PURPOSE:
Older patients with advanced cancer often have comorbidities that can worsen their cancer and treatment outcomes. We assessed how a geriatric assessment (GA)–guided intervention can guide conversations about comorbidities among patients, oncologists, and caregivers.
METHODS:
This secondary analysis arose from a nationwide, multisite cluster-randomized trial (ClinicalTrials.gov identifier: NCT02107443). Eligible patients were ≥ 70 years, had advanced cancer (solid tumors or lymphoma), and had impairment in at least one GA domain (not including polypharmacy). Oncology practices (n = 30) were randomly assigned to usual care or intervention. All patients completed a GA; in the intervention arm, a GA summary with recommendations was provided to their oncologist. Patients completed an Older Americans Resources and Services Comorbidity questionnaire at screening. The clinical encounter following GA was audio-recorded, transcribed, and coded for topics related to comorbidities. Linear mixed models examined the effect of the intervention on the outcomes adjusting for practice site as a random effect.
RESULTS:
Patients (N = 541) were 76.6 ± 5.2 years old; 94.6% of patients had at least one comorbidity with an average of 3.2 ± 1.9. The intervention increased the average number of conversations regarding comorbidities per patient from 0.52 to 0.99 (P < .01). Moreover, there were a greater number of concerns acknowledged (0.52 v 0.32; P = .03) and there was a 2.4-times higher odds of having comorbidity concerns addressed via referral, handout, or other modes (95% CI, 1.3 to 4.3; P = .004). Most oncologists in the intervention arm (76%) discussed comorbidities in light of the treatment plan, and 41% tailored treatment plans.
CONCLUSION:
Providing oncologists with a GA-guided intervention enhanced communication regarding comorbidities.
INTRODUCTION
More than 90% of Americans age 65 years or older have two or more chronic diseases including cardiovascular disease and diabetes, and patients with cancer suffer disproportionately from comorbidities.1-3 Cancer treatment can exacerbate underlying pre-existing comorbidities, and certain chronic diseases can increase the susceptibility of a patient to adverse cancer-related and cancer treatment–related effects. Comorbidities can affect a patient's prognosis, tolerability of treatment, and morbidity because of cancer and its treatment and can negatively affect survival.2,4,5 A recent call to action6 recommends clinical teams caring for patients with cancer to communicate more effectively about comorbidities to improve treatment outcomes and the patients' experience.
In the context of advanced cancer, existing comorbidities can greatly influence treatment decisions and outcomes. For example, patients who have breast cancer and diabetes are at increased risk of chemotherapy-induced peripheral neuropathy7 and all-cause mortality8 compared with patients without diabetes. Also, anthracycline chemotherapy and trastuzumab (a monoclonal antibody used for treating breast and stomach cancer) can increase the risk for potentially serious cardiovascular side effects, and therefore, patients with pre-existing cardiovascular disease have higher risk of cardiotoxicity; patients should be closely monitored, receive a modified dosing schedule, and/or potentially select an alternative treatment regimen.9-11 When prescribing potentially nephrotoxic chemotherapy (eg, platinum agents), alternative regimens or dosing approaches may need to be considered for older patients with renal insufficiency.12 Many comorbidities are treated with pharmaceuticals, and some of these drugs can have dangerous interactions with chemotherapeutic agents (eg, paclitaxel and warfarin).13,14 To further complicate treatment decisions, historically, older patients and patients with chronic diseases have been under-represented in clinical trials and comorbidities are often not well-described in therapeutic clinical trials, making it difficult to assess the appropriateness of some standard oncology interventions.2
The geriatric assessment (GA) is the gold standard for evaluating aging-related conditions and is recommended by ASCO for all patients with cancer age 65 years and older.15,16 This assessment is a multidimensional evaluation of a person's functional, cognitive, psychosocial, and medical needs so that these details can be integrated into their treatment plan. In community-dwelling older adults, the GA has been shown in some, but not all, studies to improve physical function, quality of life, and even overall survival.15 It does this by providing tools to clinical care teams to identify and address common issues of aging, some of which might not be standard in their clinical specialty. In the advanced cancer setting specifically, the GA can promote appropriate treatment modification, facilitate care coordination, and help identify potentially inappropriate medications. In our multisite cluster-randomized controlled trial Communicating about Aging and Cancer Health (COACH),17 64% of older adults with cancer reported serious comorbidities, which was defined as ≥ 3 comorbidities or at least one comorbidity that interfered with their activities a great deal. Providing a GA-guided intervention enhanced physician-patient communication about aging-related concerns (eg, cognition, social support, and physical performance) and improved patient and caregiver satisfaction.17 However, the effects of GA-guided intervention and recommendations on conversations about comorbidities specifically were not analyzed previously. Therefore, herein, we assessed whether the GA-guided intervention increased the number of conversations about comorbidities between patients, oncologists, and caregivers and whether the GA-guided intervention led to more recommendations to address comorbidities.
METHODS
Study Design and Population
This was a secondary mixed methods analysis of the COACH study (ClinicalTrials.gov identifier: NCT02107443, University of Rochester Cancer Center [URCC] 13070).17 COACH was a cluster-randomized intervention study conducted via the URCC National Cancer Institute (NCI) Community Oncology Research Program (NCORP) Research Base. NCORP is a national network of community oncology practices, as opposed to academic medical centers, with sites across the United States including Greenville Health System, Greenville, SC; Nevada Cancer Research Foundation, Las Vegas, NV; and Michigan Cancer Research Consortium, Ann Arbor, MI. The URCC NCORP Research Base served as the coordinating center. Patients were 70 years old or older, had a diagnosis of advanced solid tumor or lymphoma, were considering or receiving cancer treatment for palliative intent, had at least one GA domain impaired other than polypharmacy, and had visits planned with their oncologist for at least 3 months. The study protocol was approved by the institutional review boards at University of Rochester and all participating sites. All participants provided written informed consent.
Intervention
Oncology practices were randomly assigned to an intervention arm or a usual care arm. A GA was conducted with all patients at screening and included eight domains: comorbidity, polypharmacy, cognition, nutrition, physical performance, functional status, psychologic status, and social support.10,18,19 In the intervention arm, the GA summary along with printed recommendations specific to the patient's impaired domains was provided to oncologists, patients, and caregivers; in the usual care arm, oncologists were only alerted to clinically significant cognitive impairment and/or depression. One clinic visit within 4 weeks of the GA (between the patient, oncologist, and other attendees including caregiver[s]) was audio-recorded and transcribed. The study team at each community oncology site completed a questionnaire after the study visit regarding if they addressed various aging-related concerns and provided appropriate GA domain–specific recommendations to patients. The full methods and procedures have been published previously.17
Assessment of Comorbidities
Comorbidities were captured using the validated Older Americans Resources and Services (OARS) Comorbidity questionnaire, which inquires about the presence of 15 comorbidities (eg, diabetes, depression, and poor eyesight) and, if present, interference of the comorbidity with daily activities.18,20 Patients were considered impaired in the comorbidity domain of the GA if they reported ≥ 3 comorbidities or at least one comorbidity that interfered with their activities a great deal.17,18 For comorbidities, the summary included whether the patient was impaired and, if so, management recommendations that were integrated from experts and the National Comprehensive Cancer Network and Delphi guidelines10 (Table 1). The GA-guided intervention included the same recommendations for all patients who were impaired in the comorbidity domain, and recommendations were not specific to the type of comorbidity.
TABLE 1.
Recommendations Provided to the Oncologist in the GA-Guided Intervention for Patients Who Were Impaired in the Comorbidity Domain of the GA

Analysis of Transcripts
All transcripts of the audio-recorded clinic visits were reviewed by a team of coders for specific terms related to comorbidities defined a priori using the OARS questionnaire (eg, diabetes, hypertension, lung disease, and osteoporosis; listed in Fig 1).17 These data were used to quantify the number of conversations about each comorbidity in the intervention compared with the usual care group. In addition, coders evaluated who initiated the conversations, whether the concern was acknowledged, which was defined as exploring the issue but not implementing any care processes, or dismissed, and whether the concern was appropriately addressed via an intervention such as a patient referral (eg, physical therapy), handout (eg, nutrition and cancer), medical reconciliation (eg, for polypharmacy), or other intervention.21 Coding was performed using a priori criteria and a coding manual as described previously17,21 (Atlas.ti software, Berlin, Germany).
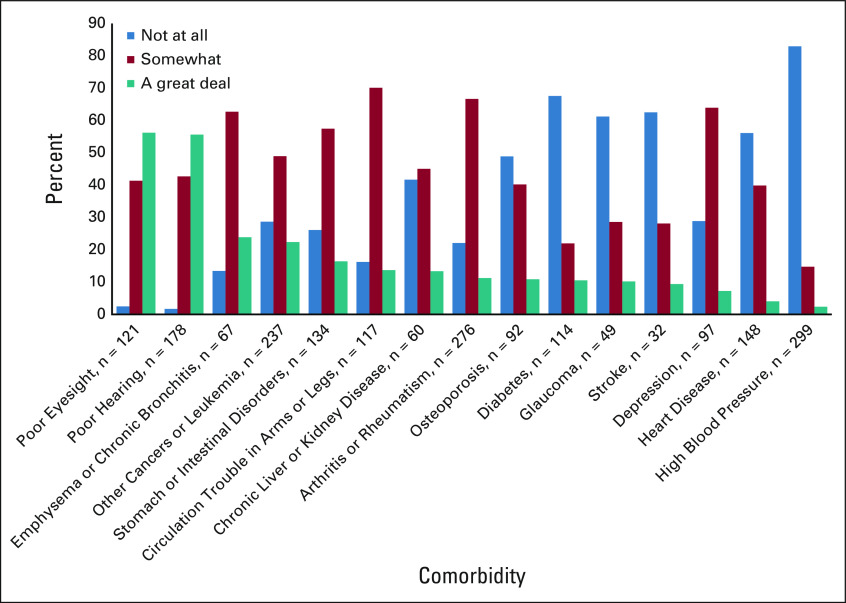
FIG 1.
The extent to which comorbidities interfere with activities of daily living (Older American Resources and Services Comorbidity questionnaire at screening, n = 540).
Statistics
The OARS Comorbidity scale was used to determine the percentage of patients with each comorbidity, the comorbidity burden (number of comorbidities per patient), and how much each comorbidity interfered with the patient's activities. A t-test was used for continuous variables, and a Pearson chi-squared test was used for categorical variables to determine differences between the usual care arm and the intervention arm. Linear mixed models were used to assess the between-group differences in the total number of conversations and number of conversations initiated by the physician per patient with practice site as a random effect. Linear mixed models were also used to compare the number of concerns related to comorbidities that were acknowledged and addressed per patient. Logistic regression was used to evaluate the odds of a participant having a comorbidity concern addressed if they were in the intervention versus usual care arm. JMP Pro 13 (SAS Institute, Cary, NC) and SAS 9.4 (SAS Institute) software was used for statistical analyses. Mean ± standard deviation is reported, and a P value < .05 was considered statistically significant.
RESULTS
Demographics and Comorbidity Characteristics
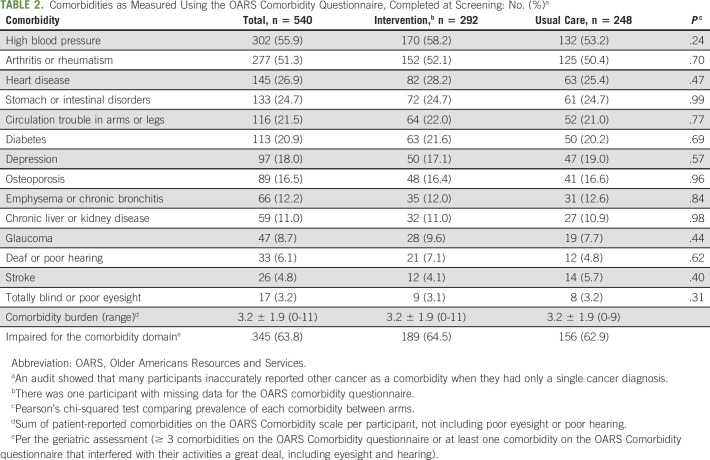
Patients (N = 541) were recruited from 30 practice clusters (17 intervention and 13 usual care). They had a mean ± SD age of 76.6 ± 5.2 years and were approximately half male and half female. Most (89.1%) were non-Hispanic White, and 7.4% were African American or Black; 12.2% had not finished high school, 36.0% had finished high school but did not attend college, and 51.6% had at least some college education; 49.0% had an income ≤ $50,000 US dollars per year, 30.3% had an income of > $50,000 US dollars per year, and 20.1% declined to report income. Participants had a range of cancer types with lung (25.9%) and GI cancers (25.5%) most represented. Full demographics and clinical characteristics for the enrolled patients were described previously.17 At screening, 94.6% of patients had at least one comorbidity; there were an average of 3.2 ± 1.9 and a median of three comorbidities per patient (Table 2). Approximately two thirds (63.8%) of all patients met the cutoff for impairment for comorbidities in the GA.17 The most reported comorbidities were high blood pressure (55.8%) and arthritis or rheumatism (51.2%). The comorbidities that interfered the most with activities of daily living were poor eyesight (56.2%), poor hearing (55.6%), and emphysema or chronic bronchitis (23.9%), whereas heart disease and high blood pressure rarely interfered with activities of daily living (Fig 1).
TABLE 2.
Comorbidities as Measured Using the OARS Comorbidity Questionnaire, Completed at Screening: No. (%)a
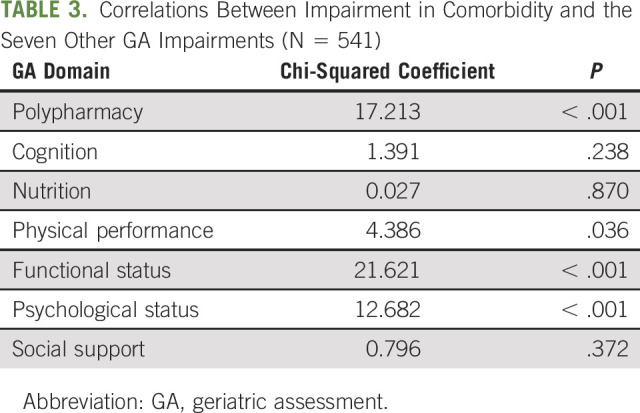
We investigated whether impairment in the comorbidity domain of the GA was related to impairment in other domains. We found that having impairment in comorbidities was associated with a higher probability of being impaired in the domains of polypharmacy, physical performance, functional status, and psychologic status (P < .05, Table 3). Furthermore, having impairment in comorbidities was associated with a greater total score on the GA (5.1 ± 1.3 including the comorbidity domain for those with impairment in the comorbidity domain v 3.5 ± 1.3 for those without impairment in the comorbidity domain on a scale of 1-8, P < .001).
TABLE 3.
Correlations Between Impairment in Comorbidity and the Seven Other GA Impairments (N = 541)
Clinical Conversations
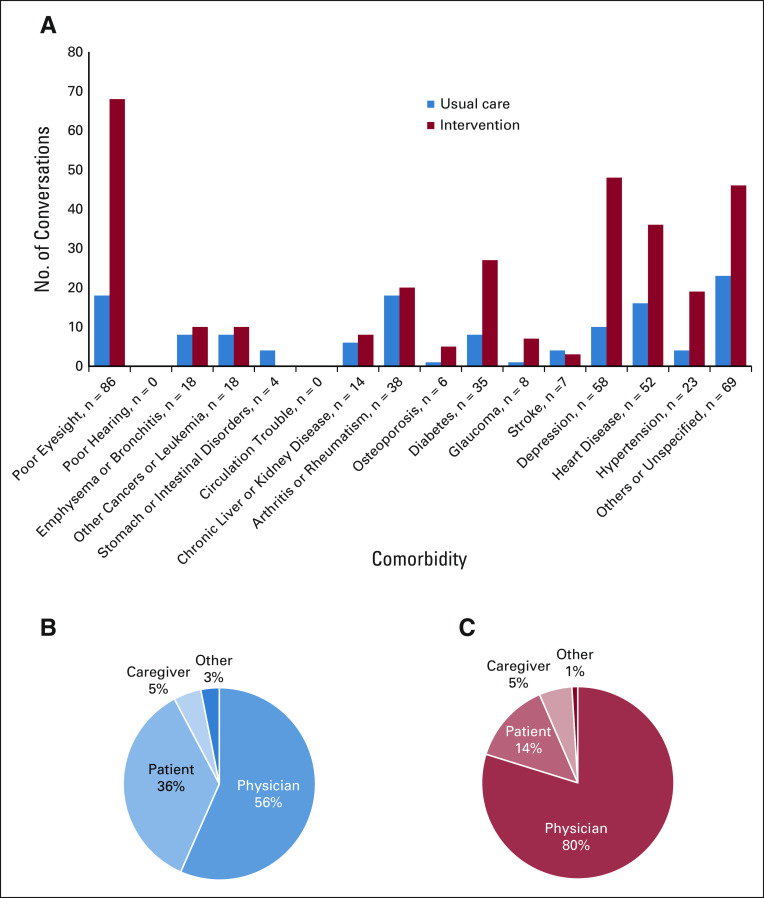
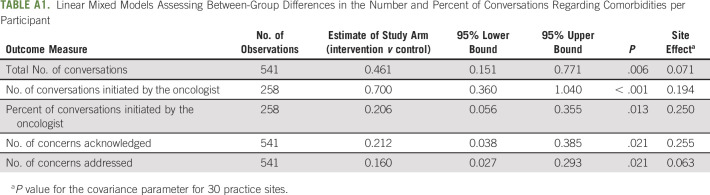
Conversations from a single clinic visit were examined for topics related to comorbidities; there were 436 topics about comorbidities among 258 participants. Providing the GA-guided intervention to oncologists increased the number of conversations regarding comorbidities 2.4-fold (129 in the usual care arm v 307 in the intervention arm; average of 0.52 per participant in the usual care arm and 0.99 in the intervention arm, P = .006, Appendix Table A1, online only). The greatest number of conversations were related to poor eyesight, diabetes, glaucoma, depression, and cardiovascular disease (Fig 2A). The percentage of conversations related to comorbidities that were initiated by oncologists was 55.1% in the usual care arm and 75.7% in the intervention arm (P = .013, Figs 2B and 2C). In the usual care arm, 81 concerns were acknowledged, and in the intervention arm, 160 concerns were acknowledged (0.32 v 0.52 per participant, respectively, P = .021). Similarly, 20 concerns were appropriately addressed in the usual care arm versus 76 in the usual care arm (0.08 v 0.24 per participant, respectively, 3.0-fold increase; P = .021). This equates to 2.39 times the odds of a patient having a comorbidity concern appropriately addressed if they were in the intervention arm versus the usual care arm (95% CI, 1.32 to 4.34; P = .004).
FIG 2.
Conversations related to comorbidities. (A) The percent of patients who had the condition at baseline and discussed the condition during their clinic visit. Comorbidities are given, with those interfering with activities a great deal on the left, and those that tend not to interfere as much on the right. (B) Who initiated the conversation in the usual care group and (C) who initiated the conversation in the intervention group. The oncologist initiated a greater number of conversations in the intervention arm than in the usual care arm (P = .01).
Management Recommendations
A questionnaire was provided to oncologists in the intervention arm after the patient's clinic visit to assess whether they or their staff considered and discussed specific aspects of comorbidities in light of the patient's treatment plan. Oncologists indicated that specific aspects of comorbidities were discussed the majority of the time—specifically, how comorbidities affect risks and benefits of treatment choices (76.3%), potential modifications to standard treatment plans (70.4%), and how cancer treatment could affect their comorbidities (73.7%, Table 4). Moreover, 81.1% of oncologists in the intervention arm recommended that their care team initiates direct communication with the patient's primary care physician about the plan for the patient's care; 40.9% indicated that they modified the dosage or schedule of cancer treatment because of concern regarding cancer treatment tolerability or worsening of comorbidities. Of patients who had chronic liver or kidney disease at screening and who were in the intervention arm, 50.0% (15 of 30) had oncologists who avoided nephrotoxic agents if another option was available and/or adjusted the dose appropriately. Also, of patients in the intervention arm who had diabetes at screening on the basis of the OARS, 36.8% (21 of 57) had oncologists who reported that they avoided neurotoxic agents if another option was equivalent. Additionally, of those with heart disease in the intervention arm, 18.6% (13 of 70) had oncologists who reported that they minimized the volume of agents and/or administered treatments at a slower infusion rate.
TABLE 4.
Percent of Oncologists Who Received the Geriatric Assessment–Guided Intervention and Who Reported Discussing Specific Aspects of Comorbidities (n = 186)
DISCUSSION
We demonstrated that providing a GA-guided intervention to oncologists more than doubled the number of conversations that occurred with patients regarding comorbidities, especially related to diabetes, cardiovascular disease, and other conditions that can directly affect cancer care. Furthermore, the GA-guided intervention prompted oncologists to adequately address patients' comorbidities three times more often than when the intervention was not provided. We were also able to capture how the GA-guided intervention directed the majority of clinicians to discuss practices that directly influence patient care (eg, modification of treatment options and engagement with the primary care physician). Overall, these data suggest that the GA summary plus guided interventions can positively influence the care of older adults with cancer and other comorbidities.
Comorbidities are highly prevalent among older patients with advanced cancer, and the GA-guided intervention provided an avenue to facilitate care coordination. The majority of participants in our study had comorbidities (95%) with an average of 3.2 and a range of 0-11; this corroborates previous work that reported a high prevalence of multimorbidity among older adults and comorbidities among patients with cancer.2,3,22-24 Practice guidelines usually address single diseases, and tailoring of cancer treatment to consider comorbid conditions is increasingly necessary for older patients with advanced cancer to improve tolerability and mitigate side effects of treatment.2 Thus, improving communication and coordination between patients, caregivers, oncologists, primary care physicians, and others in the treatment team, specifically related to comorbid conditions, is essential to optimize outcomes. Patients, oncologists, and primary care physicians are often uncertain about the role of primary care physicians and the priority of managing comorbidities during cancer treatment.25 However, it is important for primary care physicians to be present throughout cancer treatment to leverage their knowledge and insight into the management of comorbid conditions.25 Indeed, roles and priorities will likely differ depending on the patient and the situation and will change throughout the cancer experience, thus underscoring the importance of care coordination and continuous communication.6,26 Conversations between the oncologist and the primary care physician can also help identify potentially inappropriate medications because of risks of drug-drug or drug-disease interactions and deprescribe superfluous or potentially harmful medications.21,27 We demonstrate herein that providing the GA-guided intervention elicited more than twice the number of conversations regarding comorbidity and that physician-initiated conversations tended to account for the increase.
Quality conversations between patients and providers can improve health outcomes in multiple ways, many of which are indirect. For example, patients who are satisfied with communication with their providers are more trusting and adhere better to treatments.28 Data routinely show that conversations not only are simply emotionally beneficial, putting the patient at ease, but also can effectively guide decisions related to care and improve health outcomes.29,30 Conversations regarding comorbidities, specifically, can influence health outcomes by facilitating identification of appropriate interventions and treatment plans. We showed that oncologists actively addressed comorbidities three times more often (eg, patient referrals and health information) with the intervention versus usual care; future research should follow up on the effectiveness of these action plans on specific health outcomes.
Other interventions have been conducted to increase the quality of patient-provider communication, but not in the specific context of comorbidities in oncology clinics. For example, an intervention using nurse-led conversations on the basis of guided self-determination theory increased quality of life in patients with gynecologic cancer.31 Also, the Values and Options in Cancer Care intervention provided oncologists and patients with training in effective communication and demonstrated improved patient-centered communication compared with usual care.32 In a qualitative study, electronic health records facilitated communication between primary care physicians and oncologists in the context of comorbidities, although communication with patients and caregivers was not a focus.25 Although it is known that the GA increases conversations related to aging-related conditions in general and increases patient satisfaction,17 this study expands our knowledge on how GA-guided intervention increases conversations related to comorbidities specifically and provides an organized approach to care by guiding management recommendations.
This study drew from a data set of 541 older patients with advanced cancer from community oncology practices across the United States. We were able to integrate both qualitative data from transcripts of clinical encounters and quantitative data about comorbidity diagnoses in informative mixed methods analyses.33 The data are generalizable to a wide range of older patients with cancer across the country. However, our analyses were conducted on the content of a single clinic visit that might not have been representative of a typical clinic visit because of the recent completion of the GA. Patients in both arms completed the GA, which likely sparked conversations regarding comorbidities regardless of the guided management recommendations; therefore, we cannot comment on the effect of the GA on conversations per se, only the guided management recommendations. It is possible that comorbidities might have been discussed and incorporated into care at previous visits and were not relevant at this particular visit, although we believe that our large sample size provides an adequate representation of conversations in community oncology clinics with this population. In addition, 89% of our study population was non-Hispanic White, so the findings might not be generalizable to other races or ethnicities.
In conclusion, the interaction between cancer, cancer treatments, and specific comorbidities is extremely complex and management of all conditions simultaneously can be a formidable challenge for care teams. Consideration of comorbidities in cancer care is important because it can affect a patient's prognosis, tolerance of treatment, quality of life, and mortality.2,4,5 Herein, we showed that patients have a high comorbidity burden and we demonstrated that providing a GA-guided intervention to oncologists doubled the number of conversations that they had about comorbidities, leading to more concerns being acknowledged and appropriately addressed. These practices have the potential to improve patient satisfaction with cancer care17 and properly manage comorbidities during treatment of their advanced cancer.
ACKNOWLEDGMENT
We thank Teraisa Mullaney, Lisa Lowenstein, Patrick Davis, and Jennifer Peckham for coding the transcripts. We also thank Sandy Plumb for her administrative oversight of the project, Dr Eva Culakova for statistical guidance, and Dr Susan Rosenthal for editorial assistance in the preparation of this manuscript.
Appendix
TABLE A1.
Linear Mixed Models Assessing Between-Group Differences in the Number and Percent of Conversations Regarding Comorbidities per Participant
Richard F. Dunne
Consulting or Advisory Role: Exelixis
Nicholas J. Vogelzang
Employment: US Oncology
Stock and Other Ownership Interests: Caris Life Sciences
Honoraria: UpToDate, Pfizer, Novartis, Merck
Consulting or Advisory Role: Pfizer, Bayer, Genentech/Roche, AstraZeneca, Caris Life Sciences, Tolero Pharmaceuticals, Merck, Astellas Pharma, Boehringer Ingelheim, Corvus Pharmaceuticals, Modra Pharmaceuticals, Clovis Oncology, Janssen Oncology, Eisai, Myovant Sciences
Speakers' Bureau: Bayer, Sanofi, Genentech/Roche, Bristol Myers Squibb, Seattle Genetics/Astellas, Clovis Oncology, AVEO, Myovant Sciences, AstraZeneca
Research Funding: US Oncology, Endocyte, Merck, Suzhou Kintor Pharmaceuticals
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Genentech/Roche, US Oncology, Pfizer, Bayer/Onyx, Exelixis, AstraZeneca/MedImmune, Sanofi/Aventis
Luke J. Peppone
Consulting or Advisory Role: Charlotte's Web
Supriya G. Mohile
Consulting or Advisory Role: Seattle Genetics
Research Funding: Carevive
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2020 ASCO Annual Meeting.
SUPPORT
Supported by the Patient-Centered Outcomes Research Institute CD-12-11-4634 (S.G.M.); National Institutes of Health (NIH) National Cancer Institute (NCI) UG1CA189961 (Gary R. Morrow and Karen M. Mustian); NIH NCI T32CA102618 (Gary R. Morrow and Michelle C. Janelsins); NIH National Institute on Aging K24AG056589 and R33AG059206 (S.G.M.); the University of Rochester CTSA Award No. KL2TR001999 (N.J.G.); and the National Institute of Aging (NIA) K76AG064394 (A.M.).
AUTHOR CONTRIBUTIONS
Conception and design: Amber S. Kleckner, Supriya G. Mohile
Financial support: Supriya G. Mohile
Administrative support: Amber S. Kleckner, Supriya G. Mohile
Provision of study materials or patients: Elie G. Dib, Mark A. O'Rourke, Nicholas J. Vogelzang
Collection and assembly of data: Amber S. Kleckner, Megan Wells, Lee A. Kehoe, Mark A. O'Rourke, Nicholas J. Vogelzang, Elie G. Dib
Data analysis and interpretation: Amber S. Kleckner, Megan Wells, Nikesha J. Gilmore, Huiwen Xu, Allison Magnuson, Richard F. Dunne, Marielle Jensen-Battaglia, Mostafa R. Mohamed, Mark A. O'Rourke, Nicholas J. Vogelzang, Luke J. Peppone, Supriya G. Mohile
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Using Geriatric Assessment to Guide Conversations Regarding Comorbidities Among Older Patients With Advanced Cancer
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Richard F. Dunne
Consulting or Advisory Role: Exelixis
Nicholas J. Vogelzang
Employment: US Oncology
Stock and Other Ownership Interests: Caris Life Sciences
Honoraria: UpToDate, Pfizer, Novartis, Merck
Consulting or Advisory Role: Pfizer, Bayer, Genentech/Roche, AstraZeneca, Caris Life Sciences, Tolero Pharmaceuticals, Merck, Astellas Pharma, Boehringer Ingelheim, Corvus Pharmaceuticals, Modra Pharmaceuticals, Clovis Oncology, Janssen Oncology, Eisai, Myovant Sciences
Speakers' Bureau: Bayer, Sanofi, Genentech/Roche, Bristol Myers Squibb, Seattle Genetics/Astellas, Clovis Oncology, AVEO, Myovant Sciences, AstraZeneca
Research Funding: US Oncology, Endocyte, Merck, Suzhou Kintor Pharmaceuticals
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Genentech/Roche, US Oncology, Pfizer, Bayer/Onyx, Exelixis, AstraZeneca/MedImmune, Sanofi/Aventis
Luke J. Peppone
Consulting or Advisory Role: Charlotte's Web
Supriya G. Mohile
Consulting or Advisory Role: Seattle Genetics
Research Funding: Carevive
No other potential conflicts of interest were reported.
REFERENCES
- 1.King DE, Xiang J, Pilkerton CS: Multimorbidity trends in United States adults, 1988-2014. J Am Board Fam Med 31:503-513, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams GR, Mackenzie A, Magnuson A, et al. : Comorbidity in older adults with cancer. J Geriatr Oncol 7:249-257, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff JL, Starfield B, Anderson G: Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 162:2269-2276, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Patnaik JL, Byers T, Diguiseppi C, et al. : The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 103:1101-1111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao AV, Seo PH, Cohen HJ: Geratric assessment and comorbidity. Semin Oncol 31:149-159, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Rotenstein LS, Zhang Y, Jacobson JO: Chronic comorbidity among patients with cancer: An impetus for oncology and primary care collaboration. JAMA Oncol 5:1099-1100, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Hershman DL, Lacchetti C, Dworkin RH, et al. : Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:1941-1967, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Srokowski TP, Fang S, Hortobagyi GN, et al. : Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol 27:2170-2176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curigliano G, Cardinale D, Suter T, et al. : Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol 23:vii155-vii166, 2012. (suppl 7) [DOI] [PubMed] [Google Scholar]
- 10.Mohile SG, Velarde C, Hurria A, et al. : Geriatric assessment-guided care processes for older adults: A Delphi consensus of geriatric oncology experts. J Natl Compr Cancer Netw 13:1120-1130, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotrionte M, Biondi-Zoccai G, Abbate A, et al. : Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol 112:1980-1984, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Lichtman SM, Wildiers H, Launay-Vacher V, et al. : International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer 43:14-34, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Popa MA, Wallace KJ, Brunello A, et al. : Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J Geriatr Oncol 5:307-314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow A, Prusak ES, Barlow B, et al. : Interventions to reduce polypharmacy and optimize medication use in older adults with cancer. J Geriatr Oncol S1879-4068(20)30529-4, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Wildiers H, Heeren P, Puts M, et al. : International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32:2595-2603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohile SG, Dale W, Somerfield MR, et al. : Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 36:2326-2347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohile SG, Epstein RM, Hurria A, et al. : Communication with older patients with cancer using geriatric assessment: A cluster-randomized clinical trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol 6:196-204, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurria A, Gupta S, Zauderer M, et al. : Developing a cancer-specific geriatric assessment: A feasibility study. Cancer 104:1998-2005, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kehoe LA, Xu H, Duberstein P, et al. : Quality of life of caregivers of older patients with advanced cancer. J Am Geriatr Soc 67:969-977, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillenbaum GG, Smyer MA: The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 36:428-434, 1981 [DOI] [PubMed] [Google Scholar]
- 21.Ramsdale E, Lemelman T, Loh KP, et al. : Geriatric assessment-driven polypharmacy discussions between oncologists, older patients, and their caregivers. J Geriatr Oncol 9:534-539, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jørgensen TL, Hallas J, Friis S, et al. : Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer 106:1353-1360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jørgensen TL, Hallas J, Land LH, et al. : Comorbidity and polypharmacy in elderly cancer patients: The significance on treatment outcome and tolerance. J Geriatr Oncol 1:87-102, 2010 [Google Scholar]
- 24.Hurria A, Lichtman SM, Gardes J, et al. : Identifying vulnerable older adults with cancer: Integrating geriatric assessment into oncology practice. J Am Geriatr Soc 55:1604-1608, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sada Y, Street RL Jr, Singh H, et al. : Primary care and communication in shared cancer care: A qualitative study. Am J Manag Care 17:259-265, 2011 [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh J, Harrison JD, Young JM, et al. : What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res 10:132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed MR, Ramsdale E, Loh KP, et al. : Associations of polypharmacy and inappropriate medications with adverse outcomes in older adults with cancer: A systematic review and meta-analysis. Oncologist 25:e94-e108, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Street RL Jr, Makoul G, Arora NK, et al. : How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns 74:295-301, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Maly RC, Leake B, Silliman RA: Breast cancer treatment in older women: Impact of the patient-physician interaction. J Am Geriatr Soc 52:1138-1145, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Liang W: Communication between physicians and older women with localized breast cancer: Implications for treatment and patient satisfaction. J Clin Oncol 20:1008-1016, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Olesen ML, Duun-Henriksen AK, Hansson H, et al. : A person-centered intervention targeting the psychosocial needs of gynecological cancer survivors: A randomized clinical trial. J Cancer Surviv 10:832-841, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Epstein RM, Duberstein PR, Fenton JJ, et al. : Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: The VOICE randomized clinical trial. JAMA Oncol 3:92-100, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creswell JW: A Concise Introduction to Mixed Methods Research. Thousand Oaks, CA, SAGE Publications, 2015 [Google Scholar]