Abstract
Introduction
Computer-Aided Lung Informatics for Pathology Evaluation and Ratings (CALIPER) software has already been widely used in the evaluation of interstitial lung diseases (ILD) but has not yet been tested in patients affected by COVID-19. Our aim was to use it to describe the relationship between Coronavirus Disease 2019 (COVID-19) outcome and the CALIPER-detected pulmonary vascular-related structures (VRS).
Materials and methods
We performed a multicentric retrospective study enrolling 570 COVID-19 patients who performed a chest CT in emergency settings in two different institutions. Fifty-three age- and sex-matched healthy controls were also identified. Chest CTs were analyzed with CALIPER identifying the percentage of VRS over the total lung parenchyma. Patients were followed for up to 72 days recording mortality and required intensity of care.
Results
There was a statistically significant difference in VRS between COVID-19-positive patients and controls (median (iqr) 4.05 (3.74) and 1.57 (0.40) respectively, p = 0.0001). VRS showed an increasing trend with the severity of care, p < 0.0001. The univariate Cox regression model showed that VRS increase is a risk factor for mortality (HR 1.17, p < 0.0001). The multivariate analysis demonstrated that VRS is an independent explanatory factor of mortality along with age (HR 1.13, p < 0.0001).
Conclusion
Our study suggests that VRS increases with the required intensity of care, and it is an independent explanatory factor for mortality.
Key Points
• The percentage of vascular-related structure volume (VRS) in the lung is significatively increased in COVID-19 patients.
• VRS showed an increasing trend with the required intensity of care, test for trend p< 0.0001.
• Univariate and multivariate Cox models showed that VRS is a significant and independent explanatory factor of mortality.
Keywords: COVID-19, Sars-Cov2, Computed tomography, CALIPER, Texture analysis
Introduction
Since the first pneumonia cases found in Wuhan (China) in early December 2019 [1], the Coronavirus Disease 2019 (COVID-19) has widely spread all over the world with greater than 40 million cases and 1 million deaths reported as of October 2020 [2].
Clinical manifestation of disease ranges from asymptomatic to mild subclinical flu-like symptoms to severe interstitial pneumonia which can lead to organ dysfunction—shock, acute respiratory distress syndrome, and acute cardiac and kidney injury—and death [3]. COVID-19 also appears to be associated with systemic coagulopathy arising from endothelial cell damage, especially in the pulmonary vessels, with loss of vascular integrity and inflammation [4, 5]. Diffuse endovascular thrombosis with microangiopathy was found in patients who died from COVID-19 [6] and anticoagulants, such as low-molecular-weight heparin, seem to be beneficial in patients with COVID-19 [7].
Vascular alterations may present in every stage of symptomatic COVID-19 [8], since they are thought to be dependent from the virus’ interaction with the endothelial cell through the ACE-2 receptors [9].
Pulmonary complications of the infection, often requiring hospitalization, oxygenation support, and intubation, still represent an enormous challenge for worldwide health-care systems during outbreaks.
Chest CT is the most sensitive imaging approach to evaluate the extent and characteristics of pulmonary involvement and could serve as an effective way for early screening and diagnosis of COVID-19 pneumonia [10], although the CT appearance is variable and COVID-19 infection is typically confirmed with microbiological tests such as reverse-transcription polymerase chain reaction (RT-PCR).
Characteristic CT manifestations associated with COVID-19 pneumonia include ground-glass opacities and parenchymal consolidations with peripheral and lower lobe predominance [11, 12]. Linear opacities, a “crazy-paving” pattern and the “reversed halo” sign can be seen in the late phases of the disease [13]. There is some anecdotal evidence that there are morphological changes in pulmonary vasculature in COIVD-19 even in the absence of thrombi/thromboembolism [14, 15]. Although vascular enlargement in acute COVID-19 infection has been noted in several studies, the vascular changes in COVID-19 are likely difficult to detect visually and maybe underrecognized as a prognostic indicator of disease outcomes.
Computer-aided quantitative analysis of chest CT exams has already proven to be useful both in the diagnosis of COVID-19 pneumonia [16], in predicting adverse outcomes in COVID-19 pneumonia [17, 18] and to assess the progression of the disease [19]. To our knowledge, a quantitative analysis discriminating lung parenchyma involvement and disease related pulmonary vessel volume alterations has not been yet performed.
The aim of this study was to describe the relationship between COVID-19 outcome and a radiomic biomarker that assesses pulmonary vascular structures that has proven to be predictive in the assessment of idiopathic pulmonary fibrosis severity and progression [20] (CALIPER, Mayo Clinic), during the first COVID-19 outbreak in Italy in March–April 2020.
Materials and methods
The retrospective multicentric study was conducted in Azienda Ospedaliera Universitaria Pisana, Pisa, Italy (institution 1), and in Azienda Ospedaliero-Universitaria Maggiore della Carità, Novara, Italy (institution 2). The internal review boards of both institutions approved the research, protocol numbers 17368 (institution 1) and CE 130/20 (institution 2).
Data were acquired between March 1 and April 9, 2020. The inclusion criteria were as follows:
Having performed a non-contrast chest CT in the emergency setting for clinically suspected COVID-19
Having performed at least one RT-PCR test positive for SARS-COV-2 RNA no longer than a week after the CT acquisition.
The exclusion criteria were as follows:
Severe motion artifacts in the CT scan
Not technically adequate CALIPER CT segmentations
Unavailable RT-PCR outcome or date
Unavailable clinical data
We were able to identify 570 patients who met the above criteria, 268 from institution 1 and 302 from institution 2. The patient selection process is depicted in Fig. 1.
Fig. 1.
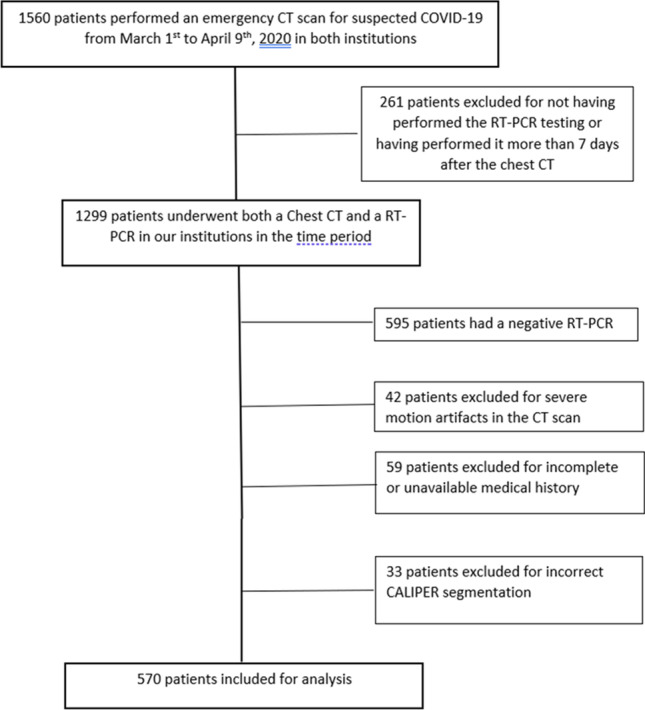
Flowchart of patient selection
We subsequently identified a subgroup of 53 sex- and age-matched controls from institution 1. These controls were selected among oncologic patients who performed a routine examination and were free of any CT-evident neoplastic or inflammatory disease in the chest cavity. We chose the controls randomly trying to reflect the sex and age distribution of COVID-19 patients. All the controls’ CT scans were non-contrast-enhanced.
Chest CT acquisition
All chest CT scans were performed during a single full inspiratory breath-hold in a supine position. In institution 1, the CTs were executed either on a 64-slice Siemens Somatom Sensation scanner (Siemens Healthineers) or on a 64-slice General Electric Light Speed scanner (General Electric Co). The Siemens technical parameters were 120 kV, 284 mAs, 1.84 spiral pitch factor, collimation width 0.6, 512 × 512 matrix, 1.5 mm reconstruction thickness, B31 kernel. The General Electric technical details were 120 kV, 169 mAs, 0.98 spiral pitch factor, collimation width 0.625, 512 × 512 matrix, 1.25 mm reconstruction thickness, standard kernel.
In institution 2, all the scans were performed on a 128-slice Philips Ingenuity Core scanner (Philips Healthcare). The scan technical parameters were 120 kV, 226 mAs, 1.08 spiral pitch factor, 0.625 collimation width, 512 × 512 matrix, 1 mm reconstruction thickness, B kernel. In younger patients (< 40 years), tube voltage was reduced in all cases to 80–100 kV and the tube current modulation was automatically modified on the basis of the scout image.
RT-PCR execution technique
The RT-PCR tests in both institutions were performed through nasopharyngeal swabs (Xpert® Nasopharyngeal Sample Collection Kit for Viruses or eNAT® Transport and Preservation Media plus One pernasal applicator swab). The RNA was first extracted (Seegene Nimbus IVD, Hamilton ELITe Galaxy, Abbott m2000sp or Biomèrieux NUCLISENS® EASYMAG®) and subsequently magnified and identified (Applied Biosystems™ 7500 Fast Dx Real-Time PCR Instrument or Abbott m2000rt) with two different sets (GeneFinder™ COVID-19 Plus RealAmp Kit or Allplex™ 2019-nCoV Assay). A rapid amplification instrument was also used (DiaSorin LIAISON® MDX). All these procedures are in full compliance with the WHO Guidelines [21].
Chest CT CALIPER analysis
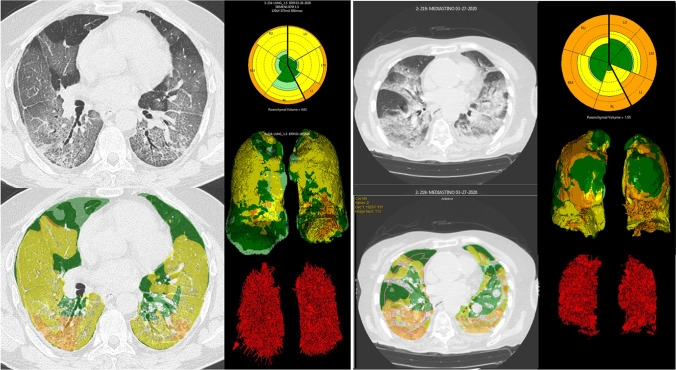
All the chest CTs were analyzed by the CALIPER software. The initial data processing steps involved extraction of the lung volume from the surrounding thoracic structures and the segmentation into upper, middle, and lower zones (see Fig. 2) based on the location of the carina and craniocaudal extent of the lung volumes. Lung segmentation was performed with an adaptive density-based morphologic approach, whereas airway segmentation involved iterative three-dimensional region growing, density thresholding, and connected components analysis. Parenchymal tissue type classification was applied to voxel volume units using proprietary texture analysis. CALIPER utilizes a computer vision-based approach to CT image characterization including the use of volumetric histogram signatures to map features and uses 3D morphology of these features for tissue classification. Specifically, CALIPER lung parenchymal analysis involved algorithmic identification and volumetric quantification of five radiological parenchymal features: normal lung, low-attenuation areas (such as hyperinflation or emphysema), ground-glass density, reticular abnormalities, and honeycombing measured in total litres for the whole lung. The volume of these radiological parenchymal patterns is also available as a percentage of total lung volume. The extent of parenchymal involvement in ILD can be considered a percent of total interstitial lung abnormalities (ILD%): a sum of percent of areas of ground glass, reticular, and HC as described by Romei et al. [20]. The CALIPER software, having been developed to assess features of COPD and interstitial lung diseases, was not designed to segment or characterize areas of lung consolidation.
Fig. 2.
Left: CALIPER lung segmentation in a 68-year-old COVID-19 patient (VRS 10.7%, ILD 56.5%) Right: CALIPER segmentation in a 91-year-old COVID-19 patient (VRS 12.4%, ILD 51.4%). VRS are shown in red in the lower right corner of both images
For vascular-related structures, the larger vessels at the hilum were automatically excluded by lung segmentation [22]. The eigenvalues of the Hessian matrix were used in order to obtain an optimized multiscale filter that enhances tubular structures, and thus segment the pulmonary vessels with a diameter approximately larger than 3 mm. The volume obtained was defined as vascular-related structures (VRS). VRS percentage was defined as the ratio of the total vessels volume divided by the total lung parenchymal volume.
Chest CT visual analysis
A total severity score (TSS) was visually calculated for each patient according to the well-established method proposed by Li et al. [23]. A radiologist with over 10 years of thoracic imaging experience evaluated all COVID-19 CT scans performing the visual quantitative evaluation. The score was based on the sum of interstitial pneumonia and consolidation percentage involvement in each lobe, which was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%), respectively. The TSS score therefore ranged from 0 to 20.
Outcome definition
We observed all the participants for up to 72 days after the CT scan. We analyzed firstly the mortality, thus dividing the COVID-19 patients between survivors and non-survivors, and secondly the required intensity of care, defining three different groups:
Patients discharged at home without the necessity of oxygenation/ventilation
Patients treated with low-flow oxygenation or non-invasive ventilation
Patients treated with invasive ventilation in the intensive care unit
Statistical analysis
Clinical and demographic variables were described with the use of frequencies and percentages for categorical variables and mean and standard deviation or median and interquartile range for quantitative variables, as appropriate. The differences between groups were evaluated with the chi-square test for categorical variables and with the unpaired Student’s t-test or with the Kruskal–Wallis test for quantitative variables, as appropriate. When more than two groups were available, the analysis of variance (ANOVA) model followed by the Bonferroni test or the Kruskal–Wallis test followed by Cuzick’s test for trend [24] were considered.
To estimate the overall survival, the Kaplan–Meier method was performed, and the log-rank tests were used for between-groups comparisons of the overall survival.
Moreover, the putative risk factors associated with death were evaluated with a univariate Cox regression model. A multivariate Cox regression model was then built according to the best Bayesian Information Criterion (BIC). All variables met the proportional-hazard assumption.
All reported p values are two-sided. p values of less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed with Stata 15 (StataCorp 2017).
Results
We studied 623 subjects: 570 COVID-19 positive, age 64.7 ± 15.6 and male sex 290 (50.9%) and 53 COVID-19 negative controls, age 61.6 ± 13.2 and male sex 33 (15.6%).
No statistically significant difference was found in the distribution of age and sexes in the two groups (p = 0.113 and p = 0.156, respectively).
As regards CALIPER analysis we found a statistically significant (p = 0.0001) difference in the distribution of VRS between COVID-19-positive patients and the controls (median [iqr] 4.05 [3.74] and 1.57 [0.40], respectively). All controls’ ILD percentage was 0.
Of the 570 COVID-19-positive patients, 115 (20.2%) required no hospitalization and spent the whole 30 days of observation in home isolation; 329 (57.7%) were hospitalized and required low-flow ventilation or NIV, while 126 (22.1%) patients required ICU admission and invasive ventilation (Table 1).
Table 1.
Characteristics of the patients
| Variable | COVID-19 group (N = 570) | Control group (N = 53) | p value | |
|---|---|---|---|---|
| Age — years | 64.7 ± 15.6 | 61.6 ± 13.2 | 0.113# | |
| Male sex — no (%) | 290 (50.9) | 33 (62.3) | 0.156† | |
| VRS **— median (iqr) | 4.05 (3.74) | 1.57 (0.40) | 0.0001‡ | |
| ILD **— median (iqr) | 15.34 (28.2) | |||
| TSS **— median (iqr) | 7(5) | |||
| Intensity of care — no (%) | 1 | 115 (20.2) | ||
| 2 | 329 (57.7) | |||
| 3 | 126 (22.1) |
Plus-minus values are Means ± SD
# Unpaired Student’s t-test
† Chi-square test
‡ Kruskal–Wallis test
** VRS (pulmonary vascular-related structures %) is defined as the ratio
between the total vessels volume and the total lung parenchymal volume
ILD interstitial lung disease extent %;
TSS total severity score
1 Discharged at home without the necessity of oxygenation/ventilation
2 Treated with low-flow oxygenation or non-invasive ventilation
3 Treated with invasive ventilation in the intensive care unit
With reference to the severity of care (groups 1, 2, and 3), the age distribution was significantly different (p < 0.0001) between the groups (group 1: 57.2 ± 15.7; group 2: 68.2 ± 15.6; group 3: 62.4 ± 12.5). The pairwise contrasts (with the Bonferroni correction) were all statistically significant (group 1 vs 2 p < 0.0001; group 2 vs 3 p = 0.001; group 1 vs 3 p = 0.024). There was no statistically different sex distribution between the groups (p = 0.052).
ILD, VRS, and TSS showed an increasing trend with the severity of care, test for trend p < 0.0001 (Table 2 and Fig. 3).
Table 2.
Characteristics of 570 COVID-19-positive patients divided according to the intensity of care
| Variable | Group 1 (N = 115) | Group 2 (N = 329) | Group 3 (N = 126) | p value |
|---|---|---|---|---|
| Age — years | 57.2 ± 15.7 | 68.2 ± 15.6 | 62.4 ± 12.5 | < 0.0001† |
| Male sex — no (%) | 57 (49.6) | 180 (54.7) | 53 (42.1) | 0.052‡ |
| VRS** — median (iqr) | 2.31 (2.06) | 4.13 (3.44) | 5.70 (4.53) | < 0.0001# |
| ILD** — median (iqr) | 3.8 (14.2) | 15.6 (27.5) | 29.7 (34.2) | < 0.0001# |
| TSS** — median (iqr) | 5 (3) | 7 (5.5) | 9 (8) | < 0.0001# |
Group 1, discharged at home without the necessity of oxygenation/ventilation
Group 2, treated with low-flow oxygenation or non-invasive ventilation
Group 3, treated with invasive ventilation in the intensive care unit
¶ Plus-minus values are means ± SD
† p value obtained from ANOVA model
Pairwise contrasts (with Bonferroni correction):
group1 vs group 2 (p < 0.0001); group 1 vs group 3 (p = 0.024);
group 2 vs group 3 (p = 0.001)
‡ Chi-square test
** VRS (pulmonary vascular-related structures percentage) is defined as the ratio
between the total vessels volume and the total lung parenchymal volume
ILD interstitial lung disease extent %; TSS total severity score
# p value obtained from Cuzick’s test for trend (23)
Fig. 3.
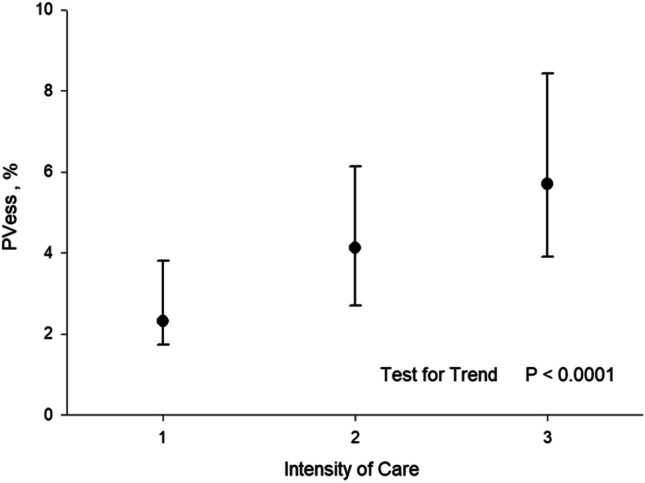
Median and interquartile range of vessels percentage (VRS) according to the intensity of care. 1: Patients discharged at home without the necessity of oxygenation/ventilation. 2: Patients treated with low-flow oxygenation or non-invasive ventilation. 3: Patients treated with invasive ventilation in the intensive care unit. PVess % = VRS %
We also evaluated the correlations (R) between ILD, VRS, and TSS: R (ILD-VRS) = 0.89; R (ILD-TSS) = 0.61; R (VRS-TSS) = 0.64.
Finally, we noted that even the group1 patients had a higher VRS than controls (p < 0.001).
We observed the 570 COVID-19 and they exited between 1 and 72 days (median 32 days). Ninety-eight patients deceased during the observation period (52 males and 46 females, 53% and 47% respectively).
Once the subjects have been divided into two groups with respect to the median age: group young, age < 66 years; group old, age > = 66, we recorded the following distribution of death of the two groups (18 group young and 80 group old, 18.4% and 81.6% respectively).
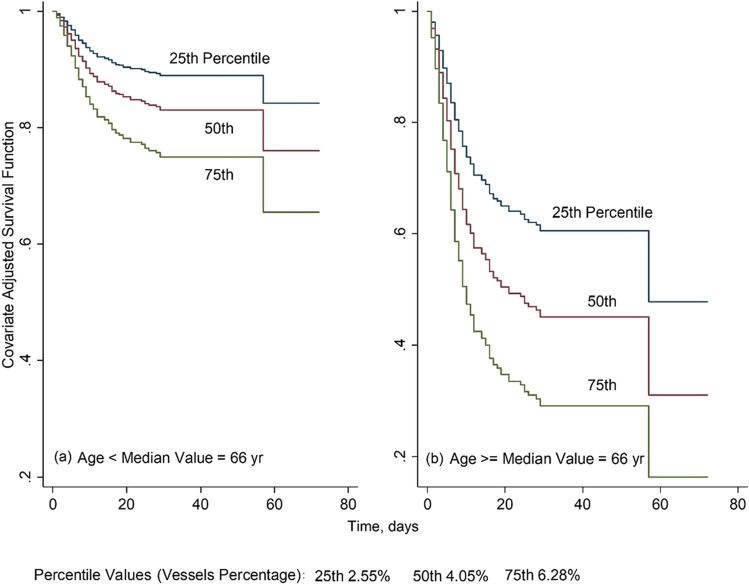
The estimated survival function with the Kaplan–Meier method is shown in Fig. 4. No statistically significant difference was found comparing survival curves between sex groups (p = 0.8 log-rank test) while a statistical difference was found among groups as younger people (< 66 years) had a significantly higher survival (p < 0.0001 log-rank test, Fig. 5).
Fig. 4.
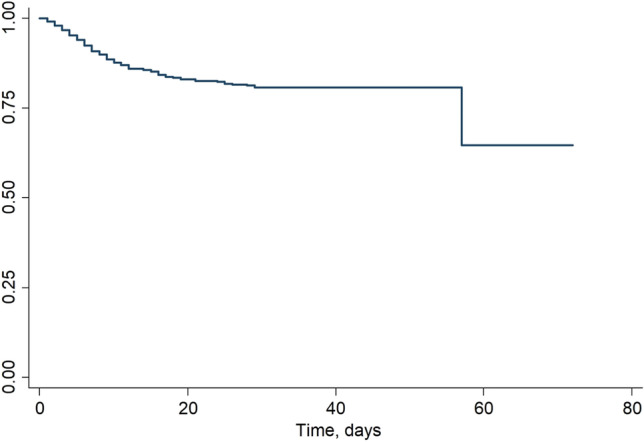
Kaplan–Meier survival estimates in 570 COVID-19-positive patients
Fig. 5.
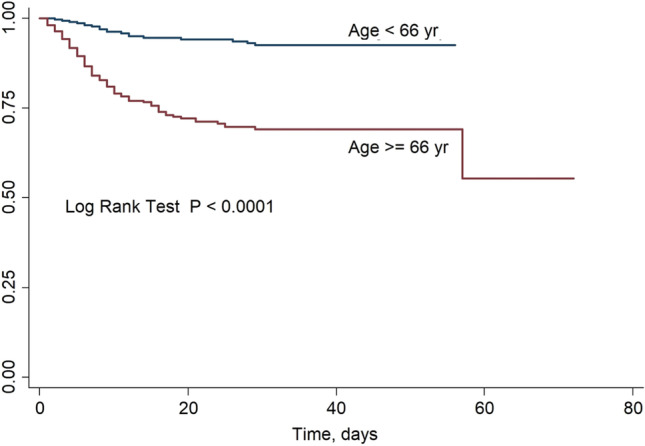
Kaplan–Meier survival estimates in 570 COVID-19-positive patients divided into two groups (age 66 years and age > 66 years)
The putative risk factors (sex, age, VRS, ILD) associated with death were evaluated with a univariate and multivariate Cox regression model; the corresponding hazard ratio and p value are shown in Table 3.
Table 3.
Risk factors considered associated with the survival in 570 COVID-19-positive patients
| Univariate model | Multivariate model* | |||
|---|---|---|---|---|
| Risk factor | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value |
| Age# | 1.06 (1.04–1.07) | < 0.0001 | ||
| median value | Reference | Reference | ||
| median value | 4.99 (2.99–8.33) | < 0.0001 | 4.28 (2.55–7.19) | < 0.0001 |
| Sex | ||||
| Female | Reference | |||
| Male | 1.05 (0.71–1.57) | 0.8 | ||
| VRS† | 1.17 (1.11–1.24) | < 0.0001 | 1.13 (1.07–1.94) | < 0.0001 |
| ILD† | 1.02 (1.017–1.03) | < 0.0001 | ||
| TSS† | 1.11 (1.07–1.16) | < 0.0001 | ||
# Median age in the 570 COVID-19-positive patients: 66 years
* Model selection: best Bayesian information criterion (BIC)
† VRS (pulmonary vascular-related structures percentage) is defined as the ratio between the total vessel
volume and the total lung parenchymal volume
ILD interstitial lung disease extent %; TSS total severity score
In the multivariate analysis, the only independent predictors were age and VRS with an HR of 4.28 [95% CI 2.55–7.19] and 1.13 [95% CI 1.07–1.94], respectively. That is, in our model, the death risk increased by 13% for every additional percentage point of VRS.
Finally, estimated survival curves based on the multivariate Cox model corresponding to specific age groups and VRS percentile values are shown in Fig. 6.
Fig. 6.
Estimated survival curves based on the multivariate cox model of Table 3 for 570 COVID-19-positive patients having (a) age 66 years and specified VRS percentile values and (b) age > 66 years and specified VRS percentile values
Discussion
In our study, the percentage of VRS over the total lung parenchyma was associated with mortality, and its increase corresponded to an increment to the required level of care.
In the multivariate analysis, the independent predictors were age and VRS.
Even with all the caution due above all to the high correlation between VRS and ILD, we can say that VRS maintains its explanatory capacity even considering ILD and TSS.
The CALIPER software has already been widely used in the analysis of the CT features of patients affected by different interstitial lung diseases [25, 26]. A recent study [20] conducted on 105 patients affected by idiopathic pulmonary fibrosis demonstrated a strong correlation between the software-evaluated disease markers (VRS and ILD) and forced vital capacity. To our knowledge, no researcher has so far tried to utilize the software for the evaluation of the CT scans of COVID-19 patients.
SARS-CoV-2 pneumonia, although different in etiology, has a close resemblance to many ILDs, since it features bilateral and peripheral ground-glass opacities, interlobular septal thickening with “crazy paving” pattern and “reversed halo sign” [11–13]. Given these premises, we decided that the CALIPER evaluation of COVID-19 patients was not only feasible but also necessary in order to individuate the risk factors of mortality.
Actually, in our study ILD% was significantly different in the patient groups with different intensity of care, and it was correlated to survival in the univariate Cox model. These results were achieved despite the fact that CALIPER is not able to properly classify areas of lung consolidation, which are a relevant part of COVID-19 pneumonia. We observed that areas of ground-glass opacity in the setting of COVID-19 are correctly segmented and classified by the CALIPER, while consolidative areas that are correctly segmented as lung parenchyma are typically classified as a reticular pattern. This is not unexpected, as consolidation is most similar in density and texture to dense/coarse reticular features in ILD. In areas of very dense peripheral consolidation, these regions can be incorrectly excluded from the lung volume segmentation by the CALIPER extraction process. These CALIPER limitations may explain why ILD% is not significant in the multivariate analysis. We also should consider that the partial exclusion of the consolidation areas in our model decreases the value of total lung volume and tends to overestimate the VRS% and ILD % value in the lung included in the analysis.
However, COVID-19 is a multisystemic pathology that does not affect only the air spaces in lung parenchyma [27]. In particular, a recently published cornerstone study [6] showed that the lungs of patients who died from the disease showed diffuse endothelial damage with diffuse thrombosis and microangiopathy. Most importantly, that study reported a new vessel growth due to intussusceptive angiogenesis that was 2.7 higher than in patients who died from influenza.
In our study, the percentage of pulmonary vascular-related structure over the total lung parenchyma was strongly associated with mortality, and its increase corresponded linearly to an increment to the required level of care. We could suppose that these results may reflect alterations in alveolo-capillary exchanges and blood oxygenation in COVID lung and provide evidence through morphological changes in the vasculature that support the role of angiopathy and neoangiogenesis in the pathophysiology of SARS-CoV-2 pneumonia.
It is also possible that the algorithm to extract the VRS could sometimes include areas of peri-vascular interstitial involvement in addition to the vascular structures. Therefore, areas of COVID-19 interstitial pneumonia or inflammatory changes next to a vessel might be captured as a “VRS” structure, increasing the amount of VRS % value in our results. This is maybe depicted in Fig. 2, where the more consolidated patient on the right part of the image shows VRS which are less linear and less “vessel like” in comparison with the less consolidated patient on the left.
As a result, we can try to explain the high positive linear correlation between VRS and ILD. This is not a novelty in CALIPER studies, since it has already been found in the setting of idiopathic pulmonary fibrosis [28, 29]. Those papers justified the correlation with pulmonary hypertension and to the morphologic alterations of the vascular structure which are typical of lung fibrosis. Both the aforementioned reasons can apply to COVID-19 patients, since a percentage of them can be affected by pulmonary hypertension [30] and vessel distortion can be present. In addition, it may reflect the previously discussed angiopathy and perivascular interstitial involvement, which are expected to be more pronounced in patients with greater ILD% involvement.
The significant increase of VRS % in patients belonging to group 1 (with low ILD involvement and few consolidations) versus normal subjects demonstrated that even if VRS % included part of the interstitial disease, it is a faithful index of the percentage of vascular volume in the normal lung and in the parenchyma affected by ILD. Vascular enlargement and congestion in that critical area in which the blood-air exchanges happen may be the pathophysiological reason for our results. Noteworthy, Menter et al. found diffuse alveolar damage and capillary congestion in the autopsies of COVID-19-deceased patients [31]. Therefore, the possibility to evaluate the volumetric characteristics of vessels in the lung with the CALIPER is particularly relevant in COVID-19 pneumonia. This is visually challenging, and an objective measure of the vascular changes that have clinical significance could be valuable in the triage and treatment of acute COVID-19 patients.
Another factor that may contribute to explain the correlation between VRS, the intensity of care, and mortality is the fact that having been developed for interstitial pneumonia evaluation, the CALIPER does not include areas of thick peripheral lung consolidation in the total lung volumed. As a consequence, VRS may be increased in patients with large consolidative areas, being therefore a biomarker that combines information from both vascular-like structures and consolidation.
Specifically, CALIPER evaluation of the CT scans in COVID-19 patients could help identify those with a higher risk of mortality who therefore need closer attention by the physician. According to our multivariate model, combining VRS and patient age to identify a subset of “high-risk” patients, has the potential to be used as a gatekeeper for earlier/more aggressive therapy. While it is certainly not a parameter to evaluate on its own, it may work in addition with the already known clinical risk factors [32] for an integrative analysis on the model of the one performed by Chao et al. [33]
In our study, we found no significant differences in mortality between the two sexes; we were then unable to reproduce the results reported by Percivale et al. [34]. Our other findings regarding the age and percentage of parenchymal involvement risk factors in mortality are largely confirmatory of the existing literature [30, 35].
Our study has several limitations. The first one is the retrospective nature of the study, in which the enrolled patients presented autonomously to the emergency department during various stages of the disease. This fact likely introduces some bias for more severe cases of COVID-19 or for patients with co-morbidities that would necessitate an emergency department visit. A second limitation is due to the nature of CALIPER, which was developed for evaluation of parenchymal lung disease and vascular-related structures but trained for classification of features in the setting of ILD and COPD. The CALIPER application does not have a “consolidation” class and, in some cases, incompletely extracts the lung parenchyma, particularly in peripheral regions of dense consolidation. The third and last limitation is the lack of laboratory parameters, such as pO2, for correlation and assessment of disease severity at the time of CT evaluation.
Conclusion
Our results indicate that VRS increases with the required intensity of care, and its increase could be a risk factor for mortality. Obviously, given the novel nature of this research, further investigations are needed in order to confirm our results. Additional development of segmentation and classification algorithms to characterize areas of consolidation in COVID-19 pneumonia would allow for a more comprehensive assessment of the pulmonary parenchymal processes and a more accurate determination of percent involvement for cases with dense consolidation.
Abbreviations
- CALIPER
Computer-aided lung informatics for pathology evaluation and rating
- COVID-19
Coronavirus Disease 2019
- ILD
Interstitial lung diseases
- RT-PCR
Reverse-transcription polymerase chain reaction
- VRS
Vascular-related structures
Funding
No funding has been released for this research. This study was conducted by the voluntary contribution of the authors using technology belonging to Azienda Ospedaliero-Universitaria Pisana (Pisa, Italy) and Ospedale Maggiore della Carità (Novara, Italy).
Declarations
Guarantor
The scientific guarantor of this publication is Dr. Chiara Romei.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors, Chiara Airoldi, has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because this research was retrospective and did not alter the treatment or the life of the patients in any way. Enrolled patients were assigned a progressive identification number (ID), which was later used to collect their personal data. This procedure guaranteed anonymity, allowing non-disclosure of sensitive data.
Ethical approval
The internal review boards of Azienda Ospedaliera Universitaria Pisana, Pisa, Italy and Azienda Ospedaliero-Universitaria Maggiore della Carità - Novara, Italy, approved the research, protocol numbers 17368 and CE 130/20.
Methodology
• retrospective
• diagnostic or prognostic study / observational
• multicenter study
Footnotes
The institution from which the work originated is the 2nd Radiology Unit, Pisa University-Hospital, Via Paradisa 2, Pisa, 56,124, Italy
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chiara Romei and Zeno Falaschi contributed equally to this work.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Situation update worldwide, as of 18 October 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed November 2020
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;325:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(1):eabe4724. doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcone M, Tiseo G, Barbieri G, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polak SB, Van Gool IC, Cohen D, et al. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nägele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Xia L. Coronavirus Disease 2019 (COVID-19): Role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020;214:1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 11.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease 2019 (COVID-19): relationship to duration of infection. Radiology. 2020;295:685–691. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endeman H, van der Zee P, van Genderen ME, van den Akker JPC, Gommers D. Progressive respiratory failure in COVID-19: a hypothesis. Lancet Infect Dis. 2020;20(12):1365. doi: 10.1016/S1473-3099(20)30366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel BV, Arachchillage DJ, Ridge CA, et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202(5):690–699. doi: 10.1164/rccm.202004-1412OC.PMID:32667207;PMCID:PMC7462405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciccarese F, Coppola F, Spinelli D, et al. Diagnostic accuracy of North America expert consensus statement on reporting CT findings in patients with suspected COVID-19 infection: an Italian single center experience radiology. Cardiothoracic Imaging. 2020;2:4. doi: 10.1148/ryct.2020200312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanza E, Muglia R, Bolengo I, et al. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur Radiol. 2020;30(12):6770–6778. doi: 10.1007/s00330-020-07013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pu J, Leader JK, Bandos A, et al. Automated quantification of COVID-19 severity and progression using chest CT images. Eur Radiol. 2021;31(1):436–446. doi: 10.1007/s00330-020-07156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romei C, Tavanti LM, Taliani A, et al. Automated computed tomography analysis in the assessment of idiopathic pulmonary fibrosis severity and progression. Eur J Radiol. 2020;124:108852. doi: 10.1016/j.ejrad.2020.108852. [DOI] [PubMed] [Google Scholar]
- 21.Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans (2020) https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance/. Accessed 03/01/2021
- 22.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Predicting outcomes in idiopathic pulmonary fibrosis using automated CT analysis. Am J Respir Crit Care Med. 2018;198:767–776. doi: 10.1164/rccm.201711-2174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K, Fang Y, Li W, Pan C, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 25.Jacob J, Hirani N, van Moorsel CHM, et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J. 2019;53(1):1800869. doi: 10.1183/13993003.00869-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Automated computer-based CT stratification as a predictor of outcome in hypersensitivity pneumonitis. Eur Radiol. 2017;27(9):3635–3646. doi: 10.1007/s00330-016-4697-4. [DOI] [PubMed] [Google Scholar]
- 27.Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology (Bethesda) 2020;35(5):288–301. doi: 10.1152/physiol.00019.2020.PMID:32783610;PMCID:PMC7426542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crews MS, Bartholmai BJ, Adegunsoye A, et al. Automated CT analysis of major forms of interstitial lung disease. J Clin Med. 2020;9(11):3776. doi: 10.3390/jcm9113776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Evaluation of computer-based computer tomography stratification against outcome models in connective tissue disease-related interstitial lung disease: a patient outcome study. BMC Med. 2016;14(1):190. doi: 10.1186/s12916-016-0739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106(17):1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao H, Fang X, Zhang J, et al. Integrative analysis for COVID-19 patient outcome prediction. Med Image Anal. 2021;67:101844. doi: 10.1016/j.media.2020.101844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Percivale I, Danna PSC, Falaschi Z, et al. Men and women affected by Sars-CoV-2 pneumonia: same CT features but different outcome. Clin Radiol. 2020;11:S0009-9260(20)30629-2. doi: 10.1016/j.crad.2020.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 inWuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]