Abstract
Objective: To evaluate interleukin-1ß (IL-1ß) and interleukin-8 (IL-8) epithelial expressions in potentially malignant disorders of the oral mucosa as malignant predictive markers.
Study design: About 55 tissues embedded in paraffin, comprising 15 oral lichen planus (OLP) lesions, 15 leukoplakias, 15 oral squamous cell carcinomas (OSCC), and 10 samples of normal oral mucosa were included in the study. IL-1ß and 8 expressions were assessed by immunohistochemistry using antibodies antihuman IL-1ß human (sc-7884, Santa Cruz® H-153) and antihuman IL-8 (ab7747, abcam®). The number of positive cells was compared using Student's t-test. Any p-value < 0.05 was considered statistically significant.
Results: Nuclear and cytoplasmatic keratinocyte staining were positive for both cytokines in all study groups. However, a statistically significant decrease was observed within all cases compared to normal mucosa, both staining for IL-1β and 8. Moreover, IL-8 showed significant differences between OLP and leukoplakia, and when compared to OSCC.
Conclusions: Oral epithelial expression of IL-1β and 8 seems to decrease when the malignant transformation of the oral mucosa increases.
Keywords: IL-1β, IL-8, oral carcinoma, leukoplakia, oral lichen planus, inflammation
Highlights
- Normal oral keratinocytes can express IL-1β and 8.
- The nuclear expression of IL-1β and 8 are possible in oral keratinocytes.
- Expression of IL-1β and 8 are present in both leukoplakia and OLP, although variable.
- As OLP is an inflammatory disease, the expression of IL-1β and 8 are present, although variable. The absence of tissular IL-1β and 8 may lead to OSCC progression.
- A decrease of tissular IL-1β and 8 may lead to OSCC progression. The saliva scenario might be different and maybe not comparable.
- New studies are needed to correlate the observed findings with the prognosis in OSCC.
Introduction
Currently, the term “precancerous” in oral mucosa has been replaced by potentially malignant disorders, recognizing the fact that not all disorders become oral carcinomas and malignant transformation may appear outside of the lesion area. Oral potentially malignant disorders include leukoplakia and proliferative verrucous leukoplakia, erythroplakia, submucous fibrosis, palatal lesions in reverse smokers, oral lichen planus (OLP), actinic cheilitis, lupus erythematosus, and most recently included chronic hyperplastic candidosis, oral lichenoid lesions, verrucous hyperplasia, and oral lesions of graft vs. host disease [1].
Leukoplakia and OLP are the most common potentially malignant disorders of the oral mucosa. Leukoplakia is defined by WHO as an oral white plaque of questionable risk having excluded known diseases or disorders that carry no increased risk for cancer [1]. OLP is a chronic mucocutaneous T-cell mediated inflammatory disease [1]. Although, it is defined by WHO as a potentially malignant disorder, there is some controversy, as a result of the malignant transformation, the rate ranges from 0% up to ~8% [1].
When any of those two disorders progress into cancer, it is done as an oral squamous cell carcinoma (OSCC), the most frequent head and neck malignancy. OSCC is the eighth most common type of cancer worldwide and the sixth most common cause of death of all malignancies, with a survival rate of around 40–50% in 5 years [2].
The role played by different cytokines in the transformation of leukoplakia and OLP into OSCC has been investigated mainly as salivary markers [3–8]. It has been shown that different tumors can produce cytokines in an autocrine manner and act by promoting angiogenesis and immunologic responses favoring the tumor [9–12].
Interleukin-1ß (IL-1β) and interleukin-8 (IL-8) are produced by a wide cell diversity, including oral keratinocytes [10, 12]. They have also been detected in high concentrations both in serum and saliva of patients with OSCC, suggesting they could serve as biomarkers for the disease [7, 8, 10].
Interleukin-1ß, a typical cancer-inflammation-linked cytokine, might play an important role in OSCC pathogenesis. Evidence shows IL-1β knockdown significantly inhibits OSCC cell growth and is regulated by genes, such as TGFβ [13]. Also, IL-1β has been identified as one of the key node genes in the tumor microenvironment during oral carcinogenesis [13].
Interleukin-8 is secreted by different stimulae among which IL-1 is included [14]. Higher levels of IL-8 have been found in the saliva of patients with OSCC, considering IL-8 a promising biomarker for oral malignant tranformation [14–18]. At this time, there is not enough evidence related to tissular inflammatory markers to be used for predictive purposes and therapeutic management of oral cancer. Therefore, the present study aimed to evaluate tissue expression of IL-1ß and 8 in leukoplakia, OLP, and OSCC.
Materials and Methods
Study Group
Biopsies of 55 subjects were included in this study. Bioethics approval for the study was obtained from the Bioethics Committee of Dental School, Universidad Central de Venezuela, under N° 0353–2012. Samples of OLP (n = 15), leukoplakias with histopathological evidence of mild to moderate epithelial dysplasia (n = 15), and well-differentiated OSCC (n = 15) were included after patient written consent. Normal gingival mucosa (n = 10) was obtained from healthy patients undergoing impacted third molar extraction, with no clinical signs of swelling or infection, and who gave informed written consent to participate in the study.
Immunohistochemistry
Sections of 4 μm were cut for indirect biotin-streptavidin-peroxidase staining. For antigen retrieval, a pH 6.1 repair solution (DAKO®, Santa Clara, CA, USA) in a steamer was used for an hour. Tissue sections were incubated with rabbit primary polyclonal anti- IL-1ß human antibodies (Santa Cruz® H-153, sc-7884, Texas, TX, USA) at 1:50 dilution and anti-IL-8 human antibody (Abcam®, ab7747) at 1:25 dilution for 1 h. Detection and amplification system EnVision (DAKO®) was applied for 30 min. Slices were exposed to diaminobenzidine chromogen (DAKO®) and finally stained with hematoxylin. Pinolidal cyst and tonsillar tissue were used as positive controls for IL-1β and IL-8, respectively.
IL-1ß and IL-8 Quantification
Slides were placed in an optic photomicroscope (OlympusCX41, San Diego, CA, USA) and observed under X10/0.25 magnifications. Digital images were taken from five different fields and saved as a jpeg file. A 6 × 6 grid was adapted to each image and examined for nuclear keratinocyte counting [19] using the digital image processing software ImageJ (1.46ª version). The mean of positive cells was calculated for each case.
Statistical Analysis
Data obtained were analyzed statistically by using SPSS statistical software (version 18.0). The number of positive cells was expressed by mean ± SD and compared using Student's t-test for independent samples. P-values <0.05 were considered statistically significant.
Results
Immunostaining for IL-1β and IL-8 was observed in the nucleus and cytoplasm of keratinocytes (Figures 1, 2).
Figure 1.
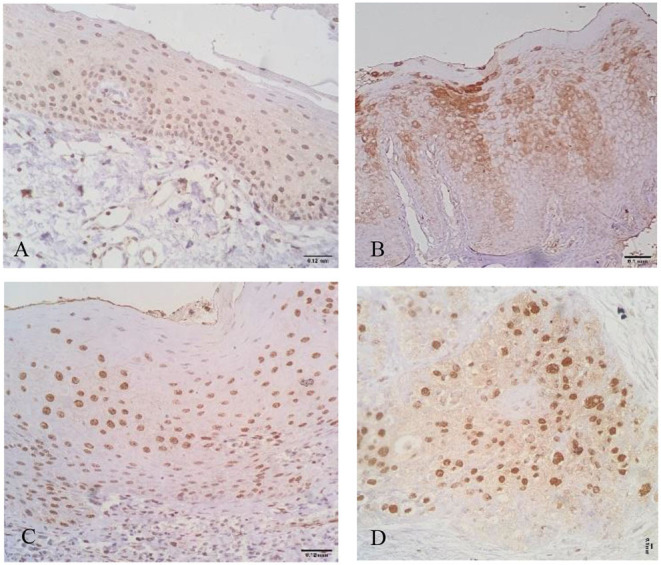
Interleukin-1β (IL-1β) + cells in oral healthy mucosa (A), leukoplakia (B), oral lichen planus (OLP) (C), and oral squamous cell carcinoma (OSCC) (D). Note different patterns of expression where cytoplasmatic and nuclear expressions are evident.
Figure 2.
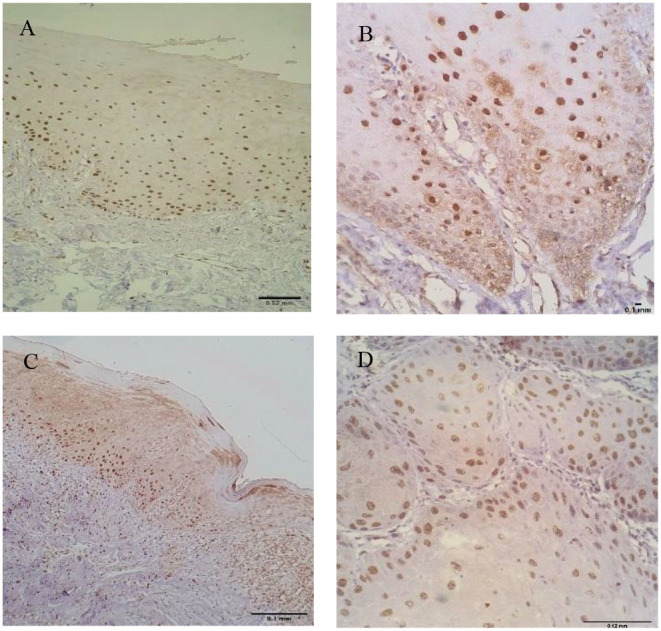
Keratinocytes expressing interleukin-8 (IL-8) in oral healthy mucosa (A), leukoplakia (B), OLP (C), and OSCC (D). Nuclear and cytoplasmatic staining was observed in all groups.
Interestingly, the highest expression of IL-1β was observed in oral healthy mucosa (mean of 329.25 ± 90.55 cells/field). Lower IL-1β positive cells were observed in leukoplakias (224.60 ± 161.05 cells/field), OLP (111 ± 101.752 cells/field), and OSCC (132.07 ± 121.951 cells/field) (Table 1, Figure 1).
Table 1.
Interleukin-1β (IL-1β) and Interleukin-8 (IL-8) keratinocyte expression.
| Interleukin | Mean ± SD positive cells/ field | p -value | |||
|---|---|---|---|---|---|
| Oral normal mucosa | OLP | Oral leukoplakia | OSCC | ||
| IL-1β | 329.25 ± 90.55 | 111 ± 101.75 | 224.60 ± 161.05 | 132.07 ± 21.95 | 0.06 |
| IL-8 | 389.8 ± 84.5 | 230.02 ± 227.67 | 413.87 ± 250.78 | 255 ± 193.9 | 0.046 |
When individual groups were compared, statistically significant decreased IL-1β expression was observed between healthy patients and OLP (p = 0.0001) and OSCC (p = 0.001) groups (Table 2).
Table 2.
Interleukin-1β p-values comparison among groups.
| Oral lesion | Oral normal mucosa | OLP | Oral leukoplakia | OSCC |
|---|---|---|---|---|
| Oral normal mucosa | - | 0.0001* | 0.001* | |
| OLP | 0.0001* | - | 0.029* | 0.611 |
| Oral leukoplakia | 0.106 | 0.029* | - | 0.087 |
| OSCC | 0.001* | 0.611 | 0.087 | - |
Statistically significant.
In relation to IL-8, the highest mean of positive cells was observed in leukoplakias (413.87 ± 250.789 cells/field) followed by normal oral mucosa (389.80 ± 84.507 cells/field), OSCC (255 ± 193.90 cells/field), and finally OLP (230.02 ± 227.67 cells/field) (p = 0.046) (Table 1, Figure 2).
Interleukin-8 was statistically decreased in OSCC when compared to the normal oral mucosa (p = 0.026), and conversely statically increased in leukoplakias (p = 0.009) (Table 3).
Table 3.
Interleukin-8 p-values comparison among groups.
| Oral lesion | Oral normal mucosa | OLP | Oral leukoplakia | OSCC |
|---|---|---|---|---|
| Oral normal mucosa | - | 0.101 | 0.009* | 0.026* |
| OLP | 0.101 | - | 0.342 | 0.958 |
| Oral leukoplakia | 0.009* | 0.342 | - | 0.043* |
| OSCC | 0.026* | 0.958 | 0.043* | - |
Statistically significant.
Discussion
Interleukin-1β is a pro-inflammatory cytokine that is implied the most in oral carcinogenesis [8, 20]. The research evidenced interesting findings related to the immunohistochemical analysis of this cytokine; nuclear and cytoplasmic keratinocyte staining was observed in all the study groups, and IL-1β was increasingly expressed by oral healthy mucosa.
Nuclear keratinocyte expression of IL-1 and IL-8 has been observed before [21], moreover, IL-1α and IL-1β have been observed in vivo and in vitro in gingival keratinocytes, probably as a result of permanent bacterial response [22–24]. It is known that inflammasome activation induces caspase-1 dependent secretion of the pro-inflammatory cytokine IL-1β in keratinocytes. Gingival keratinocytes also can produce IL-8 in response to IL-1β maintaining tissue micro-environment [22–24]. The normal mucosa samples were originated by periodontal tissue and that could explain the persistent expression of both cytokines under “healthy” or insignificant clinical inflammatory circumstances.
The oral environment is formed by a series of elements that make it an immunological organ, such as the presence of lymphoid tissue associated with the mucosa, microbiota, saliva with components, such as secretory IgA and a large variety of biomarkers [18]. Additionally, it has been shown that oral keratinocytes can express molecules of histocompatibility type II complex, which make it behave as an antigen-presenting cell [25], and make it capable of expressing and secreting pro-inflammatory cytokines such as IL-1β and IL-8 [26, 27]. The use of gingival mucosa, as mentioned, could imply a pro-inflammatory environment, which may not represent a strictly healthy control, although, the results favor a decreased expression than in oral potentially malignant disorders studied.
Wu et al. [13] observed, in a rat model, keratinocyte IL-1β positivity in healthy oral mucosa, leukoplakia, and OSCC; however, staining was more significant in cases of the disease, when comparing with healthy tissue, suggesting that cytokines increase is more closely related to malignant transformation.
Taking into account that the IL-1β expression and secretion by oral keratinocyte is inflammasome-dependent, IL-1 may have a role in cell death activation through pyroptosis, the process currently recognized as necessary for cell exchange and protection of oral mucosa epithelium [28]. Mechanism of cell death derived from the activation of the inflammasome, which involves IL-1β secretion as an oncosupressive effect [28], might explain the observations in this study, where a significant reduction in IL-1β expression by keratinocyte was observed. This might suggest that loss of IL-1β implies pyroptosis reduction, favoring cell proliferation, observed in OLP and leukoplakia, which is a determining fact of OSCC pathogenesis.
A dual role for IL-1β in carcinogenesis, tumor growth, invasion, and metastasis has also been suggested [28, 29]. Gasparoto et al. [30] chemically induced squamous cell carcinomas in mouse skin and investigated the effect of the inflammasome on the appearance and tumor development. The authors observed that proteins derived from the inflammasome were involved in the protection against squamous cell carcinoma since their loss hinders the development of the antitumor response. On the other hand, and consistent with the dual role of this cytokine, Dantas et al. [31] observed how IL-1β immunostaining was significantly higher in metastases of oral carcinomas and lymph nodes than in squamous epithelium of tumor resection margins; while Singh et al. [32] described how the immunostaining for IL-1β in non-metastatic oral carcinomas was weak in more than half of the cases. Therefore, the presence of IL-1β in oral epithelial tissues remains controversial.
Interleukin-8 is also related to carcinogenesis. It has an important angiogenic and chemotactic performance, being produced by a wide group of cells, the keratinocytes among them. Additionally, the expression of nuclear and cytoplasmatic IL-8 in OSCC keratinocytes has been described [33].
Studies assessing IL-8 in potentially malignant disorders and OSCC have been reported in saliva and serum [16, 17], however, in oral mucosa by immunohistochemical staining are scarce and results seem different. Jenkins et al. [34] assessed the intensity of the NF-κB primary transcription factor and IL-8 expression in samples of esophageal adenocarcinoma, Barrett esophagitis (as premalignant precursor lesion), and squamous tissue next to these lesions and observed IL-8 diminish, similar to results obtained in the study.
Immunohistochemical behavior of IL-8 was similar to the one observed with IL-1β, in this sense, previous studies [11, 18, 24, 25] have shown that the IL-1ß induction is within the costimulating factors which produce IL-8, so these results may have a direct relationship with this fact. Taking into account that IL-8 is a chemoattractant cytokine, for cells such as polymorphonuclear neutrophils and lymphocytes [11, 35], its absence or reduction in the expression of disease cases, mainly OSCC, could represent a loss of antitumor immune vigilance favoring carcinogenic activity.
Lee et al. [35] studied the effect of IL-8 in the tumorigenesis of human ovarian cancer in mice. Cells transfected with IL-8 exhibited poor growth. Authors related the reduction of growth and cell proliferation with the leakage of activated neutrophils, suggesting the release of oxygen and proteases might produce the death of tumor cells.
Decrease expression observed with IL-8 can be related to the IL-1ß which induces its synthesis. This finding might imply a loss of chemotactic activity reducing antitumor function induced by polymorphonuclear neutrophils and lymphocytes. IL-1ß immunohistochemical findings allow suggesting that patients with OSCC might exhibit epithelial loss of this cytokine, which might be related to deactivation of the inflammasome at the keratinocyte producing not only the reduction of its expression and secretion but also the reduction of pyroptosis favoring the carcinogenesis process.
In OLP, some studies have described abnormal expression patterns of several inflammation-related cytokines (including IL-1 β and IL-8) in tissues, saliva, and serum from patients [7, 12, 36]. In this study, OLP showed the lowest cytokine expression probably related to the chronic inflammatory nature of the pathology.
While IL-1β and IL-8 were decreased in OLP lesions, various studies have consistently shown increased levels of IL-8 in serum and saliva from patients with OLP [7, 12, 36, 37]. This disparity between tissues and fluids indicates that ILs may function mainly in the systemic immune response of patients with OLP rather than in the local lesions.
Additionally, it has been described that the level of salivary IL-8 is lower in OLP with epithelial dysplasia than patients with OSCC [12, 37], indicating that salivary IL-8 level might be useful, non-invasive method for monitoring the malignant transformation of OLP.
Conclusions
Oral epithelial expression of IL-1β and 8 seem to decrease when the malignant transformation of the oral mucosa increases. IL-1β may play a role in oral carcinogenesis via keratinocytes.
A significant discovery of the results was observed in gingival keratinocytes, showing expression of both cytokines under normal, or mild inflammatory conditions.
Statement of Clinical Relevance
Loss of IL-1β and 8 in potentially malignant disorders, as leukoplakia and OLP, might suggest the start of a malignant transformation process.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Warnakulasuriya S. Oral potentially malignant disorders: a comprehensive review on clinical aspects and management. Oral Oncol. (2020) 102:104550. 10.1016/j.oraloncology.2019.104550 [DOI] [PubMed] [Google Scholar]
- 2.Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. (2017) 67:51–64. 10.3322/caac.21384 [DOI] [PubMed] [Google Scholar]
- 3.Punyani SR, Sathawane RS. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin Oral Investig. (2013) 17:517–24. 10.1007/s00784-012-0723-3 [DOI] [PubMed] [Google Scholar]
- 4.Kaur J, Jacobs R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J Korean Assoc Oral Maxillofac Surg. (2015) 41:171–5. 10.5125/jkaoms.2015.41.4.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michailidou E, Tzimagiorgis G, Chatzopoulou F, Vahtsevanos K, Antoniadis K, Kouidou S, et al. Salivary mRNA markers having the potential to detect oral squamous cell carcinoma segregated from oral leukoplakia with dysplasia. Cancer Epidemiol. (2016) 43:112–8. 10.1016/j.canep.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 6.Brailo V, Vucicevic-Boras V, Lukac J, Biocina-Lukenda D, Zilic-Alajbeg I, Milenovic A, et al. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med Oral Patol Oral Cir Bucal. (2012) 17:e10–5. 10.4317/medoral.17323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodus NL, Cheng B, Myers S, Miller L, Ho V, Ondrey F. The feasibility of monitoring NF-kappaB associated cytokines: TNF-alpha, IL-1alpha, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol Carcinog. (2005) 44:77–82. 10.1002/mc.20113 [DOI] [PubMed] [Google Scholar]
- 8.Kamatani T, Shiogama S, Yoshihama Y, Kondo S, Shirota T, Shintani S. Interleukin-1 beta in unstimulated whole saliva is a potential biomarker for oral squamous cell carcinoma. Cytokine. (2013) 64:497–502. 10.1016/j.cyto.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 9.Turhani D, Krapfenbauer K, Thurnher D, Langen H, Fountoulakis M. Identification of differentially expressed, tumor-associated proteins in oral squamous cell carcinoma by proteomic analysis. Electrophor. (2006) 27:1417–23. 10.1002/elps.200500510 [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann TK, Sonkoly E, Homey B, Scheckenbach K, Gwosdz C, Bas M, et al. Aberrant cytokine expression in serum of patients with adenoid cystic carcinoma and squamous cell carcinoma of the head and neck. Head Neck. (2007) 29:472–8. 10.1002/hed.20533 [DOI] [PubMed] [Google Scholar]
- 11.Watanabe H, Iwase M, Ohashi M, Nagumo M. Role of interleukin-8 secreted from human oral squamous cell carcinoma cell lines. Oral Oncol. (2002) 38:670–9. 10.1016/S1368-8375(02)00006-4 [DOI] [PubMed] [Google Scholar]
- 12.Czerninski R, Basile JR, Kartin-Gabay T, Laviv A, Barak V. Cytokines and tumor markers in potentially malignant disorders and oral squamous cell carcinoma: a pilot study. Oral Dis. (2014) 20:477–81. 10.1111/odi.12160 [DOI] [PubMed] [Google Scholar]
- 13.Wu T, Hong Y, Jia L, Wu J, Xia J, Wang J, et al. Modulation of IL-1β reprogrammes the tumor microenvironment to interrupt oral carcinogenesis. Sci Rep. (2016) 6:20208. 10.1038/srep20208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan LP, Wang LF, Chiang FY, Lee KW, Kuo PL, Liang CH. IL-8 promotes HNSCC progression on CXCR1/2-meidated NOD1/RIP2 signaling pathway. Oncotarget. (2016) 7:61820–31. 10.18632/oncotarget.11445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St John MA, Li Y, Zhou X, Denny P, Ho CM, Montemagno C, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. (2004) 130:929–35. 10.1001/archotol.130.8.929 [DOI] [PubMed] [Google Scholar]
- 16.Schiegnitz E, Kämmerer PW, Schön H, Blatt S, Berres M, Sagheb K, et al. Proinflammatory cytokines as serum biomarker in oral carcinoma-A prospective multi-biomarker approach. J Oral Pathol Med. (2018) 47:268–74. 10.1111/jop.12670 [DOI] [PubMed] [Google Scholar]
- 17.Lee LT, Wong YK, Hsiao HY, Wang YW, Chan MY, Chang KW. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. (2018) 47:699–707. 10.1016/j.ijom.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Wu JY, Yi C, Chung HR, Wang DJ, Chang WC, Lee SY, et al. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol. (2010) 46:226–31. 10.1016/j.oraloncology.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 19.Bologna-Molina R, Damian-Matsumura P, Molina-Frechero N. An easy cell counting method for immunohistochemistry that does not use an image analysis program. Histopathology. (2011) 59:801–3. 10.1111/j.1365-2559.2011.03954.x [DOI] [PubMed] [Google Scholar]
- 20.Lee CH, Chang JS, Syu SH, Wong TS, Chan JY, Tang YC, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. (2015) 230:875–84. 10.1002/jcp.24816 [DOI] [PubMed] [Google Scholar]
- 21.Babiuch K, Kuśnierz-Cabala B, Kesek B, Okoń K, Darczuk D, Chomyszyn-Gajewska M. Evaluation of proinflammatory, NF-kappaB dependent cytokines: IL-1α, IL-6, IL-8, and TNF-α in tissue specimens and saliva of patients with oral squamous cell carcinoma and oral potentially malignant disorders. J Clin Med. (2020) 9:867. 10.3390/jcm9030867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sfakianakis A, Barr CE, Kreutzer DL. Actinobacillus actinomycetemcomitans-induced expression of IL-1alpha and IL-1beta in human gingival epithelial cells: role in IL-8 expression. Eur J Oral Sci. (2001) 109:393–401. 10.1034/j.1600-0722.2001.00162.x [DOI] [PubMed] [Google Scholar]
- 23.Uchida Y, Shiba H, Komatsuzawa H, Takemoto T, Sakata M, Fujita T, et al. Expression of IL-1 beta and IL-8 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans. Cytokine. (2001) 14:152–61. 10.1006/cyto.2001.0863 [DOI] [PubMed] [Google Scholar]
- 24.Sfakianakis A, Barr CE, Kreutzer D. Mechanisms of Actinobacillus actinomycetemcomitans-induced expression of interleukin-8 in gingival epithelial cells. J Periodontol. (2001) 72:1413–9. 10.1902/jop.2001.72.10.1413 [DOI] [PubMed] [Google Scholar]
- 25.Villarroel-Dorrego M, Speight PM, Barrett AW. Expression of major histocompatibility complex class II and costimulatory molecules in oral carcinomas in vitro. Med Oral Patol Oral Cir Bucal. (2005) 10:188–95. [PubMed] [Google Scholar]
- 26.Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL. Coexpression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg. (1997) 174:507–12. 10.1016/S0002-9610(97)00165-7 [DOI] [PubMed] [Google Scholar]
- 27.Cohen RF, Contrino J, Spiro JD, Mann EA, Chen LL, Kreutzer DL. Interleukin-expression by head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. (1995) 121:202–9. 10.1001/archotol.1995.01890020064013 [DOI] [PubMed] [Google Scholar]
- 28.Drexler SK, Yazdi AS. Complex roles of inflammasomes in carcinogenesis. Cancer J. (2013) 19:468–72. 10.1097/PPO.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 29.Apte RN, Voronov E. Is interleukin-1 a good or bad 'guy' in tumor immunobiology and immunotherapy? Immunol Rev. (2008) 222:222–41. 10.1111/j.1600-065X.2008.00615.x [DOI] [PubMed] [Google Scholar]
- 30.Gasparoto TH, de Oliveira CE, de Freitas LT, Pinheiro CR, Hori JI, Garlet GP, et al. Inflammasome activation is critical to the protective immune response during chemically induced squamous cell carcinoma. PLoS ONE. (2014) 9:e107–70. 10.1371/journal.pone.0107170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dantas TS, Barros Silva PG, Lima Verde MEQ, Ribeiro Junior AL, Cunha MDPSS, Mota MRL, et al. Role of inflammatory markers in prognosis of oral squamous cell carcinoma. Asian Pac J Cancer Prev. (2019) 20:3635–42. 10.31557/APJCP.2019.20.12.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh P, Augustine D, Rao RS, Patil S, Sowmya SV, Haragannavar VC, et al. Interleukin-1beta and Caspase-3 expression serve as independent prognostic markers for metastasis and survival in oral squamous cell carcinoma. Cancer Biomark. (2019) 26:109–22. 10.3233/CBM-190149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita Y, Okamoto M, Goda H, Tano T, Nakashiro K, Sugita A, et al. Prognostic significance of interleukin-8 and CD163-positive cell-infiltration in tumor tissues in patients with oral squamous cell carcinoma. PLoS ONE. (2014) 9:e110378. 10.1371/journal.pone.0110378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins GJ, Mikhail J, Alhamdani A, Brown TH, Caplin S, Manson JM, et al. Immunohistochemical study of nuclear factor-kappaB activity and interleukin-8 abundance in oesophageal adenocarcin a useful strategy for monitoring these biomarkers. J Clin Pathol. (2007) 60:1232–7. 10.1136/jcp.2006.043976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee LF, Hellendall RP, Wang Y, Haskill JS, Mukaida N, Matsushima K, et al. IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. J Immunol. (2000) 164:2769–75. 10.4049/jimmunol.164.5.2769 [DOI] [PubMed] [Google Scholar]
- 36.Rhodus NL, Cheng B, Myers S, Bowles W, Ho V, Ondrey F. A comparison of the pro-inflammatory, NF-kappaB-dependent cytokines: TNF-alpha, IL-1-alpha, IL-6, and IL-8 in different oral fluids from oral lichen planus patients. Clin Immunol. (2005) 114:278–83. 10.1016/j.clim.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 37.Chiamulera MMA, Zancan CB, Remor AP, Cordeiro MF, Gleber-Netto FO, Baptistella AR. Salivary cytokines as biomarkers of oral cancer: a systematic review and meta-analysis. BMC Cancer. (2021) 21:205. 10.1186/s12885-021-07932-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


