Abstract
Polydimethyl siloxane (PDMS) has been used extensively for microfluidic devices due to its chemical properties allowing for rapid molding and versatile biological application. Soft lithography based PDMS fabrication primarily comprises casting from patterned photoresist on a silicon wafer. The patterned photoresist is often replaced with the cast PDMS as a more durable template mold for final PDMS fabrication that is less fragile and expensive. PDMS-PDMS double casting prolongs the longevity of soft lithography molds and reduces overall costs to microfuidic applications. A common end to the lifetime of PDMS negative masters is the risk of bonding between the replicate and mold and distorted topographrical features. This review examines common chemical and physical debonding approaches between PDMS-PDMS castings to exend the lifetime of PDMS masters.
1. Introduction
Polydimethylsiloxane (PDMS) is the most commonly used elastomeric material for the rapid fabrication of devices for biological applications in microfluidics1–7, to mechanical and chemical applications such as lubricants8, anti-foaming agents9, ant-flea treatments10, and conditioners for hair products10. PDMS is widely accessible in part due to its broad temperature range for fabrication which can be initiated with a catalyst at room temperature or accelerated at higher temperatures with lab-standard hot plate or oven. Further PDMS has few requirements for specific instrumentation, primarily being a vacuum chamber or pump to degas the chamber. PDMS fabrication is also amenable to many existing techniques to enable low-cost, rapid prototyping2. In the biomedical realm, PDMS devices have enabled the miniaturization of laboratory diagnostics and modeling of complex biological systems as consolidated platforms comprising fields such as biomedical microelectromechanical systems (BioMEMs) and lab on a chip (LoC) devices11. The technology underlying BioMEMs facilitate modeling of complex biological systems (e.g. organ-on-a-chip) by spatial localization of cells and cell derived signals in arrangements and levels that recapitulate natural physiology. High throughput is achieved by redundant designs such as parallelized structures for multiplexing experimental conditions. Further low volume designs allow precise manipulation of liquid reagents and volumes to allow higher than physiological concentrations at the micro, nano, and pico scale to increase limits of detection 2. Through these features, bioMEMs and LoC devices greatly advanced research in modeling complex in vivo systems such as fluid dynamics within blood vessels11, biochemical synthesis12,13, and autonomous complex assay protocols1.
PDMS is the primary substrate for bioMEMS due to several advantages including being chemically inert, biocompatible, thermally stable properties2, and 3D patterning at cell-scale dimensions of microns or smaller14. These advantages allow for PDMS to interact with biological tissues and fluids with minimal risks of direct material induced effects on associating cells. PDMS resolves micron scale features to mimic complex biological structures 12,15. Pre-polymerized PDMS is viscous and conformable; it can be cured upon a master mold to generate a negative impression of mold topological features. Typically, these master molds are patterned photoresist on silicon wafers16–18, which is an established technique in engineering solid state devices. While there are many advantages to being able to use photoresist directly for bottom-up fabrication including high spatial resolution and fidelity of feature transfer, there are still significant challenges namely photoresist fragility. Deterioration of photoresist masters such as SU8 has been reported in as little as five replication cycles19.
Soft lithography by PDMS-PDMS casting circumvents mechanical vulnerabilities of photoresists. Structures in patterned photoresist can damage with release of the cured PDMS replica18. As reported by several groups, photoresist masters suffer from fragility and gradual loss of fidelity over time as the photoresist is used to cast replicas16,20,21. Further, both the high price and time associated with fabrication make it difficult to continually replace them if they have features crack, delaminate, or even shatter after casting 14,16–18,20–22. Protective coatings and baking strategies can strengthen photoresist structures; however, these stabilization techniques are not sufficient to protect from damage from repetitive use as a template mold. To address these shortcomings, PDMS masters can bear the replicative burden and can be remade as needed. To prolong the longevity of the original master, the PDMS replica of the photoresist can act as the master for PDMS “double casting” until it is no longer faithful to the original mold through deformation or destruction. The original photoresist master will generally be designed with the scenario of the PDMS replica, a negative cast, as the master rather than final product though alternatively the first replica can act as an intermediate master and the serial second replica can act as the ‘final’ master that is identical to the original photoresist master. Then as needed a new PDMS master can be generated from single or serial casting directly from the photoresist master14,16,19,22. In these scenarios, the photoresist master is much less prone to damage by infrequent use. In order to employ a PDMS master, the double casting technique depends on efficient separation of the cured PDMS mold from the PDMS master, which is non-trivial as PDMS tends to bond easily to other silicon containing materials such as glass and PDMS. Strategies for passivating the PDMS layering either permanently or transiently decrease stiction and bonding between two PDMS layers, to allow for cast PDMS release while preserving the precise feature of the photoresist master.
Controlling surface interactions between adjacent layers is critical to fabrication of multilayer BioMEMS devices. Several molding techniques to develop complex microfluidic devices involve serial layering which interact to enable e functional valves, mixing chambers, altered flow dynamics, and a variety of automation and control over the microfluidic system1. The bonding affinity is a major advantage to using PDMS fabrication of bioMEMS devices. However, in the case of these PDMS-PDMS double casting, it is crucial to block bonding between PDMS master and cast replica. Techniques that passivate these PDMS layers can differ greatly in the way that they introduce a “spacer” layer of treatment or functionalization between the two layers of PDMS. This “spacer” layer prevents the bonding of the two layers together while the replica layer of PDMS cures to solid form. Bonding can be mediated the migration of uncrosslinked PDMS oligomers to the surface boundary between the two PDMS layers. The spacer layer impedes uncrosslinked PDMS from both layers from crosslinking to each other and bonding. Both gas phase functionalization, including plasma treatment, and liquid phase treatments with various chemicals have been used to passivate surfaces.
Much of the information on debonding strategies for soft lithography are scattered across peer-reviewed publications and non-academic sources such as blogs and message boards, making it difficult to locate information for optimized techniques. Company websites for microfabrication instrumentation provide useful tips on debonding such as the case with manufacturers Diener and Thierry for their ion etch and oxygen plasma machines. These websites can be invaluable to novice and experienced users alike as they provide insight that may be either too banal or esoteric to be included within the literature. Blogs such as Researchgate, the Microfluidic Circle, Chips and Tips, StackExchange, as well as the manufacturer’s websites such as Henniker Plasma, Harrick Plasma, Thierry, and Diener each have information to troubleshoot PDMS applications. Each of these resources are invaluable for both naïve and veteran researchers as the information contained in them range from extremely basic to highly specialized information on wattage, percent composition of gasses, and even vacuum pressure within plasma chamber. This information is not as obvious within literature. This lack of review makes it more challenging for new researchers to enter into the field of microfabrication and microfluidics.
Here, we review debonding approaches as a central resource for bioMEMS investigators. We further discuss relevant metrics and engineering tools for comparative analysis of efficacy with the intent to help readers find techniques which would work best for their needs. The reviewer covers most common methodologies for double casting, as well as comparative analysis of each modality. As the primary application for PDMS in microfluidics is bioMEMs, we shall pay special focus on biocompatibility that the debonding in the double casting process does not restrict from biological systems or is biologically toxic.
PDMS has a low surface energy and usually requires surface modification to facilitate bonding to other substrates and plastics. Generally, these different chemical modifications focus primarily on hydrophilic modification, which attaches polar moieties upon the surface, and amphiphilic modification which have both hydrophilic and hydrophilic moieties. These double casting techniques can be broadly be separated into a few categories for comparison and understanding: 1) silanization techniques5,23,24, 2) fluorocarbon treatments19, 3) polymer coating treatments18,25, and finally 4) other miscellaneous treatment modalities, which do not fit among the other categories14,22.
2. Surface Functionalization
PDMS bonding strength is primarily governed by chemical (adhesion, reversible and irreversible bonding, and completeness of curing) and physical interactions (steric, friction, surface roughness) at the interface of the layered PDMS. Interfering with these interactions through changes to the surface chemistry, physical properties, or even passivation layers all allow for less adhesion between the two PDMS layers.
2.1. Chemical Functionalization Techniques:
2.1.1. Silane based Chemistry:
Silanization adds silanes on the PDMS surface via molecular self-assembly into a monolayer, passivating the surface from PDMS-PDMS bonding.
There is a wealth of literature on silane-based passivation treatment for PDMS. Treatments such as trimethylchlorosilane5,18,23,26 (TMCS), octadecyl trichlorosilane (OTS)27–29, tridecafluoro-1,1,2,2- tetrahydro octyl 1-tricholosilane24,30 (TFOCS) or (PFOS)31,32, 1 H, 1 H, 2 H, 2H- perfluorodecyltricholorosilane (FDTS27–29/PFDTS33) can be applied as an interspacing layer of silanes between the two PDMS layers to prevent them from crosslinking or bonding to each other. Each of these techniques have been applied to the treatment of PDMS separately. TMCS5,18,23,26 has been previously used to develop high fidelity hydrophobic surfaces for preventing bonding between PDMS layers23 or between extremely thin pieces of PDMS and the silicon mold themselves5 while OTS27–29 belongs to a large family of trichlorosilanes that can be used to functionalize surfaces and decrease the friction due to adhesion upon microscale surfaces27. TFOCS24,30/PFOS31,32 has been used with SU-8 photoresists to debond from PDMS24 but has also been used to treat other surfaces as well to prevent PDMS bonding. Also within this family of silanes is FDTS27–29 /PFDTS33 which bridges the divide between two commonly used surface functionalization techniques for debonding treatment, and thus has strengths of both systems. As FDTS is a trichloro functionalized silane, it adheres and self-assembles to the PDMS and the silicon surfaces spontaneously and reliably, as do most silane treatments upon silicon-based surfaces. However, FDTS is a fluorinated molecule and as such allows for the formation of extremely hydrophobic surfaces. It is also been shown that FDTS can enable physical nano structuring on multilayer surfaces as water content in the solvent may enable evaporation based nucleation of polymerization33, which in non-uniformities in the surface that can be seen in SEM but that are noticeably absent in the absence of water and also on monolayer surfaces. This roughness in addition to the hydrophobicity it confers both serve to repel bonding and has been used as a passivation coating for nanolithography as well as double casting.
Silanes such as 3- Aminopropyl triethoxysilane (APTES)34–38 and 3-Aminopropyl trimethoxysilane (APTMS)39 are popularly used for treatment of silicon laden surfaces like glass17,40,41. APTES is typically used to bond PDMS to other materials such as plastics and other silicon containing products due to its reactive amine functionalization and not typically used as a passivation layer. However, by the same rationale, two-step reactions can conjugate APTES to passivating molecules to mask the surface of the PDMS master to the replica. Silanes such as the ones used in these passivation treatments involve pre-treatment of the PDMS surface with oxygen plasma to add hydroxyl groups onto the surface for reaction with the silane. As silanization has been well characterized in the literature for passivation of photoresists42, as well as surface functionalization of glass40,41,43 and other silicon containing materials such as silicon masters42, its translation to double casting does not require any more materials or any altered protocols.
Silane chemistry has been well established in the literature44–47 as a chemical modification technique that is able to alter silicon surfaces such as glass and PDMS to fit whatever functionalization is necessary for the situation. As both PDMS and the silicon wafers are both able to be altered through silane chemistry, the ease of implementation allows for the large popularity of silane chemistry to double casting techniques. As there exists many different silanes with different functional groups, silanes afford a great deal of flexibility and customization to the passivation of the PDMS- PDMS interface.
2.1.2. Fluorine-based Chemistries:
Fluoride containing compounds can functionalize a surface to prevent adhesion between PDMS layers. In addition, there are many techniques for fluoride surface functionalization: from surface plasma treatment with CF4 and hydrogen gasses to generate a temporary fluorinated layer48, to permanent functionalization process using a coating of perfluorocyclobutane, also known as octafluorocyclobutate (C4F8). In the case of octafluorocyclobutate, it can be employed to reduce adhesion and serve as a debonding layer49–52 especially in PDMS molds with high aspect ratios. These fluorinated layers can also be applied with FDTS as mentioned previously. Fluorinated substrates have lower surface energies53, and reduce friction on the surface, decreasing adhesion between the PDMS layers52,53.
Fluorination techniques typically require either plasma treatment with different fluorinated gases, or a wet treatment with fluorinated silane or other alkanes. These gases can be harmful either to personnel or the environment, especially CF4 which can thermally degrade into carbon monoxide and can also generate hydrogen fluoride in presence of water, resulting in hydrofluoric acid which is extremely dangerous. Thus, some fluorination techniques using silicon and fluorine groups in liquid phases may be much safer than some gas phase treatments.
2.2. Non-Chemical Treatments:
2.2.1. Polymeric-based Modifications:
PDMS release from a master generally involves a protective coating on the photoresist master. For biological applications, an inert coating potential downstream toxicity by carryover on to the PDMS. Aside from traditional surface functionalization techniques, anti-stiction of the PDMS surface has been done using various polymeric coatings such as polydopamine, Parylene C, and Polyethylene Glycol (PEG). The biocompatibility of these techniques is high as the materials are relatively inert upon deposition, and the coating mechanisms are spontaneous reactions that merely require the application of the polymer layer to the PDMS surface. Biocompatibility is well-tested in polymers such as parylene and PEG that are chemically inert.
Polydopamine is biologically sourced and has a low risk of toxicity for bench top studies. Polydopamine forms a spontaneous debonding layer after immersion of the PDMS into the solution without the need for plasma treatment or any other chemicals17,25. A literature search did not identify a study that evaluated the shelf-life of the coated surface or its stability with repeated PDMS casting; however, its ease of application and low cost make it an amenable approach to bioMEMs. Similarly, hydrophilic hydroxypropyl-methylcellulose (HPMC) is a semi-synthetic derivative of the polysaccharide, cellulose. It can be used to resolve feature sizes of 50 microns through treatment of the PDMS master with a citric acid and HPMC solution 14. This allowed for uniform soaking and distribution of the HPMC. The mass percentage of the HPMC is designed to increase the viscosity of the surface treatment, while maintaining a thin layer upon the PDMS. Under these conditions, reproducible batches of PDMS masters were made which only differed by 4% maximally even after 4 replicas were made. This has also been done with a solution of 5 mM phosphate buffered solution (pH of 3) containing 0.1% (w/w) HPMC for ten minutes after which it was thoroughly rinsed with DI water and then dried with nitrogen16. Using SEM, the investigators were able to surmise that the thickness of the HPMC coating was below 1 micron as the microstructures on the silicon due to repetitive oxygen plasma treatment were still visible upon the PDMS masters. Biologically sourced polymers such as polydopamine and HPMC form a spontaneous biocompatible anti-stiction coating just by immersion that circumvent toxic reagents 17,25.
The parylene C which is a polymer comprised of poly(para-xylylene) is a common debonding material widely popular by its ease of use and inert chemsitry18. This treatment is easily applied using chemical vapor deposition and has been used extensively to coat silicon wafers and PDMS. Parylene has favorable properties such as optical clarity, chemical inertness, hydrophobicity, and is a biocompatible chemical18. The chemistry has been shown to be transferrable to coating PDMS, as the machinery and technology all already exist, and as PDMS has previously been shown to be capable of silanization7,54. Similarly, polyethylene glycol (PEG) have been used as a protein anti-stiction layer in a variety of biomedical applications 55–57. As a debonding layer, PEG-coatings allows PDMS-PDMS casting of high fidelity microneedles at sub-millimeter to millimeter scale 57. As PEG is used for clinical applications, it is a safe and effective long-term surface treatment for bioMEMs 2.
2.2.2. Solvent, Thermal, and Physical-based Modifications:
Some debonding strategies use solvents such as alcohols, (low) temperatures, or surface roughness to maintain the hydrophilicity of plasma treated PDMS. Immediate immersion of the plasma treated surfaces in methanol and ethanol solvents has been used as an alternate method for depleting PDMS short chains that can modify the surface by replacing hydroxyl groups. As the short chains of untreated PDMS would be leached out from the bulk to dissolve directly into the alcohol, they cannot replace the hydroxyl group laden PDMS surface, retaining the hydrophilic surface. Then, when exposed to further PDMS casting cycles, the hydrophilic surface of the alcohol bulk-passivated PDMS prevents adhesion to the PDMS replica layer 22.
Other investigators have tried to maintain surface chemistry after plasma treatment using thermal manipulation. The oxygen plasma treatment functionalizes the surface with hydroxyl groups which increases the overall hydrophilic surface. This hydrophilicity is transient as the functionalized silane groups will be replaced by the non-functionalized bulk material reverting it back to its previously hydrophobic nature. One particularly simple methodology to reducing this hydrophobic reversion is placing the plasma treated PDMS samples into a freezer to slow down the diffusion of the small molecular weight PDMS chains to the bulk, thus preventing loss of hydrophilicity58. Alternatively, heating techniques crosslink free oligomers in PDMS as well as alter physical properties of PDMS. In one study, the PDMS master was thermally aged to generating a much stiffer PDMS material than the secondary PDMS replica layer. After baking the PDMS at 100°C for 48 hours, the low molecular chains that usually allow for reaction and binding to the surface of the PDMS were crosslinked and thus could not bind as strongly to the replica layer of PDMS59. The debonding layer between the PDMS molds did not have any chemicals or treatments, the double casting method allowed for immediate generation of three-dimensional cell suspensions within the mold without concern about toxicity or biocompatibility.
In contrast to techniques which add layers to the surface to prevent the bonding of the two PDMS layers the physical roughness of the surface can be altered directly to minimize the stiction between deposited materials. In one study, the surface roughness of the photoresist master was altered by using a RIE to etch the photoresist surface on a silicon wafer using sulfur hexafluoride (SF6) 60 as well as similarly using CF4 gases to pit the surface and even functionalize a passivation layer48. As the photoresist will not etch homogenously, the surface will be pitted and roughened based on the exposure time of the RIE. The authors show increasing roughness with longer etching time enhances debonding between deposited layers. The surface properties can effectively be altered without chemical functionalization60. While amenable to many materials, the roughness approach extends the lifetime of the photoresist master rather than the PDMS directly.
These techniques passivate the PDMS surface without complex layers of chemical reagents, and rather maintain an adhesive barrier through plasma or physical treatment. As many of the other chemical techniques require plasma treatment to pretreat the PDMS before any further modification, techniques that do not utilize chemical reactions in order to passivate are much easier to implement as they do not require any additional reagents, and can effectively be passivated through use of placement in the freezer, thermally aging, or through immersion in alcohols. In addition, as these processes do not functionalize the surface, these techniques could even be used in conjunction with chemical techniques if need be, allowing for long term storage of samples until functionalization with another technique. Since there are not any chemical modifications here, these described passivating approaches can be transient eventually reverting to a hydrophobic surface. Thermal aging elicits a more stable modification, at the cost of altering the physical parameters of the PDMS mold. Immersion in alcohols does not permanently alter the surface chemistry but would require several rinses and drying steps to make sure that no alcohols remain within the PDMS which could be toxic to cells downstream.
3. Comparison of Modification Techniques:
The mechanism (chemistry) for debonding varies across the surface treatments. While treatments such as fluorine and silane chemistries alter the surface to add groups that are highly hydrophobic, many of the polymeric treatments as well as the “miscellaneous treatments” aim to attach a anti-stiction layer upon the surface of the PDMS to prevent adhesion between the two layers. In contrast to the more chemical intensive functionalization techniques, the polymer and solvent techniques primarily make the surface less reactive to the silinol chemistries that the PDMS would use to bind to each other. As such, comparing these surfaces must be done on parameters that would be consistent across all their different chemistries. Parameters such as ease of application, biocompatibility, techniques used for verification, as well as resolution limits for each of these techniques to compare these techniques accurately and fairly.
3.1. Ease of Applications:
Each of these different surface treatments for effective PDMS double casting have varying degrees of ease in applying the debonding layers, in terms of required instrumentation, technical expertise, and reagents. In the chemical modification techniques, some specialized instrumentation is required for chemical vapor deposition and oxygen plasma treatment. The required instruments are generally available to most MEMs cleanroom facilities. While the liquid immersion (e.g. polydopamine) may appear to be the simplest in application, the effectiveness of the debonding layer must be taken into consideration as discussed further on when selecting a debonding treatment. Relative to access to necessary equipment and safety measures, the debonding techniques are generally amenable to most novice users with training and protocols.
3. 2. Biocompatibility:
The discussed debonding techniques were identified to be suitable for bioMEMs. A crucial parameter for PDMS-PDMS double casting for bioMEMs applications is biocompatibility. This requirement predicates that any additive that is used to passivate the PDMS surface does not cause toxicity for the PDMS replicates that will eventually interface with cells in the microfluidic devices. These surface modifications techniques have been investigated for cellular toxicity using live dead staining with calcein, propidium iodide, and ethidium homodimer59 61. An ex vivo assay for fish embryo toxicity provides a physiologically relevant assessment of complex biocompatibility that not obvious by cultured cells alone 62–64. For new derivations of surface modifications, it is important to investigate the biological effects on cells, as downstream effects of the chemicals used could result in non-compatible environments for cell culture and experimentation.
3.3. Verification Techniques:
There are several analytical strategies to characterize the surface coverage and the lowering of bonding strength. Debonding tests have been conducted showing using Atomic Force Microscopy (AFM) cantilever beam adhesion and using passivated coatings to reduce the amount of energy needed to detach the beam from the surface48. Other tests for delamination include peel tests65 and other mechanical testing such as compression and young’s modulus61 measurements. Tests for verifying surface chemistry and stiffness involve X-ray Photoelectron Spectroscopy (XPS)14,66, Fourier Transform Infrared Spectroscopy (FTIR)67,68, and AFM69,70 most commonly. This allows for validation of functionalization as well as confirmation that there are no unknown or unexpected reactions happening on the PDMS surface which could affect biocompatibility or device fabrication, or in the case of changes to the stiffness of the PDMS- fluid profiles. Scanning Electron Microscopy51,71–73 (SEM) can also be used in place of, or to supplement AFM in quantifying the surface features, dimensions, and properties.
3.4. Resolution Limits:
Resolution limits are different for each modification or technique. Many papers that go into depth about their resolutions when using the double casting methods do not speak of the limits that their debonding technique can reach but rather the resolution that they could achieve in their hands. Using that metric, chemical techniques such as silanization and fluorination techniques typically can resolve device resolutions on the scale of 20 microns19. In contrast, polymeric treatments such as polydopamine show resolution limits for devices of 10 microns74, while parylene has been shown on scale of 10 microns26. Treatments using alcohols or heat treatments or solvents report single micron resolution22 which may be reaching the nanoscale and the limits of what patterns can be transposed to PDMS. These debonding treatments do not add appreciable thickness to the PDMS surface which would otherwise impede the transposable features for soft lithography PDMS casting. Investigating the resolution limits of each of these techniques can help researchers decide which treatment technique would be suitable for their needs and is a direction that our field should expand into.
With each treatment, the debonding efficacy can affect the resolution of features sizes with repeated PDMS-PDMS casting. Some of these techniques show the casting resolution changes with each repetitive casting14,16,19. Our lab has also evaluated HPMC treatment techniques by SEM to show successful non-destructive debonding with repeated PDMS-PDMS double casting (data not shown).
3.5. Double Casting Methodology:
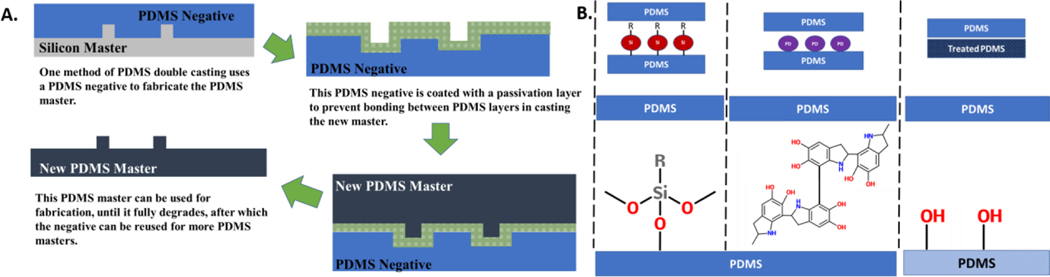
Deterioration of photoresist masters such as SU8 has been reported in as little as five replication cycles19. Double casting with PDMS extends the number of replicates using PDMS as master. A cast of the master generates a negative pattern which can be referred to as a first replica. This first replica is coated with the debonding layer, and then cast with PDMS again to generate the second replica which is an exact copy of the master19. Passivation of the first replica can be facilitated by HPMC14, parylene26, thermal aging59,75 and solvent treatment22 as summarized in Figure 1. Subsequent steps are the same.
Figure 1:
Schematic of PDMS double casting using derived from an initial photoresist mold and examples of functionalization techniques for debonding layering. In double casting the PDMS negative is used as an intermediary to produce PDMS masters which can be used until deterioration. The PDMS negative can then be used to produce further copies of the PDMS master until deterioration which both multiplies the number of masters and extends the lifetime of individual PDMS molds. The debonding layer (in green) can be generated through a variety of chemistries (B) such as silane, polymers such as polydopamine, or even treatments such as heat or alcohol washes.
We have summarized the above discussions (Table 1) to allow for ease of comparison between the different functionalization schemes.
Table 1:
Comparison of Debonding Techniques
| Technique | Functional Group of Interest | Examples of Technique | References | Requires O2 Plasma Pre-Treatment? | Chemical Treatment? | Biocompatible? | Known Number of Consecutive Castings | Advantages | Weaknesses |
|---|---|---|---|---|---|---|---|---|---|
| Silanization | Silicon chemicals acting as a coupling agent or to lower surface energy | TFOCS/PFOS, TMCS, OTS, FDTS | 22,28–30; 5,18,21, & 24; 25–27; 25–27, 31 | Yes | Yes | Not universally; ideally should not affect replica | Not seen in the literature | Well characterized chemistries; Spontaneous self-assembly for monolayer coating; Flexibility of functionality | May be hazardous (lack biocompatibility if there is carryover) and requires prior plasma treatment |
| Fluorination | Fluorine Groups to lower surface energy | CF4, C4F8, FDTS, Surface Roughness Treatments with SF 6 or CF4 | 36;37–40;25–27, 31;47–48 | Yes | Yes | Not universally; ideally should not affect replica | Not seen in the literature | Well characterized chemistries; Spontaneous self-assembly for monolayer coating; Flexibility of functionality | May be hazardous (lack biocompatibility if there is carryover) and requires prior plasma treatment |
| Polymer Treatments | Inert polymer coating to prevent adhesion | Polydopamine, Parylene C, PEG | 17,23,61;18;43–45 | No | Yes | Yes, all of these are biocompatible | 30 castings for polydopamine (23) | Directly biocompatible; No pre-treatment (plasma) Do Not Require Constant Recoating to Maintain Anti-stiction | Limited formulations tested |
| Miscellaneous Treatments | Non-chemical treatments that add in a separation layer or affect the physical interface between the two PDMS layers | Alcohol Treatments, HPMC, low or high temperature treatments | 19;14,16;46,47; | No | No | Yes, all of these are biocompatible | Not seen in the literature | All Lack Chemical Treatments-Only Requiring Heat/Cold/Alcohols as Techniques Do Not Coat PDMS, They Are Biocompatible and Do Not Change Resolution of Printing | Many Can Revert Hydrophobicity May Alter Stiffness of the PDMS Limited Options for Non-Chemical Treatments |
4. Main Take-Aways and Conclusions:
PDMS double casting is a useful technique that benefits from modifying surface chemistry. Defining the surface chemistry of PDMS masters controls adhesion between the PDMS master and replicates. The stability of the surface functionalization and integrity of patterned features is critical to increasing the repeat use of PDMS masters. In the increasing complexity of bioMEMS systems, adjacent concerns include biocompatibility, ease of application, as well as debonding efficiency.
Ideally, several features should be achieved from a debonding strategy: minimal force is required to release the replicate layer of PDMS, negligible alteration to surface roughness and patterned topography, debonding layer is uniform, and the debonding treatment is stable or can be regenerated to maintain the debonding layer and integrity of the PDMS master. In contrast, concerns of the debonding layer include: deterioration of the debonding layer, detachment of the debonding layer with the replica; build-up of PDMS or conversely removal of PDMS with casting, surface roughening, dust attraction to the passivation layer; inefficient release based on surface area (high aspect ratio) or nanotopography; and inefficient chemical debonding and anti-stiction. To our knowledge no one has considered the debonding layers effects on physical deterioration of the PDMS with time due to for example changes in water content, gas permeation and last repeated casting where physical stress from bending/peeling leads to hysteresis that gradually alters the master surface.
Debonding extends the lifetime of PDMS masters. PDMS double casting generates a PDMS master to substitute the fragile photoresist master. Photoresist masters are known to crack or delaminate in 1–5 casts. This PDMS master can cast several replica before it is eroded or worn down showing negligible change in feature dimensions even up to 10 consecutive casting19. This strategy preserves the photoresist master and PDMS intermediary master by limited use. As such double casting has benefits in reducing costs and overhead in PDMS device fabrications without risk or loss of feature resolution.
Biocompatibility is generally accepted to preserved for PDMS based fabrication of bioMEMs devices. With the application of new improved debonding layers, characterization of the PDMS surface after double casting techniques ensures no transfer of surface chemistry (or alterations to the replica surface chemistry) that negatively affect biological interactions including cells. We have described approaches to confirming cell viability.
Surface chemistry characterization can be done by FTIR, XPS, and (water) contact angle measurements to name a few. While surface chemistry characterization is not a focus of this article we direct attention to excellent reviews on surface chemistry76,77 analysis.
There remains a gap in the literature comparing the relative longevity of each of these techniques, and showing which ones allow for the greatest increase in longevity of the master molds. There has not been a definitive comparison of techniques that chemically alter the surface, techniques that coat the surface in polymer without chemically reacting with the surface, and techniques that physically alter the surface through solvent or thermal techniques. Knowing the amount of cycles that the master and replicas could be used for, would give direction on which techniques would be ideal for application. While current methodologies for double casting may not need this knowledge to apply these techniques to improve their fabrication set-up, more complex techniques and niche applications would need to have this information to maximize their design. To summarize, any of these techniques could be ideal for the intended application due to the unique parameters the user may need.
When prioritizing a PDMS passivation treatment, the transmission of the original patterning is the most critical feature. High aspect ratios, complex designs, roughness, and surface area may all affect the integrity of debonding. Distinct material types between PDMS master and replica will present new challenges as well. For example, for applications with living cells, there is a greater interest in altering mechanical properties of the underlying synthetic substrate. Peeling force and stiction may require more attention with distinct formulations of elastomeric replicas and PDMS master mold. Debonding layers should be considered also for heterogeneous interfaces as well. In all cases, debonding longevity must be evaluated. Some treatments such as polydopamine coating, and solvent treatments can be regenerated with periodic treatments to replenish the anti-stiction layering. In contrast, techniques such as thermal treatment may permanently alter the physical properties of the master mold. These permanent physical changes in the PDMS master could become important for molding in terms of feature or rigidity, which could affect longevity of the molding, as well as the faithfulness of the molding design. Lastly, knowing what the order of resolution magnitude of your needed feature dimensions are can also widen or restrict your choices for ideal debonding layer.
To conclude, PDMS double casting techniques allow for inexpensive generation of masters without the need for repetitive use of more fragile silicon or photoresist masters. These techniques utilize various chemistries to passivate the PDMS- PDMS bonding interface, and as such, these different chemistries have their own advantages and disadvantages when it comes to ease, repeat use, and resolution. To decide which technique is ideal for the application, it is important to understand the different classes of techniques as well as the application that the researcher needs. Further characterization of these techniques will allow for users to decide which double casting techniques would be ideal for their own use, enabling lower cost of entry into the microfluidic field as well furthering knowledge about surface treatments of PDMS for microfluidic processes. In addition, the easier the entry in the field of bioMEMs, the closer that our field moves toward solving clinical challenge using for example microfluidics for drug treatment, personalized medicine, and organ-on-a-chip systems. Hopefully with the influx of new users and the different expertise that they bring, some of the largest questions and problems in our field can be solved.
Acknowledgements
This work was supported by Faculty Investment Fund RES221997 from Case Western Reserve University (CWRU) (S.E.S), NIH NHLBI SPN00586 (S.E.S), NIH 1 C06 RR12463-01, and 5T32HL134622 (A.A.). We thank Chao Liu of the Senyo laboratory for helpful edits.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Watson C. and Senyo S, HardwareX, 2019, 5, e00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthier E, Young EWK and Beebe D, Lab Chip, 2012, 12, 1224. [DOI] [PubMed] [Google Scholar]
- 3.Toepke MW and Beebe DJ, Lab Chip, 2006, 6, 1484–1486. [DOI] [PubMed] [Google Scholar]
- 4.Halldorsson S, Lucumi E, Gómez-Sjöberg R. and Fleming RMT, Biosens. Bioelectron, 2015, 63, 218–231. [DOI] [PubMed] [Google Scholar]
- 5.Tonin M, Descharmes N. and Houdré R, Lab Chip, 2016, 16, 465–470. [DOI] [PubMed] [Google Scholar]
- 6.Ansari A, Schultheis K, Patel R, Al-Qadi KI, Chen S, Jensen CR, Schad SR, Weddell JC, Vanka SP and Imoukhuede PI, AIChE J, , DOI: 10.1002/aic.16844. [DOI] [Google Scholar]
- 7.Séguin C, McLachlan JM, Norton PR and Lagugné-Labarthet F, Appl. Surf. Sci, 2010, 256, 2524–2531. [Google Scholar]
- 8.Zolper T, Li Z, Chen C, Jungk M, Marks T, Chung YW and Wang Q, Tribol. Lett, 2012, 48, 355–365. [Google Scholar]
- 9.Kekevi B, Berber H. and Yıldırım H, J. Surfactants Deterg, 2012, 15, 73–81. [Google Scholar]
- 10.Mehlhorn H, in Handbook of Hair in Health and Disease, 2012, pp. 355–385. [Google Scholar]
- 11.Sackmann EK, Fulton AL and Beebe DJ, Nature, 2014, 507, 181–189. [DOI] [PubMed] [Google Scholar]
- 12.Gupta K, Kim DH, Ellison D, Smith C, Kundu A, Tuan J, Suh KY and Levchenko A, Lab Chip, 2010, 10, 2019–2031. [DOI] [PubMed] [Google Scholar]
- 13.Jensen KF, Reizman BJ and Newman SG, Lab Chip, 2014, 14, 3206–3212. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Hao X, Wang C, Zhang B. and Wang W, Microsyst. Technol, 2014, 20, 1933–1940. [Google Scholar]
- 15.Kamei KI, Kato Y, Hirai Y, Ito S, Satoh J, Oka A, Tsuchiya T, Chen Y. and Tabata O, RSC Adv, 2017, 7, 36777–36786. [Google Scholar]
- 16.Gitlin L, Schulze P. and Belder D, Lab Chip, 2009, 9, 3000–3002. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Zhang LL, Sun JH, Li H. and Cui DF, J. Micromechanics Microengineering, , DOI: 10.1088/0960-1317/24/9/095006. [DOI] [Google Scholar]
- 18.Heyries KA and Hansen CL, Lab Chip, 2011, 11, 4122–4125. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang G. and Kutter JP, J. Micromechanics Microengineering, , DOI: 10.1088/0960-1317/21/10/105020. [DOI] [Google Scholar]
- 20.Briones MPP, Honda T, Yamaguchi Y, Miyazaki M, Nakamura H. and Maeda H, J. Chem. Eng. Japan, 2006, 39, 1108–1114. [Google Scholar]
- 21.Koerner T, Brown L, Xie R. and Oleschuk RD, Sensors Actuators, B Chem, 2005, 107, 632–639. [Google Scholar]
- 22.Kim SH, Lee S, Ahn D. and Park JY, Sensors Actuators, B Chem, , DOI: 10.1016/j.snb.2019.04.145. [DOI] [Google Scholar]
- 23.Sun M, Luo C, Xu L, Ji H, Ouyang Q, Yu D. and Chen Y, Langmuir, 2005, 21, 8978–8981. [DOI] [PubMed] [Google Scholar]
- 24.Sidorova JM, Li N, Schwartz DC, Folch A. and Monnat RJ Jr, Nat. Protoc, 2009, 4, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuah YJ, Koh YT, Lim K, Menon NV, Wu Y. and Kang Y, Sci. Rep, , DOI: 10.1038/srep18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Pei W, Tang R, Chen S. and Chen H, Sensors Actuators, A Phys, , DOI: 10.1016/j.sna.2012.09.024. [DOI] [Google Scholar]
- 27.Srinivasan U, Houston MR, Howe RT and Maboudian R, J. Microelectromechanical Syst, 1998, 7, 252–259. [Google Scholar]
- 28.Ashurst WR, Yau C, Carraro C, Lee C, Kluth GJ, Howe RT and Maboudian R, Sensors Actuators, A Phys, 2001, 91, 239–248. [Google Scholar]
- 29.Fréchette J, Maboudian R. and Carraro C, J. Microelectromechanical Syst, 2006, 15, 737–744. [Google Scholar]
- 30.Qin D, Xia Y. and Whitesides GM, Nat. Protoc, 2010, 5, 491–502. [DOI] [PubMed] [Google Scholar]
- 31.Xiu Y, Zhu L, Hess DW and Wong CP, 2006, 127, 9676–9681. [DOI] [PubMed] [Google Scholar]
- 32.Xiu Y, Zhu L, Moon J, Hess DW and Wong CP, Proc. Int. Symp. Exhib. Adv. Packag. Mater. Process. Prop. Interfaces, 2007, 172–179. [Google Scholar]
- 33.Raza MA, Kooij ES, Silfhout A. and Poelsema B, in Trends in Colloid and Interface Science XXIV, eds. Starov V. and Procházka K, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011, vol. 138, pp. 81–84. [Google Scholar]
- 34.Hu B, Zhu Q, Xu Z. and Wu X, Biomed. Res, 2015, 26, 452–455. [Google Scholar]
- 35.Andree KC, Barradas AMC, Nguyen AT, Mentink A, Stojanovic I, Baggerman J, Van Dalum J, Van Rijn CJM and Terstappen LWMM, ACS Appl. Mater. Interfaces, 2016, 8, 14349–14356. [DOI] [PubMed] [Google Scholar]
- 36.Ansari A, Patel R, Schultheis K, Naumovski V. and Imoukhuede PI, J. Vis. Exp, 2016, 2016, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansari A, Lee-Montiel FT, Amos JR and Imoukhuede PI, Biotechnol. Bioeng, 2015, 112, 2214–2227. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Jones P. and Haswell SJ, Chem. Eng. J, 2007, 135, 82–88. [Google Scholar]
- 39.Padmanabhan S, Coughlin JE and Iyer RP, Tetrahedron Lett, 2005, 46, 343–347. [Google Scholar]
- 40.Saneinejad S. and Shoichet MS, J. Biomed. Mater. Res, 1998, 42, 13–19. [DOI] [PubMed] [Google Scholar]
- 41.Ibarlucea B, Fernández-Sánchez C, Demming S, Büttgenbach S. and Llobera A, Analyst, 2011, 136, 3496–502. [DOI] [PubMed] [Google Scholar]
- 42.Ting LH, Feghhi S, Han SJ, Rodriguez ML and Sniadecki NJ, J. Nanotechnol. Eng. Med, 2011, 2, 1–5. [Google Scholar]
- 43.Antoniou A, Herlem G, André C, Guillaume Y. and Gharbi T, Talanta, 2011, 84, 632–7. [DOI] [PubMed] [Google Scholar]
- 44.Kuddannaya S, Chuah YJ, Lee MHA, Menon NV, Kang Y. and Zhang Y, ACS Appl. Mater. Interfaces, 2013, 5, 9777–9784. [DOI] [PubMed] [Google Scholar]
- 45.Lee KS and Ram RJ, 12th Int. Conf. Miniaturized Syst. Chem. Life Sci. - Proc. MicroTAS 2008 Conf., 2008, 919–921. [Google Scholar]
- 46.Yadav AR, Sriram R, Carter J. a.and Miller BL, Mater. Sci. Eng. C, 2014, 35, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ansari A. and Imoukhuede PI, Nano Res, 2018, 11, 5107–5129. [Google Scholar]
- 48.Lopera S. and Mansano RD, ISRN Polym. Sci, 2012, 2012, 1–5. [Google Scholar]
- 49.Yeo LP, Yan YH, Lam YC and Chan-Park MB, Langmuir, 2006, 22, 10196–10203. [DOI] [PubMed] [Google Scholar]
- 50.Argyrakis P, Teo L, Stevenson T. and Cheung R, in Microelectronic Engineering, 2005. [Google Scholar]
- 51.Ebert D. and Bhushan B, J. Colloid Interface Sci, 2016, 481, 82–90. [DOI] [PubMed] [Google Scholar]
- 52.Zhuang YX and Menon A, J. Vac. Sci. Technol. A Vacuum, Surfaces, Film., 2005, 23, 434–439. [Google Scholar]
- 53.Kasai T, Bhushan B, Kulik G, Barbieri L. and Hoffmann P, J. Vac. Sci. Technol. B Microelectron. Nanom. Struct., 2005, 23, 995–1003. [Google Scholar]
- 54.Ren K, Zhao Y, Su J, Ryan D. and Wu H, Anal. Chem, 2010, 82, 5965–5971. [DOI] [PubMed] [Google Scholar]
- 55.Guan X, Guo Z, Wang T, Lin L, Chen J, Tian H. and Chen X, Biomacromolecules, 2017, 18, 1342–1349. [DOI] [PubMed] [Google Scholar]
- 56.Chandradoss SD, Haagsma AC, Lee YK, Hwang JH, Nam JM and Joo C, J. Vis. Exp, 2014, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu KT and Chung CK, J. Micromechanics Microengineering, , DOI: 10.1088/0960-1317/26/6/065015. [DOI] [Google Scholar]
- 58.Jahangiri F, Hakala T. and Jokinen V, Microfluid. Nanofluidics, 2020, 24, 1–11. [Google Scholar]
- 59.Kwapiszewska K, Zukowski K, Kwapiszewski R. and Brzózka Z, AIMS Biophys, 2016, 3, 553–562. [Google Scholar]
- 60.Lee CC and Hsu W, J. Vac. Sci. Technol. B Microelectron. Nanom. Struct., 2003, 21, 1505–1510. [Google Scholar]
- 61.Gökaltun A, (Abraham) Kang YB, Yarmush ML, Usta OBand Asatekin A, Sci. Rep, 2019, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu F, Macdonald NP, Cooper JM and Wlodkowic D, Proc. SPIE - Int. Soc. Opt. Eng, 2013, 8923, 892344. [Google Scholar]
- 63.Ulhaq M, Carlsson G, Örn S. and Norrgren L, Environ. Toxicol. Pharmacol, , DOI: 10.1016/j.etap.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Seiler TB, Best N, Fernqvist MM, Hercht H, Smith KEC, Braunbeck T, Mayer P. and Hollert H, Chemosphere, , DOI: 10.1016/j.chemosphere.2014.02.064. [DOI] [PubMed] [Google Scholar]
- 65.Tang L. and Lee NY, Lab Chip, 2010, 10, 1274–1280. [DOI] [PubMed] [Google Scholar]
- 66.Kurkuri MD, Al-Ejeh F, Shi JY, Palms D, Prestidge C, Griesser HJ, Brown MP and Thierry B, J. Mater. Chem, 2011, 21, 8841–8848. [Google Scholar]
- 67.Li J, Li G, Zhang K, Liao Y, Yang P, Maitz MF and Huang N, Appl. Surf. Sci, 2013, 273, 24–31. [Google Scholar]
- 68.Hettlich HJ, Otterbach F, Mittermayer C, Kaufmann R. and Klee D, Biomaterials, 1991, 12, 521–524. [DOI] [PubMed] [Google Scholar]
- 69.Bhattacharya S, Datta a., Berg JM and Gangopadhyay S, J. Microelectromechanical Syst, 2005, 14, 590–597. [Google Scholar]
- 70.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S. and Langer R, Biomaterials, 2004, 25, 3583–3592. [DOI] [PubMed] [Google Scholar]
- 71.Yoon HJ, Kim TH, Zhang Z, Azizi E, Pham TM, Paoletti C, Lin J, Ramnath N, Wicha MS, Hayes DF, Simeone DM and Nagrath S, Nat. Nanotechnol, 2013, 8, 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park K, Akin D. and Bashir R, Biomed. Microdevices, 2007, 9, 877–883. [DOI] [PubMed] [Google Scholar]
- 73.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET and Beebe DJ, Lab Chip, 2009, 9, 2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu H, Wu L, Zhou X, Liu B. and Zheng B, Small, 2018, 14, 1–9. [DOI] [PubMed] [Google Scholar]
- 75.Ziółkowska K, Zukowski K, Chudy M, Dybko A. and Brzózka Z, 15th Int. Conf. Miniaturized Syst. Chem. Life Sci. 2011, MicroTAS 2011, 2011, 2, 1164–1166. [Google Scholar]
- 76.Zhou J, Ellis AV and Voelcker NH, Electrophoresis, 2010, 31, 2–16. [DOI] [PubMed] [Google Scholar]
- 77.Gokaltun A, Yarmush ML, Asatekin A. and Usta OB, TECHNOLOGY, 2017, 05, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]