorioallantoic membrane (CAM) route [16] and incubated for 5–8 days at 37°C in an egg incubator. Embryos that died after 24 h were chilled. The CAM from the dead embryo was collected.
According to the manufacturer’s instruction, the DNA was extracted from CAM homogenate by a commercial DNA extraction kit (Gene Proof, Brno, Czech Republic). A previously described PCR assay targeting 446-bp fragments of the DNA polymerase gene of DVEV was carried out for molecular detection of DVEV [17].
A phylogenetic study was carried out for determining the genetic relationship of DVEV of Australian black swans with global DVEV available in the GenBank. The partially amplified DNA polymerase gene product (446-bp) was sequenced from a commercial company (Apical Scientific Selangor, Malaysia). The DNA sequences were aligned with 16 published sequences of DVEV reported in the GenBank, and a phylogenetic tree was constructed by the maximum likelihood method using MEGA X [18].
Results
The clinical signs observed in Australian black swans included depression, ruffled feathers, ocular and nasal discharge, anorexia, labored breathing, greenish watery diarrhea, extreme thirst, and ataxia, followed by death. The morbidity and mortality rates were 50% (10 of 20) and 30% (6 of 20), respectively.
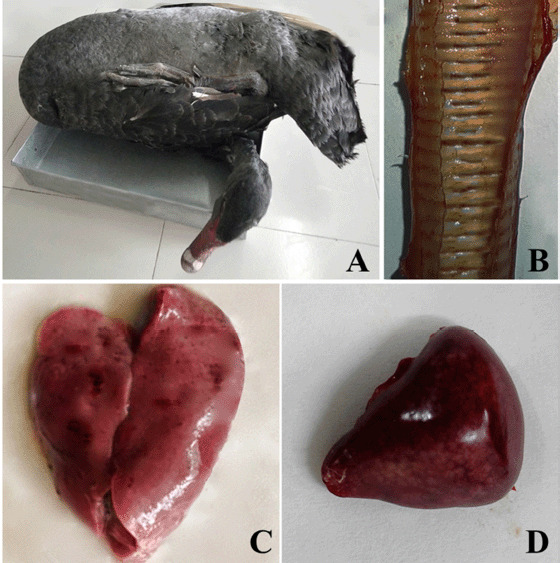
During postmortem examination, the gross pathological lesions observed in dead Australian black swans included enlarged hemorrhagic liver and spleen and hemorrhage of the annular tracheal bands (Fig. 1).
Figure 1. Gross pathological lesions of a dead Australian black swan suspected to be infected with DVE. A dead Australian black swan (A), hemorrhage on the annular tracheal bands (B), pale and enlarged liver with pin point hemorrhages on its surface (C), and dark enlarged spleen with numerous petechial hemorrhages on its surface (D).
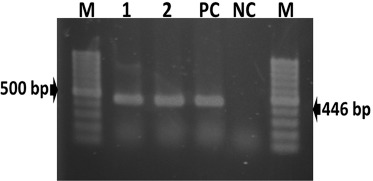
The DNA extracted from CAM homogenate were successfully amplified in PCR assay with the production of a 446-bp fragment of DNA polymerase gene (Fig. 2).
Figure 2. Amplification of a partial fragment of DNA polymerase gene (446-bp) of duck viral enteritis virus by PCR. Lane M: 100 bp size DNA ladders (Thermo Scientific, USA); Lanes 1–2: DNA extracted from samples of Australian black swans; Lane PC: positive control; and Lane NC: negative control.
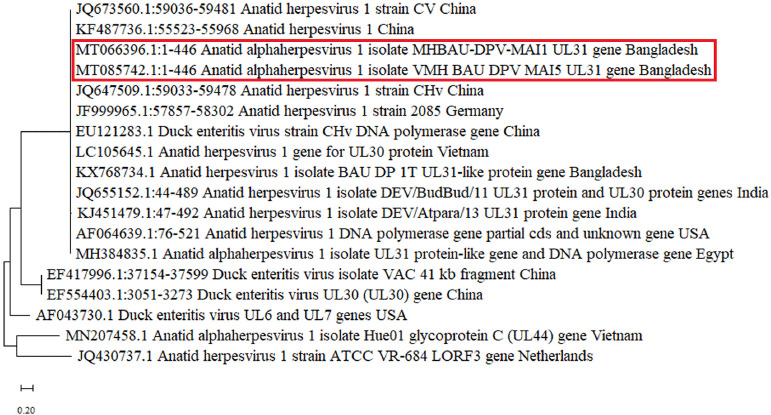
The phylogenetic tree derived from the sequence data is shown in Figure 3. The phylogenetic tree showed that sequenced strains of the two DVEVs of this study (GenBank accession no. MT066396 and MT085742) were similar to Anatid herpesvirus 1 isolates previously reported from Bangladesh, India, Vietnam, China, Germany, USA, and Egypt.
Figure 3. Phylogenetic analysis based on partial sequences (446-bp) of DNA polymerase gene of DVEV isolates from 16 reference strains obtained from GenBank. There are two sequences reported in the tree from this study (MT066396 and MT085742) that are found similar to the DVEV previously reported in Bangladesh, India, Vietnam, China, Germany, the USA, and Egypt. The tree is constructed by the maximum likelihood method using MEGA X software.
Discussion
DVE is a viral illness that is lethal to ducks and swans in Southeast Asia [19]. DVE causes significant economic losses in commercial, domestic duck farms due to carcass condemnation and decreased egg production [20]. The current study evaluated a possible DVE outbreak in Australian black swans in a safari park.
DVE is highly lethal to both domestic and wild ducks [2]. The DVE produces death and morbidity rates of up to 100% in ducks and swans [9], depending on the severity of the infecting virus and the duck’s immunological condition [20]. The recent investigation discovered a 30% death rate among Australian black swans. Keymer and Gough [21] reported a 22.5% mortality rate in DVEV-infected captive mute swans, with substantial clinical symptoms including polydipsia, photophobia, wing drooping, inappetence, watery nasal discharge, and yellowish diarrhea [21]. Clinical symptoms almost identical to those seen in the affected Australian black swans were observed in this investigation. Viral replication results in increased vascular permeability, which results in lesions and hemorrhages in the spleen, thymus, liver, and bursa of Fabricius [22,23]. Postmortem lesions almost identical to those observed in the reported Australian black swans were also observed. Clinical symptoms and gross pathological lesions observed in this study’s DVE-infected flock and other flocks described by numerous investigators vary according to the afflicted birds’ species, immunological status, age, and sex, as well as the virulence of the field virus strain [24].
Migratory waterfowls are the key risk factors for disease dissemination [9,25,26]. Ducks that were sick or recovered transmit viruses through their droppings, polluting nearby bodies of water [8]. The viruses can survive and stay for an extended period of time in dirty, stagnant, and slow-moving water bodies [24]. A susceptible duck may contract DVEV by oral, nasal, or cloacal routes, as well as through skin wounds [2]. The suspected swan flock may have contracted the virus by drinking or eating in the safari park lake’s tainted water. Wild birds have also been observed to carry the virus that causes this disease to spread to susceptible flocks. DVE outbreaks have been recorded often in duck flocks that cohabitate with free-range waterfowl [9]. During the spring season, from March to June, outbreaks of DVE are regularly observed in susceptible duck and swan flocks [2]. During the spring season, convalescent ducks were reported to release the virus spontaneously [2]. DVE outbreaks occurred in March in the reported swan flock. Exotic migrating ducks freely interacted and scavenged with the lake’s swan flock. Numerous tourists approached the premises of the safari park’s swan flock and were frequently provided food. Wild birds were spotted in the woods surrounding the safari park’s lake. DVEV has been detected in wild birds [27].
A phylogenetic analysis was conducted to assess the genetic link between the DVEV isolates and those in the GenBank. The phylogenetic study revealed that the sequenced strains of DVEV were highly similar to DVEV isolates from Bangladesh, India, Vietnam, China, Germany, the USA, and Egypt. Ahamed et al. [28] reported a genetic similarity between Bangladeshi DVEV isolates and DVEVs from China.
Conclusion
This study established that DVEV was the cause of death in an Australian black swan flock at a safari park. To combat this lethal viral infection, regular surveillance of DVEV in swan flocks, effective immunization, and biosecurity protocols must be implemented.
List of abbreviations
CAM, chorioallantoic membrane; DVE, duck virus enteritis; DVEV, duck virus enteritis virus; PCR, polymerase chain reactions; PBS, Phosphate-buffer solution.
Acknowledgments
The authors thank Dr. Stephen M Boyle, Virginia Tech, USA, for editorial comments on this manuscript. The research work was funded by the Bangladesh Agricultural Research Council (NATP Phase-2, Grants no. livestock 367). The authors acknowledge the safari park authority for providing dead swan samples.
Conflict of interests
The authors declare no conflict of interests.
Author contribution
MMI conceived of the study, gathered the sample, carried out the experiment, analyzed the data, and drafted the manuscript. JI was involved in sample collection, research, and data analysis. MSI devised the technique and assisted in editing the initial draft. TA helped develop the methodology, carried out the phylogenetic analysis, and analyzed the findings. MRI conceptualized the experiment and assisted with writing, reviewing, and editing. MMK contributed to the methodology, writing the initial draft, reviewing and revising the final draft. MAI was involved in the idea and design of the experiment, the formal analysis, the funding acquisition, the research, the methodology, the supervision, the writing-original draft, the writing-review and editing.
References
- [1].Liu F, Cao Y, Yan M, Sun M, Zhang Q, Wang J, et al. Development of a colloidal gold immunochromatographic assay for duck enteritis virus detection using monoclonal antibodies. Pathogens. 2021;10:365. doi: 10.3390/pathogens10030365. https://doi.org/10.3390/pathogens10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dhama K, Kumar N, Saminathan M, Tiwari R, Karthik K, Kumar MA, et al. Duck virus enteritis (duck plague)–a comprehensive update. Vet Q. 2017;37(1):57–80. doi: 10.1080/01652176.2017.1298885. https://doi.org/10.1080/01652176.2017.1298885. [DOI] [PubMed] [Google Scholar]
- [3].El-Tholoth M, Hamed MF, Matter AA, Abou El-Azm KI. Molecular and pathological characterization of duck enteritis virus in Egypt. Transb Emerg Dis. 2019;66(1):217–24. doi: 10.1111/tbed.13002. https://doi.org/10.1111/tbed.13002. [DOI] [PubMed] [Google Scholar]
- [4].Yao M, Zhang X, Gao Y, Song S, Xu D, Yan L. Development and application of multiplex PCR method for simultaneous detection of seven viruses in ducks. BMC Vet Res. 2019;15(1):103. doi: 10.1186/s12917-019-1820-1. https://doi.org/10.1186/s12917-019-1820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Makhija A, Kumar S. Characterization of duck plague virus stability at extreme conditions of temperature, pH and salt concentration. Biologicals. 2017;45:102–5. doi: 10.1016/j.biologicals.2016.09.009. https://doi.org/10.1016/j.biologicals.2016.09.009. [DOI] [PubMed] [Google Scholar]
- [6].Elmberg J, Berg C, Lerner H, Waldenström J, Hessel R. Potential disease transmission from wild geese and swans to livestock, poultry and humans: a review of the scientific literature from a one health perspective. Infect Ecol Epidemiol. 2017;7(1):1300450. doi: 10.1080/20008686.2017.1300450. https://doi.org/10.1080/20008686.2017.1300450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Woźniakowski G, Samorek-Salamonowicz E. First survey of the occurrence of duck enteritis virus (DEV) in free-ranging Polish water birds. Arch Virol. 2014;159(6):1439–44. doi: 10.1007/s00705-013-1936-8. https://doi.org/10.1007/s00705-013-1936-8. [DOI] [PubMed] [Google Scholar]
- [8].Shawky S, Schat KA. Latency sites and reactivation of duck enteritis virus. Avian Dis. 2002;46(2):308–13. doi: 10.1637/0005-2086(2002)046[0308:LSAROD]2.0.CO;2. https://doi.org/10.1637/0005-2086(2002)046. [0308:LSAROD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [9].Sandhu TS, Shawky SA. Duck virus enteritis (duck plague) In: Saif YM, Barnes HJ, Glission JR, Fadly AM, McDougald LR, Swayne DE, editors. Diseases of poultry. 11th. Iowa State University Press; Ames, IA: 2003. pp. 354–63. [Google Scholar]
- [10].Altizer S, Foufopoulos J, Gager A. Diseases, conservation and encyclopedia of biodiversity. 2001:109–26. https://doi.org/10.1016/B0-12-226865-2/00072-9. [Google Scholar]
- [11].Sarker AJ. Duck plague in Bangladesh. Indian Vet J. 1980;57(10):787–91. [Google Scholar]
- [12].Khan KA, Saha S, Hossain MT, Haque ME, Haq MM, Islam MA. Epidemiological investigation of recurrent outbreaks of duck plague in selected Haor (wetland) areas of Bangladesh. J Adv Vet Anim Res. 2018;5(2):131–9. https://doi.org/10.5455/javar.2018.e256. [Google Scholar]
- [13].Jacobsen GS, Pearson JE, Yuill TM. An epornitic of duck plague on a Wisconsin game farm. J Wildl Dis. 1976;12(1):20–6. doi: 10.7589/0090-3558-12.1.20. https://doi.org/10.7589/0090-3558-12.1.20. [DOI] [PubMed] [Google Scholar]
- [14].Montali RJ, Bush M, Greenwell GA. An epornitic of duck viral enteritis in a zoological park. J Am Vet Med Assoc. 1976;169(9):954–58. [PubMed] [Google Scholar]
- [15].Kaleta EF, Kuczka A, Kühnhold A, Bunzenthal C, Bönner BM, Hanka K, et al. Outbreak of duck plague (duck herpesvirus enteritis) in numerous species of captive ducks and geese in temporal conjunction with enforced biosecurity (in-house keeping) due to the threat of avian influenza A virus of the subtype Asia H5N1. Dtsch Tierarztl Wochenschr. 2007;114(1):3–11. [PubMed] [Google Scholar]
- [16].OIE. World organization for animal health; Paris, France: 2012. Duck virus enteritis. Manual of diagnostic tests and vaccines for terrestrial animals; pp. 508–9. [Google Scholar]
- [17].Hansen WR, Nashold SW, Docherty DE, Brown SE, Knudson DL. Diagnosis of duck plague in waterfowl by polymerase chain reaction. Avian Dis. 2000;44(2):266–74. https://doi.org/10.2307/1592539. [PubMed] [Google Scholar]
- [18].Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9. doi: 10.1093/molbev/msy096. https://doi.org/10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zou Z, Ma J, Huang K, Chen H, Liu Z, Jin M. Live attenuated vaccine based on duck enteritis virus against duck hepatitis A virus types 1 and 3. Front Microbiol. 2016;7:1613. doi: 10.3389/fmicb.2016.01613. https://doi.org/10.3389/fmicb.2016.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rani RU, Muruganandan B. Outbreak of duck viral enteritis in a vaccinated duck flock. Scholars J Agric Vet Sci. 2015;2(3):253–5. [Google Scholar]
- [21].Keymer IF, Gough RE. Duck virus enteritis (anatid herpesvirus infection) in mute swans (Cygnus Olor) Avian Pathol. 1986;15(1):161–70. doi: 10.1080/03079458608436275. https://doi.org/10.1080/03079458608436275. [DOI] [PubMed] [Google Scholar]
- [22].Hansen WR, Gough RE. Duck plague (duck virus enteritis) In: Thomas NJ, Hunter DB, Atkinson CT, editors. Infectious diseases of wild birds. Blackwell Publishing; Ames, IA: 2007. pp. 87–107. https://doi.org/10.1002/9780470344668.ch4. [Google Scholar]
- [23].Li N, Hong T, Li R, Guo M, Wang Y, Zhang J, et al. Pathogenicity of duck plague and innate immune responses of the Cherry Valley ducks to duck plague virus. Sci Rep. 2016;6:32183. doi: 10.1038/srep32183. https://doi.org/10.1038/srep32183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kumar SK, Sivakumar M, Selvaraj P, Yogeshpriya S, Veeraselvam M. Duck viral enteritis in chronically infected and partially immune duck flock of nomadic. J Entomol Zool Studies. 2018;6:2698–700. [Google Scholar]
- [25].Pearson GL, Cassidy DR. Perspectives on the diagnosis, epizootiology, and control of the 1973 duck plague epizootic in wild waterfowl at Lake Andes, South Dakota. J Wildl Dis. 1997;33(4):681–705. doi: 10.7589/0090-3558-33.4.681. https://doi.org/10.7589/0090-3558-33.4.681. [DOI] [PubMed] [Google Scholar]
- [26].Khan KA, Islam MA, Sabuj AAM, Bashar MA, Islam MS, Hossain MG, et al. Molecular characterization of duck plague virus from selected Haor areas of Bangladesh. Open Vet J. 2021;11(1):42–51. doi: 10.4314/ovj.v11i1.8. https://doi.org/10.4314/ovj.v11i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ziedler K, Hlinak A. The occurrence of virus enteritis (duck plague) in wild birds. Berl Munch Tierarztl Wochenschr. 1992;105(4):122–5. [PubMed] [Google Scholar]
- [28].Ahamed MM, Hossain MT, Rahman M, Nazir KHMNH, Khan MF, Parvej MS, et al. Molecular characterization of duck plague virus isolated from Bangladesh. J Adv Vet Anim Res. 2015;2(3):296–303. https://doi.org/10.1016/j.vetmic.2016.07.025. [Google Scholar]


