Abstract
Two hepatitis C antibody assays were used to test diluted positive sera. Dilutions of 1 in 5, 1 in 10, and 1 in 20 all resulted in loss of reactivity, with the greatest losses occurring in samples with low and moderate reactivities. These results disqualify pooling as a strategy for seroprevalence studies and screening programs.
Pooling of serum samples for population screening was first employed in 1943 by the U.S. Army as a cost-saving method of screening soldiers for syphilis. Serum pooling eliminates the need to test individual specimens unless the pool is found to be reactive. Pooling, usually in lot sizes of 5 to 20, has since been successfully applied to studies of human immunodeficiency virus (HIV) seroprevalence (1, 3, 5, 6). Serum pooling is appropriate for such studies because knowledge of the infectious status of any individual sample is not critical. Hepatitis C virus (HCV) infection is an important public health problem worldwide; seroprevalence surveys are being conducted in many countries, and pooling has been suggested as a cost-effective strategy for HCV screening in developing countries (2). We were interested to know if sample pooling might be applicable to HCV seroprevalence studies.
We selected two widely used, conventional enzyme immunoassays (EIAs) for this study: the UBI EIA 4.0 (Organon Teknika [OT], Durham, N.C.) and the HCV 3.0 enzyme-linked immunosorbent assay (ELISA) (Ortho Clinical Diagnostics [Ortho], Raritan, N.J.). The OT test uses synthetic peptides corresponding to the core, NS3, NS4, and NS5 regions of the genome, whereas the Ortho test uses recombinant proteins of the same antigens. Specimens used in this study had been submitted for diagnostic HCV testing during the previous 6 months and were stored at −20°C until use. Forty-one HCV antibody-positive serum samples exhibiting reactivities ranging from weak to strong were selected for testing. The serum samples were chosen and categorized according to the simple optical density (OD) readings from the initial Ortho assay as follows: weak (OD < 1), moderate (OD of ≥1 and ≤2), or strong (OD ≥ 3). Pool sizes of 5, 10, and 20 specimens were created by diluting each reactive sample 1:5, 1:10 and 1:20 with pooled negative serum samples. Serum samples in the negative pool were confirmed as non-HCV-reactive by the two antibody assays used in this study and by qualitative HCV RNA testing (Roche Amplicor; Roche Diagnostics Canada, Laval, Quebec, Canada). The manufacturer's kit insert instructions were followed without modification. All samples were tested in duplicate. The ratio of the OD to the cutoff (OD/CO ratio) for each test was determined for each individual and pooled specimen.
The results showed that loss of positive reactions occurred with dilution of the reactive samples. The OT ELISA failed to record a positive reaction for 39, 59, and 76% of reactive serum samples diluted in pool sizes of 5, 10, and 20, respectively. The Ortho ELISA failed to record a positive reaction for 22, 46, and 51%, respectively. Although the Ortho test was less affected by dilution, both kits failed to detect a significant number of reactive serum samples even at the lowest dilution. For purposes of analysis, the samples were stratified by the undiluted level of reactivity expressed as the OD/CO ratio.
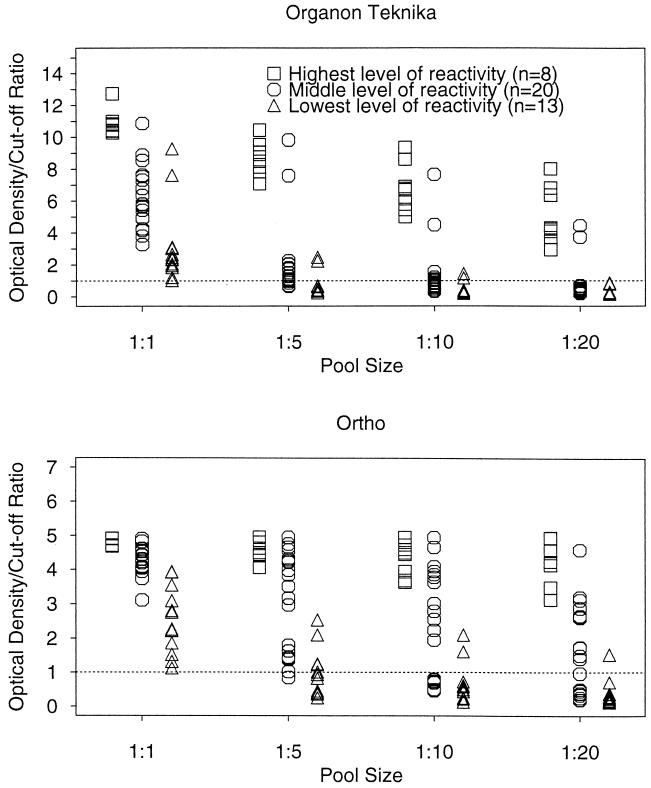
Figure 1 shows the data stratified by pool size and by the original level of reactivity of the undiluted specimen. All of the strongly reactive specimens were detected by both tests, regardless of pool size (Fig. 1). However, loss of positive reactions occurred for moderately reactive specimens and was most pronounced for pooled weakly reactive specimens.
FIG. 1.
OD/CO ratios for study sera, by test kit and dilution pool size, stratified by undiluted reactivity.
These results contrast with those reported for HIV seroprevalence surveys, where serum pooling has been shown to be an effective method for screening samples in pool sizes of 5, 10, and 20. The sensitivity of HIV tests is lower for individuals in the early stage of seroconversion or the late stage of disease, when antibody concentrations are lower (5), but this slight decrease in sensitivity is acceptable in seroprevalence surveys, where the HIV status of individuals is not critical. The cost-effectiveness of pooling diminishes as the disease prevalence increases, however (7).
We are aware of only two reports of pooling in HCV seroprevalence studies. Garcia and colleagues (2) found pool sizes of 5 to be acceptable for determining HCV seroprevalence with an Abbott EIA, but the sensitivity of pooling decreased at dilutions greater than 1:5 and with indeterminate specimens. These investigators did not study the relationship between the original level of reactivity and loss of reactivity upon dilution. Liu and colleagues (4) investigated pooling with a large number of blood donors but used only one EIA kit and one dilution: 1 in 5. In addition, there was no analysis of loss of reactivity according to the undiluted level of reactivity. In contrast, our study has shown that there is significant loss of reactivity in positive specimens with lower antibody levels, even at a 1-in-5 dilution. Of greater concern is the fact that the majority of all samples that initially were weakly reactive experienced loss of reactivity at dilutions of 1 in 10 and higher.
Our own experience with the two HCV assays used in this study leads us to conclude that pooling would not be a reliable strategy for HCV seroprevalence studies or for blood collection purposes. Our results clearly show a significant loss of reactivity, even at low dilutions, in samples that were moderately and weakly reactive when undiluted. Such samples are likely to be present in any mass testing setting and could fatally skew the results of a seroprevalence study or result in false negatives in a screening program.
REFERENCES
- 1.Emmanuel J C, Bassett M T, Smith H J, Jacobs J A. Pooling of sera for human immunodeficiency virus (HIV) testing: an economical method for use in developing countries. J Clin Pathol. 1988;41:582–585. doi: 10.1136/jcp.41.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia Z, Taylor L, Ruano A, Pavon L, Ayerdis E, Luftig R B, Visona K A. Evaluation of a pooling method for routine anti-HCV screening of blood donors to lower the cost burden on blood banks in countries under development. J Med Virol. 1996;43:218–222. doi: 10.1002/(SICI)1096-9071(199607)49:3<218::AID-JMV10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Kline R L, Brothers T A, Brookmeyer R, Zeger S, Quinn T C. Evaluation of human immunodeficiency virus seroprevalence in population surveys using pooled sera. J Clin Microbiol. 1989;27:1449–1452. doi: 10.1128/jcm.27.7.1449-1452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu P, Shi Z X, Zhang Y C, Xu Z C, Shu H S, Zhang X Y. A prospective study of a serum-pooling strategy in screening blood donors for antibody to hepatitis C virus. Transfusion. 1997;37:732–736. doi: 10.1046/j.1537-2995.1997.37797369450.x. [DOI] [PubMed] [Google Scholar]
- 5.Raboud J M, Major C, Sherlock C H, O'Shaughnessy M V. The effects of pooling serum samples from seroconverting individuals or individuals with end stage disease for HIV antibody testing: a comparison of four screen tests and three pool sizes. Serodiagn Immunother Infect Dis. 1996;8:19–24. [Google Scholar]
- 6.Sherlock C H, Strathdee S A, Le T, Sutherland D, O'Shaughnessy M V, Schechter M T. Use of pooling and outpatient laboratory specimens in an anonymous seroprevalence survey of HIV infection in British Columbia, Canada. AIDS. 1995;9:945–949. doi: 10.1097/00002030-199508000-00017. [DOI] [PubMed] [Google Scholar]
- 7.WHO Global Programme on AIDS and Global Blood Safety Initiative. Recommendations for testing for HIV antibody on serum pools. Wkly Epidemiol Rec. 1991;44:326–327. [PubMed] [Google Scholar]