PURPOSE:
This study aimed to investigate the impact of early versus not-early palliative care among cancer decedents on end-of-life health care costs.
METHODS:
Using linked administrative databases, we created a retrospective cohort of cancer decedents between 2004 and 2014 in Ontario, Canada. We identified those who received early palliative care (palliative care service used in the hospital or community 12 to 6 months before death [exposure]). We used propensity score matching to identify a control group of not-early palliative care, hard matched on age, sex, cancer type, and stage at diagnosis. We examined differences in average health system costs (including hospital, emergency department, physician, and home care costs) between groups in the last month of life.
RESULTS:
We identified 144,306 cancer decedents, of which 37% received early palliative care. After matching, we created 36,238 pairs of decedents who received early and not-early (control) palliative care; there were balanced distributions of age, sex, cancer type (24% lung cancer), and stage (25% stage III and IV). Overall, 56.3% of early group versus 66.7% of control group used inpatient care in the last month (P < .001). Considering inpatient hospital costs in the last month of life, the early group used an average (±standard deviation) of $7,105 (±$10,710) versus the control group of $9,370 (±$13,685; P < .001). Overall average costs (±standard deviation) in the last month of life for patients in the early versus control group was $12,753 (±$10,868) versus $14,147 (±$14,288; P < .001).
CONCLUSION:
Receiving early palliative care reduced average health system costs in the last month of life, especially via avoided hospitalizations.
INTRODUCTION
Palliative care is an approach to care that focuses on improving quality of life and controlling physical and psychologic symptoms for patients with life-threatening illnesses and their families.1 Unfortunately, data show palliative care is often applied very late in the disease trajectory or not at all. In the United States, palliative care is used in 45% of all deaths for a median of 17 days before death.2 Research from several cancer randomized trials has shown that the provision of early palliative care improves patient outcomes, such as reduced anxiety and depression.3-5 The integration of palliative care with standard oncologic care earlier in the cancer trajectory has even been endorsed by ASCO.6 A systematic review of 28 early palliative care trials showed, besides patient benefits, that it reduced both aggressive care, such as hospitalizations and emergency department (ED) visits in the last weeks of life, and hospital deaths.7 However, the review found mixed evidence that costs were different from usual care. Thus, although there was strong evidence of reduced hospital deaths and utilization at end of life, it was unclear whether early palliative care led to cost savings at a health system level.
There are a few studies that concluded that early palliative care led to cost savings in the last month of life, mostly through lower hospital costs.8-11 A large systematic review specifically examined the cost-effectiveness of home-based palliative care programs but found that compared with usual care, cost-effectiveness was inconclusive.12 Similar to the aforementioned review,7 and other systematic reviews,13 the methodologic challenges comparing multiple trials were small sample sizes, selection bias during recruitment, and most importantly varied definitions of the timing of early, differing elements of the palliative care intervention, and different or unclear contexts of usual care.
The health administrative data in Ontario, Canada, present a unique opportunity to address these limitations. Specifically because Ontario has a universal health system, including cancer care, we are able to standardize definitions for early palliative care, usual care, and the palliative care intervention in a large retrospective cohort including all cancers. Previously, we conducted a large propensity score–matched cohort study to examine the effect of early palliative care on late-life health services utilization.14 We used propensity score matching to address the selection bias that can occur when using observational data. Thus, although prior evidence from randomized trials provides high internal validity within controlled settings, our study design provides high external validity in real-world settings. In this study, we sought to investigate the overall mean health system costs per individual among a group who received early palliative care (at least 6 months before death) versus a matched group who did not receive early palliative care (ie, not-early group, a.k.a. control group).
METHODS
Study Design and Data Sources
Using linked administrative health databases, we performed population-based, retrospective cohort study of all cancer decedents in Ontario, Canada, from 2004 to 2014. We used propensity score matching to match decedents having received palliative care early (ie, between 12 months and 6 months before death)—referred to as the early group—to those not having received palliative care early (which includes both those who had palliative care initiated late and not initiated at all)—referred to as the control group.
To be included in the study, decedents needed to have a cancer diagnosis in the Ontario Cancer Registry before the death and a death caused by cancer as per the provincial Vital Statistics Registry (where 2014 was the most recent data available during data analysis). Other databases linked were the Discharge Abstract Database (hospitalizations), National Ambulatory Care Reporting System (ED use), Continuing Care Reporting System (complex continuing care use), Home Care Database, physician billings, Statistics Canada (sociodemographic data such as income and rurality), and the Resident Assessment Instrument-Home Care (RAI-HC) assessment database. Home care assessments used in Ontario, Canada, are validated and standardized and developed by interRAI (akin to the Minimum Data Set assessment tools used in US nursing homes and other settings).15-18 These data sets were linked using unique encoded identifiers and analyzed at ICES (formerly known as the Institute for Clinical Evaluative Sciences).
Exposure
Access to early palliative care was defined as having received home care with an end-of-life intent, an outpatient or home-visit physician billing for palliative care, or a hospitalization with a palliative care service code between 12 months and 6 months before death (the exposure period). These methods to determine palliative care service use within administrative data were validated previously.19 Generally, these services are independent of one another and uncoordinated.20 This contrasts the community-based, multidisciplinary team approach in the United States via home hospice care or in the United Kingdom via Macmillan Cancer Support program. Although a minority of patients might have access to a home-visiting, multidisciplinary, specialist palliative care team or a residential hospice, especially if they lived in a major city, this is haphazard and typically accessed in the last weeks of life.21
Outcome
The main outcome was the overall average health care costs per individual in their last month of life (CDN$). Health care costs were derived using validated costing macros22 and included the following sector costs: inpatient hospital, ED visit, physician billing, home care, and complex continuing care (ie, inpatient subacute or palliative care unit). We examined the outcome within the entire early–palliative care group compared with the matched control group, as per previous research.23 As a secondary outcome, we examined the average sector-specific costs per individual in the last month of life, comparing the early group with the control group.
Propensity Score Matching Analytic Plan
To reduce selection bias for decedents who were exposed to early palliative care in our cohort, we used propensity score matching to create a similar comparison group of unexposed decedents (not-early). The propensity score is an individual's probability of receiving early palliative care, given the values of the individual's baseline covariates measured either during or before the exposure period. Matching on the propensity score aids in balancing the distributions of measured characteristics between intervention and control groups, which helps to minimize confounding bias when estimating the effect of the intervention on an outcome.24,25
We estimated the propensity score using a logistic regression model with exposure to early palliative care as the dependent variable. The covariates in the propensity score regression included income quintile, rurality, health region, prior hospital utilization in months 24 to 12 before death, Deyo-modified Charlson comorbidity score (in the months 24 to 12 before death),26 year of death, and having had radiation or cancer surgery before death (from diagnosis to 6 months before death). In the end, all pairs were hard matched on age at death, sex (male or female), cancer type, and cancer stage at diagnosis (where available) and also matched on the logit of the propensity score (calipers of width equal to 0.2 of the standard deviation of the logit of the propensity score).27,28
Subgroup Analysis
A priori we examined separately the group who received a home care assessment in the exposure period; thus, among all those who used home care in the exposure period, individuals who also received early palliative care services were propensity score–matched with an individual who did not. The rationale was because of the concern of selection bias on unmeasured covariates within retrospective observational cohort studies. Specifically, palliative care may have been offered to those who were seemingly sicker, were more symptomatic, or had a worse physical decline and function—all reasons why someone might start home care services earlier, and thus, these act as unmeasured confounders in the matching process. To address this concern, the RAI-HC home care assessment has several unique variables that are associated with patient need for palliative care (eg, high pain, depression, and poor functional status)—and in this subgroup, we could further match on these. In other words, we identified individuals with similar levels of pain, depression, and functional status while using home care during the exposure period, although the exposed also received palliative care services, whereas the controls did not.
Therefore, we have two mutually exclusive groups of matched pairs, each pair has an exposed and unexposed decedent. The first group is the main analysis (which had no home care assessments); the other group is the subanalysis (which had home care assessments in the exposure period). So in addition to the variables in the main analysis, in the subanalysis, pairs were hard matched on their score on a prognostic physical function scale, ie, the validated CHESS scale, which measures changes in activities of daily living (ADL) status, cognition, shortness of breath, and life expectancy of < 6 months.29,30 Moreover, other covariates available in the home care assessment that were included in the propensity score regression for the subanalysis were as follows: (1) functional performance, using the validated ADL self-performance hierarchy scale, which examines the help required to eat, toilet, complete personal hygiene, etc to determine dependency31; (2) depressive symptoms, using a validated scale to measure signs and symptoms of depression32-34; (3) cognitive performance, using a validated scale to measure cognitive impairment (no impairment to severe impairment)35; (4) pain, using a validated scale of intensity of pain (ranging from no pain to severe daily pain)36; (5) having a caregiver present at home (yes or no); and (6) the number of RAI-HC assessments in the exposure period.
Analysis Plan
We calculated the average overall health care costs within the early–palliative care group and the control group. This was done separately for the main analysis and the subanalysis. Differences in mean costs (standardized to 2016) between exposed and control groups were compared using standardized differences and P values obtained from two-sample t tests. Furthermore, as a sensitivity analysis, we divided the control group (ie, not-early palliative care) into late palliative care (ie, only received palliative care in the last six months of life) and never received palliative care. We then compared our outcomes among our groups of matched pairs by early (exposed) versus late and early versus never separately. The purpose of the sensitivity analysis was to address unmeasured patient preferences. Comparing the late users paired to their early user matches specifically was an attempt to separate out those patients who might have refused palliative care as per their preference. Analysis was performed using SAS, version 9.4 (SAS Institute, Cary, NC). The study was approved by the Hamilton Integrated Research Ethics Board (#3039).
RESULTS
Demographics of the Groups Before and After Matching
In the overall cohort, there were 144,306 cancer decedents in Ontario between 2004 and 2014, of which 37.4% received early palliative care in the exposure period (12 months to 6 months before death).
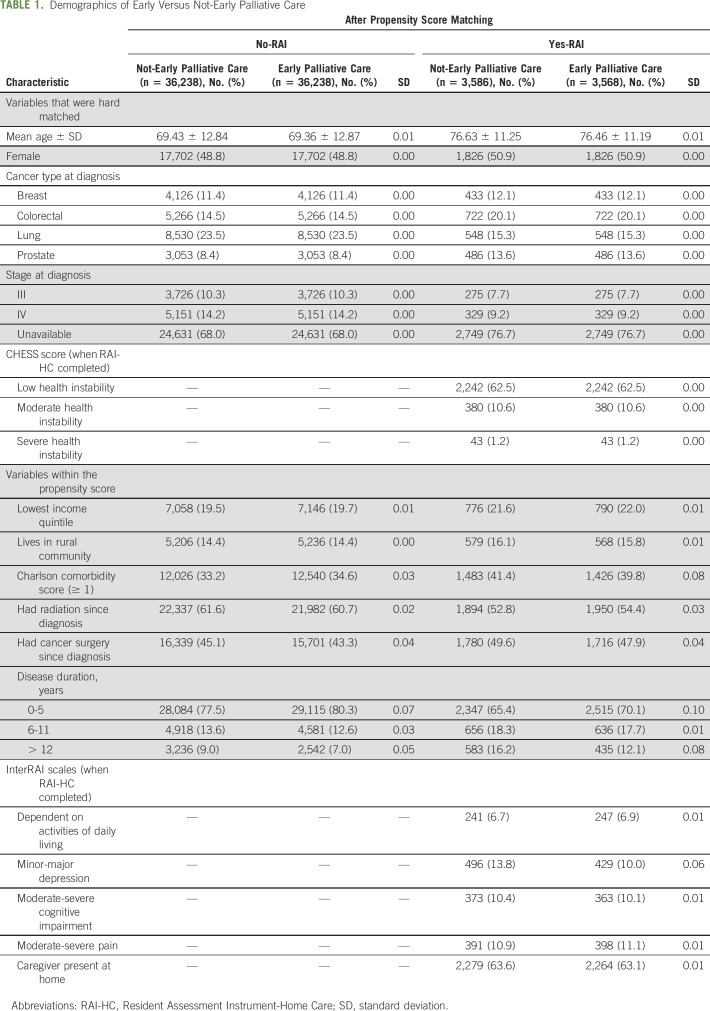
In the main analysis (comprising 89% of overall cohort, n = 128,548), we matched 83% of patients who received early palliative care for a total of 36,238 matched pairs. Before matching, those who received palliative care early, compared with those who did not, were more likely to have lung cancer (24.2% v 19.3%), stage IV cancer (20.1% v 8.1%), moderate to severe health instability (1.2% v 0.1%), symptoms of depression (3.3% v 0.9%), and moderate to severe pain (2.7% v 0.7%). After matching, the groups had nearly identical distributions with respect to the measured covariates: age at death was 69 years, 23.5% had lung cancer, 14.2% had stage IV disease, 34% had a comorbidity, and 44% had ever had cancer surgery (Table 1).
TABLE 1.
Demographics of Early Versus Not-Early Palliative Care
In the subanalysis, where individuals also had a home care assessment in the exposure period (comprising mutually exclusive 11% of overall cohort, n = 16,055), we matched 60% of patients who received home care in the exposure period for a total of 3,586 matched pairs. After matching, the groups had nearly identical distributions with respect to the measured covariates: age at death was 76 years, 15.3% had lung cancer, 9.2% had stage IV disease, 40% had a comorbidity, and 48% had ever had cancer surgery. In addition, using the RAI-HC variables, 11.8% had a CHESS score of 3 or higher, 7% were fully dependent on their ADLs, 10%-13% had signs or symptoms of minor-major depression, 10% had moderate-severe cognitive impairment, 11% had moderate-severe pain, and 63% had a caregiver living at home.
Use of Health Care Services in Last Month of Life
In the main analysis, the early group compared with the control group had a lower proportion who used inpatient hospitalizations (56.3% v 66.7%; P < .001) and ED visits (42.2% v 51.9%; P < .001) and a higher proportion who used home care (75.5% v 63.3%; P < .001) in the last month of life (Fig 1). There were no major differences in the use of physician services (98.0%) or use of complex continuing care (15.5%-18.2%) in both groups.
FIG 1.
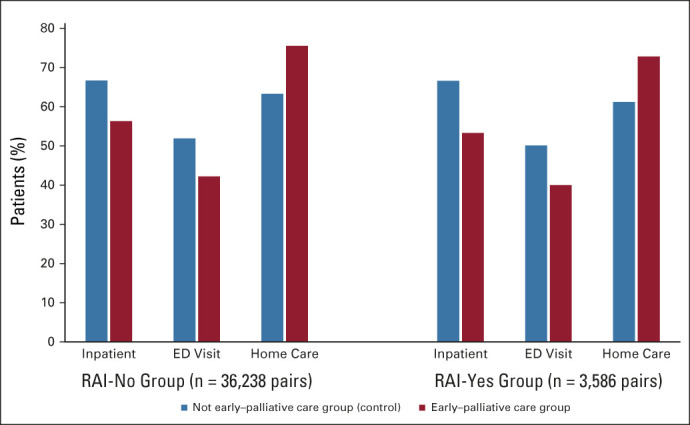
Percent of patients who used a health care sector in last month of life. ED, emergency department; RAI, Resident Assessment Instrument.
In the subanalysis, similar results emerged. In the last month of life, the early group compared with the control group had a lower proportion who used inpatient hospitalizations (53.3% v 66.6%; P < .001) and ED visits (40.0% v 50.1%; P < .001) and a higher proportion who used home care (72.8% v 61.2%; P < .001). There were no major differences in the use of physician services (98.0%) or use of complex continuing care (19.5%) in both groups.
Mean Total and Sector-Specific Health Care Costs in the Last Month of Life
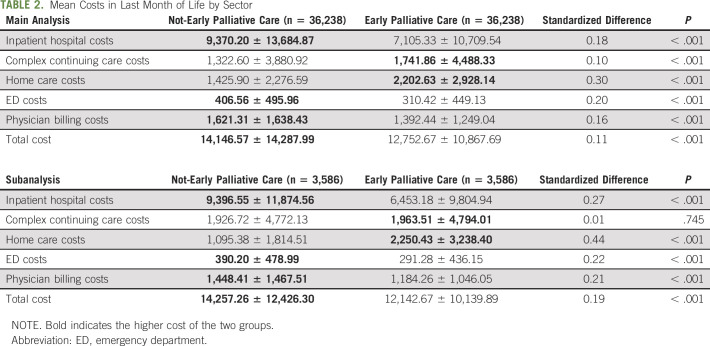
In the main analysis, the overall combined total health care costs was $462,131,424 for the entire early group and $512,643,233 for the entire control group in the last month of life. This equates to an average individual cost of $12,753 (±$10,868) in the early group versus $14,147 (±$14,288) in the control group (P < .001; Table 2). Examining sector-specific costs, the biggest differences were in home care and inpatient costs. The early group had higher average home care costs per person ($2,203 ± $2,928) in the last month of life compared with the control ($1,426 ± $2,277; P < .001). However, the early group had lower average inpatient costs per person ($7,105 ± $10,710) compared with the control group ($9,307 ± $13,685; P < .001). Trends were the same when the median was examined. With respect to the other sectors, the early group also had slightly higher complex continuing care costs but slightly lower ED costs and physician billing costs compared with the control group.
TABLE 2.
Mean Costs in Last Month of Life by Sector
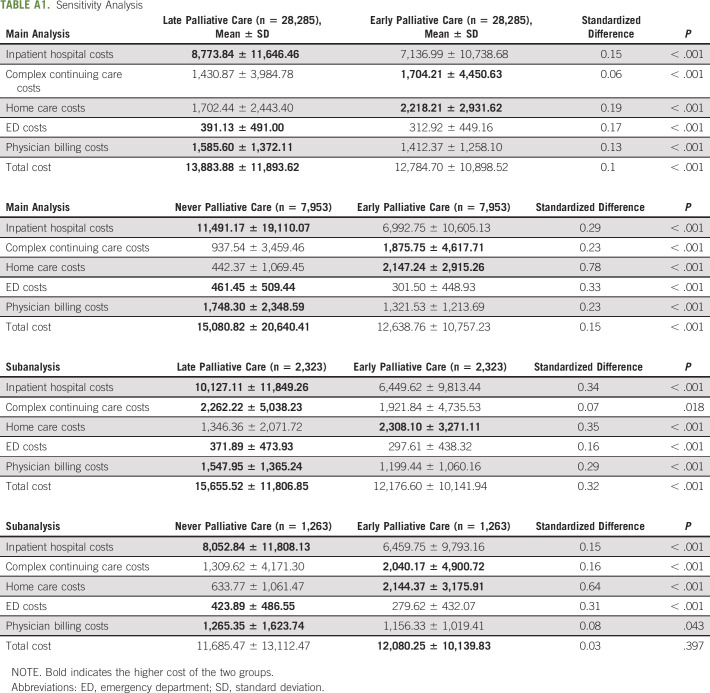
In the subanalysis, nearly identical trends were found. Average individual total health care cost in the last month of life was $12,143 ± $10,140 for the early group and $14,257 ± $14,288 for the control group (P < .001). Sector-specific trends were similar where the early group had higher average home care costs and lower average inpatient hospital costs than the control group. Finally, in the sensitivity analyses looking at early versus late paired groups, it showed the same statistically significant trends as the main and subanalysis (Appendix Table A1, online only). However, in the early versus never paired groups, the never users had lower overall costs, although this was not statistically significant. Note, total standardized costs per person increased by approximately $1,000 from 2004 to 2014, which was consistent in either exposure group.
DISCUSSION
In our large population-based cohort study of cancer decedents, those who received early palliative care had lower overall health care costs than those who did not, in the last month of life. The main differences in costs were the early–palliative care group used more home care services and less inpatient acute care services. This suggests that cost savings is driven by increased home care services use, which serves to prevent late-life hospitalizations.
Although our main findings were consistent with other studies examining palliative care and costs, our study's methods uniquely contribute to the evidence base. We addressed limitations noted in previous meta-analyses by using consistent exposure, intervention, and outcome definitions over an 11-year period of time. Our palliative care definition included a broad array of palliative care services from multiple settings, not just within inpatient hospital admissions.37-39 As well, although there are other observational cohort studies comparing early versus late palliative care, we used propensity score matching to reduce selection bias.11 Moreover, our home care subanalysis allowed us to control uniquely for additional prognostic covariates (eg, high pain or poor health instability) that are known to be associated with referrals to palliative care but are typically unmeasured confounders in other studies.40 A major strength of our study is the use of a population-based cohort of all cancers, which creates a sample size considerably greater than previous randomized trials examining this topic8 and contributes to the external validity and real-world evidence that outside controlled settings, palliative care can reduce health system costs. In other words, the large population-based sample strengthens the credibility that the results were not because of any particular cancer center,11 any specific palliative care program,23 or cancer type.8
Our data seem to support the common hypothesis of a causal pathway: those who are receiving earlier palliative care seem to access palliative home care services earlier and more often which, while increasing home care costs, seems to help some patients avoid end-of-life hospitalizations and ED visits altogether. Physician costs were similar between both groups. Ultimately, given there are statistically significant average savings at an individual level between those exposed early or not-early, the savings at a health system level across a population would seem to be sizeable. Across our early group of 36,236 individuals, there was a $50 million dollar savings than the control group. Our study, combined with the growing evidence base, underscores the need to invest in hospital and home-community palliative care programs as a strategy to not only save the health system money but also address hospital bed overcrowding.
This study has several limitations. Although we used propensity score matching to compare between those with a similar probability of receiving early palliative care, this may not represent the entire population of cancer decedents. We also matched on individual characteristics but did not examine physician propensity to provide palliative care. In addition, administrative data are limited in that providers may have been providing palliative approaches to care that are not captured in billing codes. There are other confounders that we could not directly measure, such as patient or provider preferences, and caregiver availability or private care, which may support patients to remain at home. Last, our cost analysis does not capture out-of-pocket costs or informal caregiving contributions of the patient or family. Future research could examine impacts of early palliative care on patient and caregiver well-being and quality of life, optimal timing for palliative care benefits, and physician propensity for providing palliative care.
In conclusion, in our large population-based cancer cohort study, we found strong evidence that receiving early palliative care reduced the average health system costs in the last month of life, in particular via avoided hospitalizations.
ACKNOWLEDGMENT
The authors would like to acknowledge the following people for their feedback during the preparation of this manuscript: Erin O'Leary and Urun Erbas-Oz.
APPENDIX
TABLE A1.
Sensitivity Analysis
Lisa C. Barbera
Travel, Accommodations, Expenses: Elekta
No other potential conflicts of interest were reported.
DISCLAIMER
The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding and data-providing sources.
SUPPORT
Supported by the Canadian Centre for Applied Research in Cancer Control (ARCC). ARCC receives core funding from the Canadian Cancer Society Research Institute (Grant No. 2015-703549). The lead author is also supported by the Canada Research Chairs Program.
DATA SHARING STATEMENT
Data may be obtained from a third party and are not publicly available. A data request can be sent to ICES (formerly the Institute for Clinical Evaluative Sciences): https://www.ices.on.ca/About-ICES/ICES-Contacts-and-Locations/contact-form.
AUTHOR CONTRIBUTIONS
Conception and design: Hsien Seow, Lisa C. Barbera, Kimberlyn McGrail, Fred Burge, Stuart J. Peacock, Rinku Sutradhar
Financial support: Hsien Seow
Administrative support: Hsien Seow
Collection and assembly of data: Hsien Seow, Fred Burge, Beverley Lawson, Rinku Sutradhar
Data analysis and interpretation: Hsien Seow, Lisa C. Barbera, Kimberlyn McGrail, Fred Burge, Dawn M. Guthrie, Beverley Lawson, Kelvin K. W. Chan, Rinku Sutradhar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Early Palliative Care on End-of-Life Health Care Costs: A Population-Based, Propensity Score–Matched Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lisa C. Barbera
Travel, Accommodations, Expenses: Elekta
No other potential conflicts of interest were reported.
REFERENCES
- 1.WHO : World Health Organization. Palliative care: The solid facts, in Davies E, Higginson I. (eds), 2004 [Google Scholar]
- 2.National Hospice and Palliative Care Organization : NHPCO's Facts & Figures on Hospice Care in America. Alexandria, VA, 2016 [Google Scholar]
- 3.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann C, Swami N, Krzyzanowska M, et al. : Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 383:1721-1730, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The project ENABLE II randomized controlled trial. JAMA 302:741-749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: ASCO clinical practice guideline update summary. JCO Oncol Pract 13:119-121, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Davis MP, Temel JS, Balboni T, et al. : A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 4:99-121, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Greer JA, Tramontano AC, McMahon PM, et al. : Cost analysis of a randomized trial of early palliative care in patients with metastatic nonsmall-cell lung cancer. J Palliat Med 19:842-848, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Seow H, Pataky R, Lawson B, et al. : Temporal association between home nursing and hospital costs at end of life in three provinces. Curr Oncol 23:S42-S51, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowery WJ, Lowery AW, Barnett JC, et al. : Cost-effectiveness of early palliative care intervention in recurrent platinum-resistant ovarian cancer. Gynecol Oncol 130:426-430, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Scibetta C, Kerr K, McGuire J, et al. : The costs of waiting: Implications of the timing of palliative care consultation among a cohort of decedents at a comprehensive cancer center. J Palliat Med 19:69-75, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Gomes B, Calanzani N, Higginson IJ: Benefits and costs of home palliative care compared with usual care for patients with advanced illness and their family caregivers. JAMA 311:1060-1061, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Abedini NC, Hechtman RK, Singh AD, et al. : Interventions to reduce aggressive care at end of life among patients with cancer: A systematic review. Lancet Oncol 20:e627-e636, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Seow H, Sutradhar R, Burge F, et al. : End-of-life outcomes with or without early palliative care: A propensity score matched, population-based cancer cohort study. BMJ Open 11:e041432, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirdes JP, Ljunggren G, Morris JN, et al. : Reliability of the interRAI suite of assessment instruments: A 12-country study of an integrated health information system. Biomed Cent Health Serv Res 8:1-11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Jung YI, Sung M, et al. : Reliability of the interRAI long term care facilities (LTCF) and interRAI home care (HC). Geriatr Gerontol Int 15:220-228, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Morris JN, Bernabei R, Ikegami N, et al. : RAI-Home Care (RAI-HC) Assessment Manual for Version 2.0. Washington, DC, interRAI Corporation, 1999 [Google Scholar]
- 18.Hawes C, Fries BE, James ML, et al. : Prospects and pitfalls: Use of the RAI-HC assessment by the Department of Veterans Affairs for home care clients. Gerontologist 47:378-387, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Tanuseputro P, Budhwani S, Bai YQ, et al. : Palliative care delivery across health sectors: A population-level observational study. Palliat Med 31:247-257, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brazil K, Bainbridge D, Sussman J, et al. : Coordination of palliative cancer care in the community: "unfinished business". Support Care Cancer 17:819-828, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Seow H, Brazil K, Sussman J, et al. : Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: A pooled analysis. BMJ 348:g3496, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wodchis W, Bushmeneva K, Nikitovic M, et al. : Guidelines on Person-Level Costing Using Administrative Databases in Ontario. Toronto, ON, Health System Performance Research Network, 2012 [Google Scholar]
- 23.Look Hong NJ, Liu N, Wright FC, et al. : Assessing the impact of early identification of patients appropriate for palliative care on resource use and costs in the final month of life. JCO Oncol Pract 16:e688-e702, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum P, Rubin D: The central role of the propensity score in observational studies for causal effects. Biometrika 70:41-55, 1983 [Google Scholar]
- 25.Rosenbaum P, Rubin D: Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Statistician 39:33-38, 1985 [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613-619, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083-3107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC: Type I error rates, coverage of confidence intervals, and variance estimation in propensity-score matched analyses. Int J Biostatistics 5:13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirdes JP, Poss JW, Mitchell L, et al. : Use of the interRAI CHESS scale to predict mortality among persons with neurological conditions in three care settings. PLoS One 9:e99066, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirdes JP, Frijters DH, Teare GF: The MDS-CHESS scale: A new measure to predict mortality in institutionalized older people. J Am Geriatr Soc 51:96-100, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Morris JN, Fries BE, Morris SA: Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci 54A:M546-M553, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Burrows AB, Morris JN, Simon SE, et al. : Development of an MDS-based depression rating scale for use in nursing homes. Age Ageing 29:165-172, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Martin L, Poss JW, Hirdes JP, et al. : Predictors of a new depression diagnosis among older adults admitted to complex continuing care: Implications for the depression rating scale (DRS). Age Ageing 37:51-56, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Koehler M, Rabinowitz T, Hirdes JP, et al. : Measuring depression in nursing home residents with the MDS and GDS: An observational psychometric study. BMC Geriatr 5:1, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JN, Fries BE, Mehr DR, et al. : MDS cognitive performance scale. J Gerontol A Biol Sc Med Sci 49:M174-M182, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Fries BE, Schneider D, Foley WJ, et al. : Refining a case-mix measure for nursing homes: Resource Utilization Groups (RUG-III). Med Care 32:668-685, 2001 [DOI] [PubMed] [Google Scholar]
- 37.May P, Garrido MM, Cassel JB, et al. : Cost analysis of a prospective multi-site cohort study of palliative care consultation teams for adults with advanced cancer: Where do cost-savings come from? Palliat Med 31:378-386, 2017 [DOI] [PubMed] [Google Scholar]
- 38.May P, Garrido MM, Cassel JB, et al. : Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: Earlier consultation is associated with larger cost-saving effect. J Clin Oncol 33:2745-2752, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May P, Normand C, Cassel JB, et al. : Economics of palliative care for hospitalized adults with serious illness: A meta-analysis. JAMA Intern Med 178:820-829, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maetens A, Beernaert K, De Schreye R, et al. : Impact of palliative home care support on the quality and costs of care at the end of life: A population-level matched cohort study. BMJ Open 9:e025180, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available. A data request can be sent to ICES (formerly the Institute for Clinical Evaluative Sciences): https://www.ices.on.ca/About-ICES/ICES-Contacts-and-Locations/contact-form.