Abstract
Background:
From the end of 2019 to now, coronavirus disease 2019 (COVID-19) has put enormous strain on the world's health systems, causing significant deaths and economic losses worldwide. Nasal congestion, one of the symptoms of COVID-19, poses considerable problems for patients. In China, acupuncture has been widely used to treat nasal congestion caused by COVID-19, but there is still a lack of evidence-based medical evaluation.
Methods:
According to the retrieval strategies, randomized controlled trials on the acupuncture for COVID-19 nasal congestion were obtained from China National Knowledge Infrastructure, WanFang, VIP, PubMed, Embase, and Cochrane Library, regardless of publication date, or language. Studies were screened based on inclusion and exclusion criteria, and the Cochrane risk bias assessment tool was used to evaluate the quality of the studies. The meta-analysis was performed using Review Manager (RevMan 5.3) and STATA 14.2 software. Ultimately, the evidentiary grade for the results will be evaluated.
Results:
The study will provide a high-quality and convincing assessment of the efficacy and safety of acupuncture in the treatment of COVID-19's nasal congestion and will be published in peer-reviewed journals.
Conclusion:
Our findings will provide references for future clinical decision and guidance development.
PROSPERO registration number:
NO.CRD42021299482.
Keywords: acupuncture, COVID-19, meta-analysis, nasal congestion, protocol, systematic review
1. Introduction
The coronavirus disease 2019 (COVID-19), named by the World Health Organization, was first detected in Wuhan, Hubei, China in December 2019.[1] As the outbreak has progressed, it has infected large numbers of people and is increasing worldwide, disrupting normal medical services, causing serious illness and associated long-term health sequelae, and leading to death and excess deaths.[2–4] According to Johns Hopkins University statistics as of December 20, 2021, globally, there are 273,900,334 confirmed cases of COVID-19 and more than 5,351,812 deaths. The Centers for Disease Control highlights key symptoms that can indicate COVID-19, including cough, shortness of breath or difficulty breathing, fever, chills, muscle pain, sore throat, and loss of a new sense of smell or taste.[5] There have been increasing reports of people with COVID-19 having trouble smelling, with many suffering from nasal congestion. Because anosmia caused by a blocked nose often has a significant impact on their quality of life, it can cause sufferers to fail to properly perceive the taste of food. It may directly lead to malnutrition, weight loss, food poisoning, and even depression.[6] Currently, there are no specific drugs to treat nasal congestion in COVID-19 patients. It is the majority of clinical patients who receive topical or oral decongestants to reduce congestive symptoms. Frequent use of topical decongestants often results in significant rebound congestion when the effects of the drug wear off, leading to continued use of decongestants and a vicious cycle of worsening congestion (drug-induced rhinitis). Long-term use of decongestants can cause serious side effects such as headache, bronchospasm, or dizziness.[7] Acupuncture is an external treatment of traditional Chinese medicine with a history of more than 3000 years. A large number of studies have proved that acupuncture has unique advantages in the treatment of nasal congestion and has been widely used in the world.[8–10] During the COVID-19 outbreak, acupuncture was used as an adjunctive therapy for COVID-19 in China, and its efficacy in treating COVID-19 was confirmed under traditional protocols.[11] To date, there is no high-quality evidence that acupuncture treats COVID-19 nasal congestion. Therefore, we designed this study to better understand the efficacy and safety of acupuncture in treating nasal congestion with COVID-19.
2. Methods and analysis
2.1. Study registration
This systematic review protocol has been registered in the PROSPERO (No. CRD42021299482). We will follow recommendations outlined in The Cochrane Handbook of Systematic Review of Interventions and the preferred reporting items for systematic reviews and meta-analysis protocol (PRISMA-P) statement guidelines. If amendments are needed, we will update our protocol to include any changes in the whole process of research.
2.2. Inclusion criteria for study selection
2.2.1. Types of studies
There are no restrictions on the publication language. Non-randomized controlled trial, reviews, case reports, experimental study, and animal study will be excluded.
2.2.2. Participants
COVID-19 patients with recorded nasal congestion lasted for 1 week or more. There are no restrictions on gender, race, and stage of disease. Patients with a history of nasal congestion before COVID-19 infection will be excluded. The diagnosis of COVID-19 includes Chinese or international diagnostic criteria.[12,13]
2.2.3. Types of interventions
In addition to the treatment of COVID-19, treatment group interventions comprised acupuncture, and comparator groups intervention: comfort therapy (placebo, pseudo-acupuncture, or blank control), other therapies (Western medicine, usual care or non-drug therapy, etc).
2.2.4. Types of outcomes
In this meta-analysis, the main outcome is the frequency of nasal congesture, duration of nasal congestion, the degree of nasal congestion, and quality of life.
2.3. Search strategy
Randomized controlled trials will be extracted from PubMed, EMBASE, Cochrane Library, Web of Science, Chinese Biomedical Databases, China National Knowledge Infrastructure, Wanfang database, and VIP database. The complete PubMed search strategy is summarized in Appendix 1, Supplemental Digital Content.
2.4. Data collection and analysis
2.4.1. Selection of studies
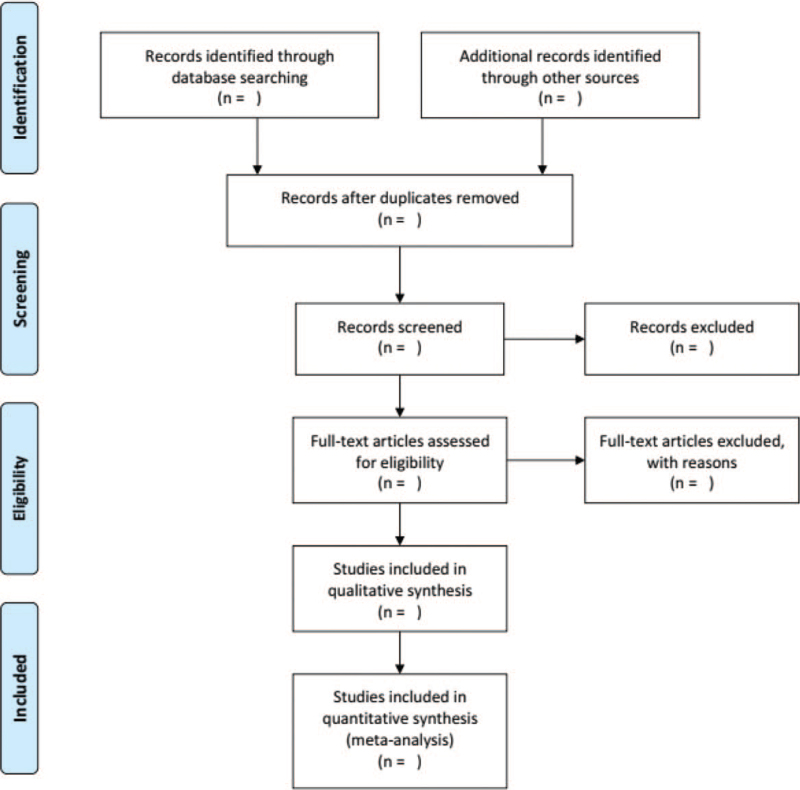
Two independent reviewers will screen and evaluate the relevant abstracts and titles of all studies against pre-determined inclusion criteria, then exclude duplicates or unqualified articles and explain why. A third investigator will resolve any differences between the 2 examiners. The process for filtering selections is shown in Figure 1.
Figure 1.
PRISMA flow diagram.
2.4.2. Data extraction and management
Two reviewers will be responsible for the extraction and management of data according to the retrieval strategy, including study title, journal, year of publication, name of first author, general information, study design, experimental intervention and timing of intervention, results, and adverse events. If there is any disagreement between the 2 reviewers during the data extraction process, the panel will jointly arbitrate and make a decision.
2.4.3. Dealing with missing data
If complete literature or relevant data is not available, we will contact the corresponding author. However, if the missing data cannot be obtained, then the study will be excluded from the analysis.
2.4.4. Assessment of risk of bias
The Cochrane Handbook for Systematic Reviews of Interventions Version 6 will be performed to assess a broad category of biases in the included studies. We will evaluate biases from the following 7 aspects: random sequence generation, allocation concealment, blinding of the participants and personnel, blinding of the outcome assessments, incomplete outcome data, selective reporting, and other sources of bias. These studies will be assigned as low risk, high risk, or unclear risk. Inconsistencies will be resolved by discussion with other reviewers.
2.4.5. Measures of treatment effect
Review Manager (RevMan 5.3, Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark) software and Stata 14.2 (Stata Corp, College Station, Texas, USA) will be used to conduct this meta-analysis. Dichotomous outcomes will be presented as risk ratios with 95% confidence intervals. When continuous outcomes exist, mean differences or standardized mean differences will be calculated.
2.4.6. Assessment of heterogeneity
Cochrane X2 and I2 tests will be used for the evaluation of heterogeneity. It is acknowledged that if P ≥ .05 and I2 ≤ 50%, the assessment of heterogeneity can be neglected; and there is great heterogeneity between included studies if P < .05 and I2 > 50%.
2.4.7. Assessment of reporting bias
If there are over 10 studies included in the meta-analysis, funnel plots will be used to detect the reporting biases.[14]
2.4.8. Data synthesis
We will take advantage of Review Manager (RevMan) software V.5.3 for data analysis and synthesis. Data will be processed with a fixed-effect model if no statistical heterogeneity was observed among the results (P ≥ .05 and I2 ≤ 50%). Meanwhile, the random-effect model will be put into use, if P < .05 and I2 > 50%.
2.4.9. Subgroup analysis
Based on the results of data synthesis, a subgroup analysis or meta-regression analysis will be performed to analyze the source of any heterogeneity.
2.4.10. Sensitivity analysis
Sensitivity analysis will be performed to examine the robustness of the study's conclusions. Will include methodological quality, sample size, and the impact of missing data. Therefore, the impact of low-quality studies on overall results will be assessed.
2.4.11. Quality of evidence evaluation
The quality of evidence will be independently assessed by 2 reviewers and graded for recommendation evaluation, development and evaluation. Evidence quality will be rated as “high”, “medium”, “low”, or “very low” according to rating criteria based on 5 parameters (publication bias, inconsistencies, inaccuracies, and research limitations).
2.4.12. Ethics and dissemination
Since this study does not involve the patient privacy, ethical approval is not required. Our research results will be shared and shown through conference reports and peer-reviewed journals.
3. Discussion
COVID-19, caused by SARS-CoV-2, is a serious global public health threat that puts people around the world at risk.[15] Breathing difficulties, fatigue, fever, and coughing are very common in COVID-19. However, SARS-CoV-2 invades the nasopharynx and causes loss of smell and taste,[16,17] most typically with nasal obstruction. The appearance of nasal congestion will directly affect the physical and mental health of patients, seriously affecting the quality of life of patients.[18] Acupuncture is recognized as an auxiliary technology, and has been widely used to treat nasal congestion and runny nose at home and abroad. Acupuncture is simple, convenient, and cheap. Acupuncture treatment can be very beneficial for patients with nasal congestion caused by COVID-19. In this work, we will conduct a systematic evaluation of the efficacy of acupuncture in the treatment of COVID-19 nasal congestion to verify the effectiveness of acupuncture. We hope that the results of this review will provide more appropriate evidence-based decision-making to help clinicians manage novel coronary pneumonic nasal obstruction in their decision-making process.
Author contributions
Data curation: Jin yun Guo.
Formal analysis: Yuxia Ma.
Methodology: Jingwen Zhang, Xinwei Lv.
Resources: Kaixin Zhang.
Software: Tingting Deng.
Visualization: Zhibin Dong.
Writing – original draft: Zhibin Dong.
Writing – review & editing: Yuning Ma.
Supplementary Material
Footnotes
Abbreviation: COVID-19 = coronavirus disease 2019.
How to cite this article: Dong Z, Guo J, Deng T, Zhang J, Lv X, Zhang K, Ma Y, Ma Y. Acupuncture for nasal congestion in COVID-19: a protocol for systematic review and meta-analysis. Medicine. 2022;101:2(e28600).
This study was supported in part by the National Natural Science Foundation of China (No. 81774402).
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Supplemental digital content is available for this article.
References
- [1].Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. Med Virol 2020;92:401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zuo Z, Wu T, Pan L, et al. Modalities and mechanisms of treatment for coronavirus disease 2019. Front Pharmacol 2021;11:583914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shah SMA, Rasheed T, Rizwan K, et al. Risk management strategies and therapeutic modalities to tackle COVID-19/SARS-CoV-2. Infect Public Health 2020;14:331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mehrzadi S, Karimi MY, Fatemi A, Reiter RJ, Hosseinzadeh A. SARS-CoV-2 and other coronaviruses negatively influence mitochondrial quality control: beneficial effects of melatonin. Pharmacol Ther 2021;224:107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Symptoms of coronavirus. US Centers for Disease Control and Prevention website. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed February 22, 2021. [Google Scholar]
- [6].Glezer I, Bruni-Cardoso A, Schechtman D, Malnic B. Viral infection and smell loss: the case of COVID-19. Neurochem 2021;157:930–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sertel S, Bergmann Z, Ratzlaff K, Baumann I, Greten HJ, Plinkert PK. Acupuncture for nasal congestion: a prospective, randomized, double-blind, placebo-controlled clinical pilot study. Rhinol Allergy 2009;23:e23–8. [DOI] [PubMed] [Google Scholar]
- [8].Kahn CI, Huestis MJ, Cohen MB, Levi JR. Evaluation of acupuncture's efficacy within otolaryngology. Ann Otol Rhinol Laryngol 2020;129:727–36. [DOI] [PubMed] [Google Scholar]
- [9].Drews T, Hummel T, Rochlitzer B, Hauswald B, Hähner A. Acupuncture is associated with a positive effect on odour discrimination in patients with postinfectious smell loss-a controlled prospective study. Eur Arch Otorhinolaryngol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vent J, Wang DW, Damm M. Effects of traditional Chinese acupuncture in post-viral olfactory dysfunction. Otolaryngol Head Neck Surg 2010;142:505–9. [DOI] [PubMed] [Google Scholar]
- [11].Badakhsh M, Dastras M, Sarchahi Z, Doostkami M, Mir A, Bouya S. Complementary and alternative medicine therapies and COVID-19: a systematic review. Rev Environ Health 2021;36:443–50. [DOI] [PubMed] [Google Scholar]
- [12].Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–93. [DOI] [PubMed] [Google Scholar]
- [13].National Health Commission of the People's Republic of China. Guideline on diagnosis and treatment of COVID-19 (Trial 8th edition). Available at: http://www.nhc.gov.cn/xcs/fkdt/202008/475d0199d34c4cac840eb7998fad444f.shtml. Accessed August 18, 2020. [Google Scholar]
- [14].Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- [15].Majumder J, Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J 2021;23:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yachou Y, El IA, Belapasov V, Ait BS. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci 2020;41:2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim GU, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect 2020;26:941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luers JC, Rokohl AC, Loreck N, et al. Olfactory and gustatory dysfunction in coronavirus disease 2019 (COVID-19). Clin Infect Dis 2020;71:2262–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.