PURPOSE:
We identified (1) differences in localized prostate cancer (PCa) risk group at presentation and (2) disparities in access to initial treatment for Asian American, Native Hawaiian, and Pacific Islander (AANHPI) men with PCa after controlling for sociodemographic factors.
METHODS:
We assessed all patients in the National Cancer Database with localized PCa with low-, intermediate-, and high-risk disease who identified as Thai, White, Asian Indian, Chinese, Vietnamese, Korean, Japanese, Filipino, Hawaiian, Pacific Islander, Laotian, Pakistani, Kampuchean, and Hmong. Multivariable logistic regression defined adjusted odds ratios (AORs) with 95% CI of (1) presenting at progressively higher risk group and (2) receiving treatment or active surveillance with intermediate- or high-risk disease, adjusting for sociodemographic and clinical factors.
RESULTS:
Among 980,889 men (median age 66 years), all AANHPI subgroups with the exception of Thai (AOR = 0.84 [95% CI, 0.58 to 1.21], P > .05), Asian Indian (AOR = 1.12 [95% CI, 1.00 to 1.25], P > .05), and Pakistani (AOR = 1.34 [95% CI, 0.98 to 1.83], P > .05) men had greater odds of presenting at a progressively higher PCa risk group compared with White patients (Chinese AOR = 1.18 [95% CI, 1.11 to 1.25], P < .001; Japanese AOR = 1.36 [95% CI, 1.26 to 1.47], P < .001; Filipino AOR = 1.37 [95% CI, 1.29 to 1.46], P < .001; Korean AOR = 1.32 [95% CI, 1.18 to 1.48], P < .001; Vietnamese AOR = 1.20 [95% CI, 1.07 to 1.35], P = .002; Laotian AOR = 1.60 [95% CI, 1.08 to 2.36], P = .018; Hmong AOR = 4.07 [95% CI, 1.54 to 10.81], P = .005; Kampuchean AOR = 1.55 [95% CI, 1.03 to 2.34], P = .036; Asian Indian or Pakistani AOR = 1.15 [95% CI, 1.07 to 1.24], P < .001; Native Hawaiians AOR = 1.58 [95% CI, 1.38 to 1.80], P < .001; and Pacific Islanders AOR = 1.58 [95% CI, 1.37 to 1.82], P < .001). Additionally, Japanese Americans (AOR = 1.46 [95% CI, 1.09 to 1.97], P = .013) were more likely to receive treatment compared with White patients.
CONCLUSION:
Our findings suggest that there are differences in PCa risk group at presentation by race or ethnicity among Asian American, Native Hawaiian, and Pacific Islander subgroups and that there exist disparities in treatment patterns. Although AANHPI are often studied as a homogenous group, heterogeneity upon subgroup disaggregation underscores the importance of further study to assess and address barriers to PCa care.
INTRODUCTION
The American Cancer Society projects approximately 250,000 new cases of prostate cancer (PCa) and more than 30,000 PCa deaths in 2021.1 Localized PCa accounts for approximately 80% of new diagnoses.2 Within localized PCa, risk groups—which categorize disease severity on the basis of clinicopathologic characteristics3—aid treatment and prognostication.
Disparities in diagnosis, treatment, and mortality among men with PCa are extensively studied in the literature and tend to be associated with sociodemographic and genetic factors.4-7 Of particular importance, race and ethnic group have been shown to influence PCa severity and treatment disparities.7-9 These differences are likely mediated by a complex mix of tumor-specific and societal factors that map with race or ethnicity, including access to care, genomic ancestry, cultural preferences, and systemic barriers to care.4-6 Notably less is known about disparities in the risk group at presentation and subsequent treatment disparities among Asian American, Native Hawaiian, and Pacific Islander (AANHPI) men with localized PCa.
AANHPI men in the United States, as well as British Asian men,10 have a lower incidence of PCa compared with their counterparts of other ethnic groups.2 For instance, the incidence of localized PCa in the United States is as follows: AANHPI (aggregated data) at 48.9 men per 100,000, Black or African American at 153.8 men per 100,000, and White American at 94.5 men per 100,000.2 However, much of the data on AANHPI men with PCa are presented in aggregate2 and therefore mask disparities among different subgroups, as suggested by studies among Asian men in Asia.8 In addition, the vast inequities in income and educational attainment among AANHPI groups,11 as well as significant variation in sociocultural beliefs, immigration patterns, lifestyles, health behaviors, and barriers to care,12,13 may manifest in PCa disparities across the disease continuum.
Recent events during the COVID-19 pandemic highlight systematic and societal racism toward Asian Americans,14-16 underscoring existing health disparities that extend well beyond the pandemic.17 Similarly, Native Hawaiian populations faced racism and violence upon European colonization,18,19 with sociodemographic disparities that persist today. Native Hawaiians experience lower income, educational attainment, and access to care, as well as higher rates of diabetes, obesity, and cancer than other racial and ethnic groups,19,20 often paralleling the experiences of Pacific Islanders who identified as Native Chamorros, Māori, Kanak, and others.19 These past and present experiences of systemic and societal racism may create and contribute to disparities that affect AANHPI communities.
Therefore, examining patterns of racial and ethnic disparities in PCa can further elucidate subgroups of AANHPI patients who may benefit from improved access to screening and policies designed to mitigate barriers to care.21 Furthermore, with Asian men accounting for 23.3% of new PCa diagnoses worldwide,22 a study of disaggregated ethnicity data would not only elucidate disparities within the United States but also may provide insight into clinical features of these different ethnic groups in the countries to which AANHPI men can trace their ancestry. Using the National Cancer Database (NCDB), the present study aimed to identify differences in PCa risk group at presentation for Asian American, Native Hawaiian, and Pacific Islander men after controlling for sociodemographic factors. We also assessed disparities in access to initial treatment for men with localized PCa.
METHODS
Data Source and Patients
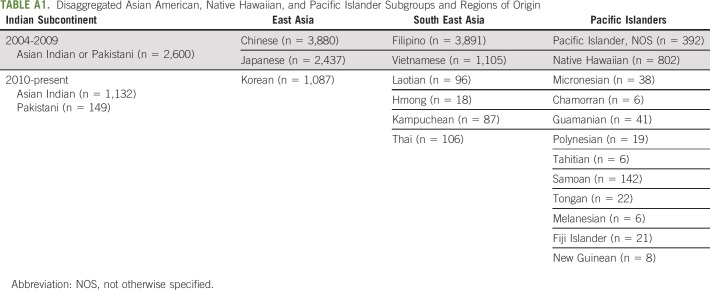
We performed a retrospective cohort analysis of data from 2004 to 2017 using the PCa Participant User File from the NCDB.23 Established in 1989, the NCDB is a nationwide US hospital–based cancer registry sponsored by the American Cancer Society and the American College of Surgeons. The NCDB captures approximately 70% of new cancer diagnoses in the United States, and importantly, it allows for disaggregation by Asian American subgroup given the relatively large sample size. We assessed all patients in the NCDB with localized (TxN0M0) prostate adenocarcinoma with low-, intermediate-, and high-risk disease who identified as Thai, White, Asian Indian or Pakistani, Asian Indian, Chinese, Vietnamese, Korean, Japanese, Filipino, Hawaiian, Pacific Islander, Laotian, Pakistani, Kampuchean, and Hmong. Notably, before 2010, South Asian patients who identified as Indian or Pakistani were grouped together. In 2010 and onward, patients who identified as Asian Indian or Pakistani were coded separately.23 Furthermore, given relatively low sample sizes and consistent with their disaggregation as a distinct ethnogeographic group from Asian Americans in the US Census in 2000, patients who identified as Micronesian, Chamorran, Guamanian, Polynesian, Tahitian, Samoan, Tongan, Melanesian, Fiji Islander, New Guinean, and Pacific Islander not otherwise specified were grouped together as a single cohort under Pacific Islander, recognizing that these groups represent distinct and unique Pacific Island cultures. Because of a relatively larger cohort size, patients who identified as Hawaiian were considered separately.
Men were categorized as having low- (Gleason ≤ 6, prostate-specific antigen [PSA] < 10 ng/mL, and cT1-T2a), intermediate- (Gleason 7, PSA 10-20 ng/mL, or cT2b-T2c), or high-risk disease (Gleason 8-10, PSA > 20 ng/mL, or cT3-T4) on the basis of AUA/ASTRO guidelines24,25 and consistent with previous studies.26 We excluded patients for whom there were incomplete data for the assignment of risk groups.
Clinical and Sociodemographic Covariates
The primary dependent variables of interest were TxN0M0 PCa risk group upon presentation and status of treatment by a provider (no treatment given, active surveillance or watchful waiting, or treatment given). Lack of treatment is coded in the NCDB as “Treatment is refused or the physician decides not to treat for any reason such as the presence of comorbidities.” Additionally, treatment after a period of active surveillance is considered subsequent treatment and is not encoded as active surveillance or watchful waiting.23
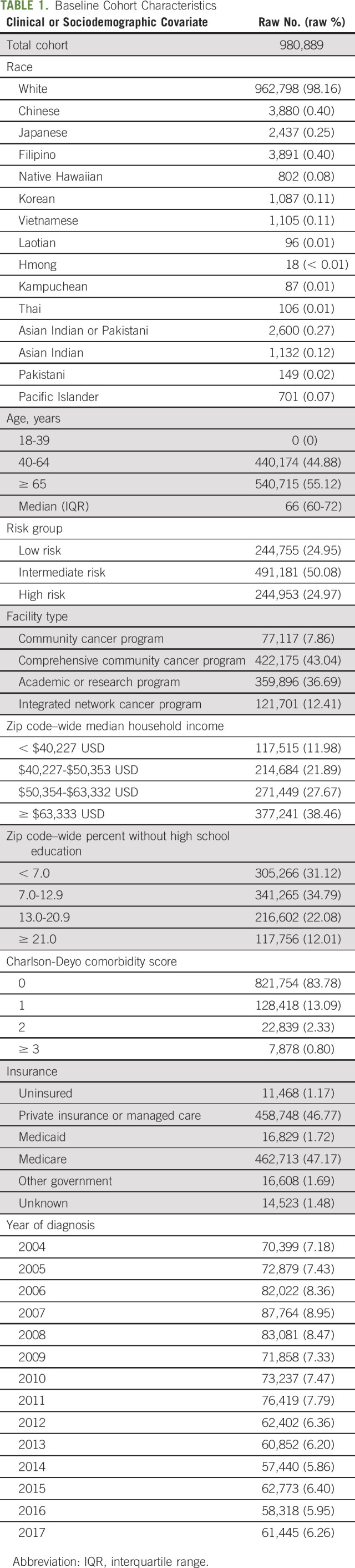
The primary independent variables of interest were race or ethnicity (Vietnamese, Korean, Thai, White, Asian Indian or Pakistani, Asian Indian, Chinese, Japanese, Filipino, Native Hawaiian, Pacific Islander, Laotian, Pakistani, Kampuchean, and Hmong), age, facility type, median household income for each patient's ZIP code (< $38,000 in US dollars [USD], $38,000-$47,999 USD, $48,000-$62,999 USD, or ≥ $63,000 USD), percentage of adults in the patient's ZIP code who did not graduate from high school (≥ 21.0%, 13.0%-20.9%, 7.0%-12.9%, or < 7.0%), Charlson-Deyo comorbidity coefficient (0, 1, 2, or ≥ 3), insurance status (uninsured, private insurance or managed care, Medicaid, Medicare, other government, or unknown), and year of diagnosis. Data on income and percentage of high-school graduates and proxies for socioeconomic status made use of quartiles that were derived from the 2012 American Community Survey for each patient's home ZIP code (Table 1).
TABLE 1.
Baseline Cohort Characteristics
Statistical Analysis
The statistical analyses carried out in this study involved ordinal logistic regressions to compare the effects of categorical and quantitative independent variables on ordinal dependent variables. Ordinal logistic regression models defined adjusted odds ratios (AOR) with 95% CI of (1) presenting in a progressively higher TxN0M0 PCa risk group, adjusting for all independent variables listed above, and (2) receiving treatment or active surveillance with intermediate- or high-risk disease, adjusting for all independent variables listed above and risk group.7 Such a regression model implicitly assumes that the difference between low- and intermediate-risk diseases is equivalent to that between intermediate- and high-risk diseases and that the difference between no treatment and active surveillance is equivalent to that between active surveillance and treatment. For both primary dependent variables, separate models defined AORs with 95% CIs, initially with White patients as the referent group given that they represent the largest race or ethnicity group in the NCDB; subsequently, analyses were conducted in which White patients were excluded from the regression to allow for comparison between Asian American subgroups, Native Hawaiians, and Pacific Islanders, with Chinese Americans as the referent given the sample size. Additionally, supplemental analyses for both primary dependent variables were conducted to retrieve odds ratios (ORs), only adjusting for race or ethnicity, age, and risk group to allow for the maximum possible patients despite disaggregation by race or ethnicity. Analyses were performed using Stata/SE 16.1 (StataCorp, College Station, TX). The hospital institutional review board deemed the study to be exempt given the use of deidentified data.
RESULTS
Baseline Characteristics
Of 980,889 men included in the study, the median age was 66 (interquartile range: 60-72) years; 244,755 (24.95%) had low-risk disease, 491,181 (50.08%) had intermediate-risk disease, and 244,953 (24.97%) had high-risk disease. White Americans made up 98.16% (n = 962,798), Chinese 0.40% (n = 3,880), Japanese 0.25% (n = 2,437), Filipino 0.40% (n = 3,891), Hawaiian 0.08% (n = 802), Korean 0.11% (n = 1,087), Vietnamese 0.11% (n = 1,105), Laotian 0.01% (n = 96), Hmong < 0.01% (n = 18), Kampuchean 0.01% (n = 87), Thai 0.01% (n = 106), Asian Indian or Pakistani 0.27% (n = 2,600), Asian Indian 0.12% (n = 1,132), Pakistani 0.02% (n = 149), and Pacific Islander 0.07% (n = 701; Appendix Table A1, online only). In the study cohort, 28,297 men (2.89%) had no insurance or were on Medicaid (Table 1).
Disparities in Risk Group at Presentation
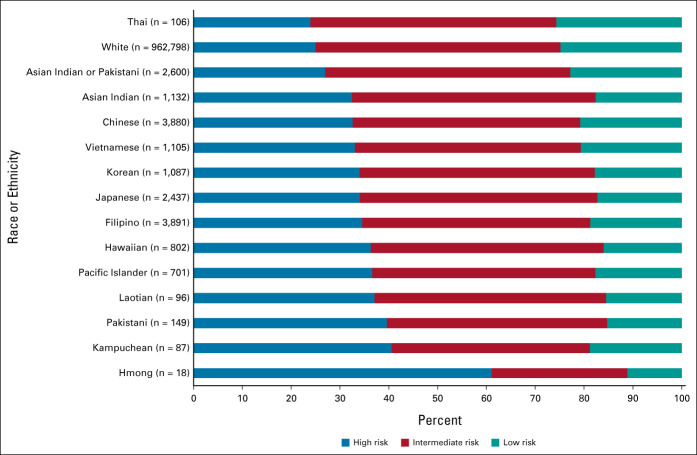
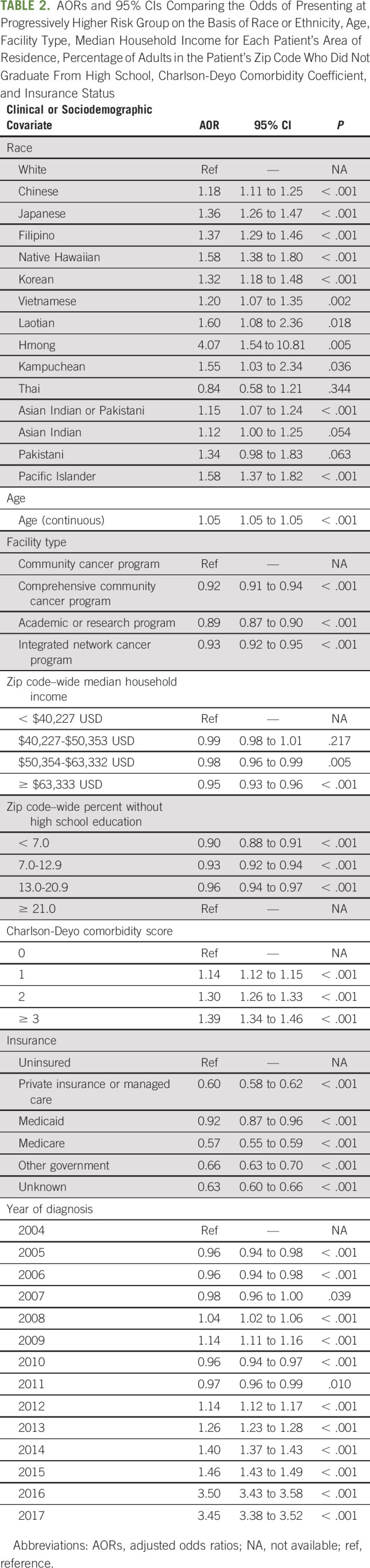
Ordinal logistic regression modeling indicated that, with the exception of Thai (AOR = 0.84; 95% CI, 0.58 to 1.21; P > .05), Asian Indian (AOR = 1.12; 95% CI, 1.00 to 1.25; P > .05), and Pakistani (AOR = 1.34; 95% CI, 0.98 to 1.83; P > .05) men, all AANHPI subgroups had greater odds of presenting at a progressively higher TxN0M0 PCa risk group compared with White patients (Fig 1 and Table 2; Chinese AOR = 1.18, 95% CI, 1.11 to 1.25, P < .001; Japanese AOR = 1.36, 95% CI, 1.26 to 1.47, P < .001; Filipino AOR = 1.37, 95% CI, 1.29 to 1.46, P < .001; Korean AOR = 1.32, 95% CI, 1.18 to 1.48, P < .001; Vietnamese AOR = 1.20, 95% CI, 1.07 to 1.35, P = .002; Laotian AOR = 1.60, 95% CI, 1.08 to 2.36, P = .018; Hmong AOR = 4.07, 95% CI, 1.54 to 10.81, P = .005; Kampuchean AOR = 1.55, 95% CI, 1.03 to 2.34, P = .036; Asian Indian or Pakistani AOR = 1.15, 95% CI, 1.07 to 1.24, P < .001; Native Hawaiians AOR = 1.58, 95% CI, 1.38 to 1.80, P < .001; and Pacific Islanders AOR = 1.58, 95% CI, 1.37 to 1.82, P < .001). ORs from analyses adjusting for race or ethnicity and age only demonstrated similar racial and ethnic disparities in risk group at presentation, with Asian Indian and Pakistani Americans becoming significantly more likely to present at a progressively higher risk group than White patients (Asian Indian OR = 1.48, 95% CI, 1.32 to 1.65, P < .001; Pakistani OR = 2.03, 95% CI, 1.50 to 2.76, P < .001; Appendix Table A2, online only).
FIG 1.
Proportion of men with low-, intermediate-, and high-risk prostate cancer at presentation, stratified by race or ethnicity.
TABLE 2.
AORs and 95% CIs Comparing the Odds of Presenting at Progressively Higher Risk Group on the Basis of Race or Ethnicity, Age, Facility Type, Median Household Income for Each Patient's Area of Residence, Percentage of Adults in the Patient's Zip Code Who Did Not Graduate From High School, Charlson-Deyo Comorbidity Coefficient, and Insurance Status
On subgroup analysis including only AANHPI patients, with Chinese as the referent group, Japanese (AOR = 1.15; 95% CI, 1.04 to 1.27; P = .005), Filipino (AOR = 1.18; 95% CI, 1.08 to 1.28; P < .001), Hmong (AOR = 3.45; 95% CI, 1.30 to 9.17; P = .013), Native Hawaiians (AOR = 1.37; 95% CI, 1.19 to 1.59; P < .001), and Pacific Islanders (AOR = 1.38; 95% CI, 1.18 to 1.61; P < .001) were more likely to present with greater risk disease (Appendix Table A4, online only).
Disparities in Treatment
Ordinal logistic regression modeling defined AORs for receiving treatment or active surveillance with intermediate- or high-risk disease, with White patients as the comparison group (AOR > 1 indicates greater odds of receiving treatment; AOR < 1 indicates lower odds of receiving treatment), and found that Japanese Americans were more likely to receive treatment compared with White patients (Japanese AOR = 1.46; 95% CI, 1.09 to 1.97; P = .013; Table 3). Hmong Americans were nominally more likely to receive treatment (Hmong AOR undefined, 0 no treatment, 0 active surveillance, and 12 treatment given).
TABLE 3.
AORs and 95% CIs Comparing the Odds of Receiving Treatment or Active Surveillance on the Basis of Race or Ethnicity, Age, Risk Group, Facility Type, Median Household Income for Each Patient's Area of Residence, Percentage of Adults in the Patient's Zip Code Who Did Not Graduate From High School, Charlson-Deyo Comorbidity Coefficient, and Insurance Status
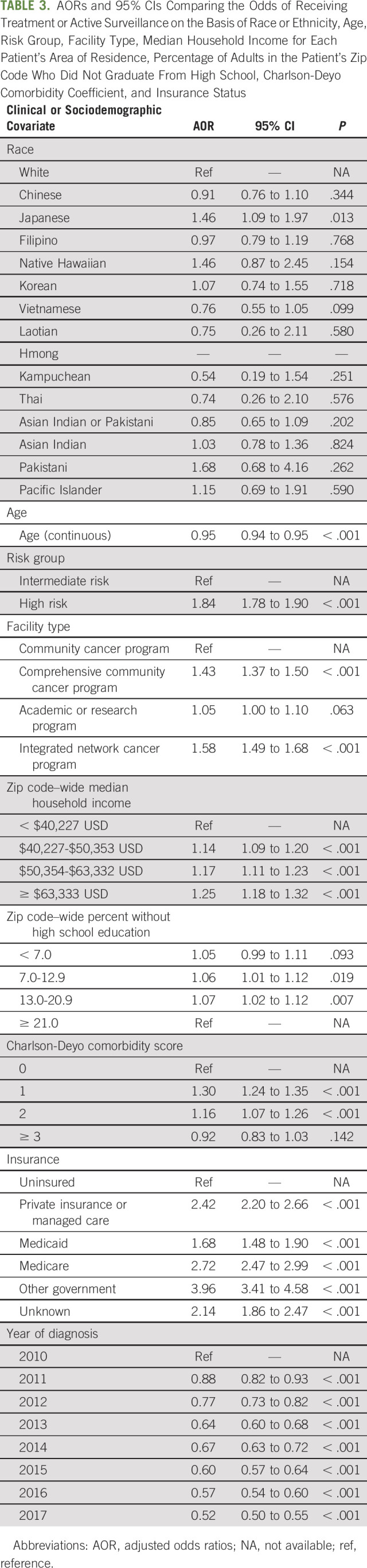
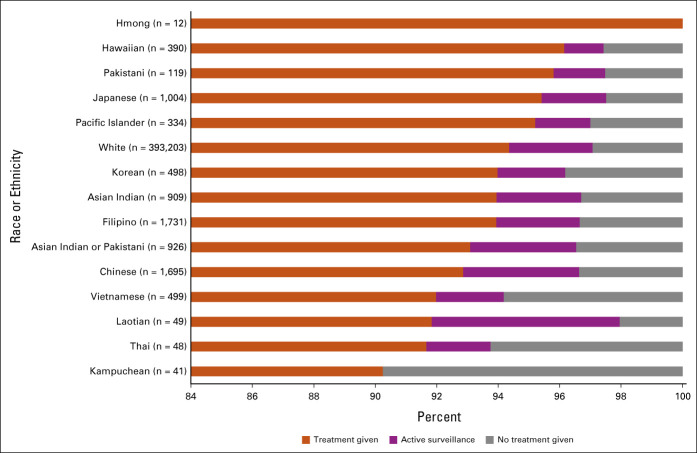
There was no statistically significant difference between the rate of treatment receipt between the other ethnic subgroups and their White counterparts. However, there was a trend suggesting that many of these ethnic groups were less likely to receive treatment, particularly among the Southeast Asian communities, i.e., Thai, Laotian, and Kampuchean (Fig 2 and Table 3; all P values > .05; Chinese AOR = 0.91, 95% CI, 0.76 to 1.10; Filipino AOR = 0.97, 95% CI, 0.79 to 1.19; Korean AOR = 1.07, 95% CI, 0.74 to 1.55; Vietnamese AOR = 0.76, 95% CI, 0.55 to 1.05; Laotian AOR = 0.75, 95% CI, 0.26 to 2.11; Kampuchean AOR = 0.54, 95% CI, 0.19 to 1.54; Thai AOR = 0.74, 95% CI, 0.26 to 2.10; Asian Indian or Pakistani AOR = 0.85, 95% CI, 0.65 to 1.09; Asian Indian AOR = 1.03, 95% CI, 0.78 to 1.36; Pakistani AOR = 1.68, 95% CI, 0.68 to 4.16; Native Hawaiians AOR = 1.46, 95% CI, 0.87 to 2.45; and Pacific Islanders AOR = 1.15, 95% CI, 0.69 to 1.91). ORs from analyses adjusting for race or ethnicity, age, and risk group only demonstrated similar racial and ethnic disparities for receiving treatment or active surveillance, with Chinese and Vietnamese Americans becoming significantly less likely to receive treatment than White patients (Chinese OR = 0.82, 95% CI, 0.68 to 0.99, P = .043; Vietnamese OR = 0.69, 95% CI, 0.50 to 0.95, P = .025; Appendix Table A3, online only).
FIG 2.
Proportion of men with prostate cancer given treatment, active surveillance, and no treatment, stratified by race or ethnicity.
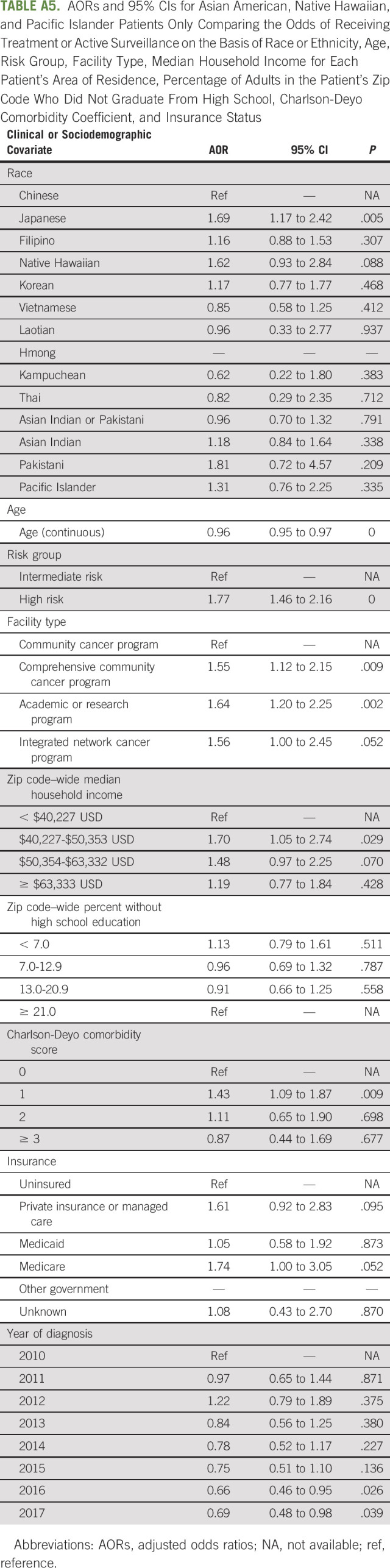
On subgroup analysis including only AANHPI patients, with Chinese as the referent group, Japanese Americans (AOR = 1.69; 95% CI, 1.17 to 2.42; P = .005) received active surveillance or treatment more frequently upon presentation (Appendix Table A5, online only).
DISCUSSION
In this study of 980,889 US men diagnosed with prostate adenocarcinoma from 2004 to 2017, we found that Chinese, Japanese, Filipino, Korean, Vietnamese, Laotian, Hmong, Kampuchean, Asian Indian or Pakistani, Native Hawaiian, and Pacific Islander race or ethnicity groups were associated with increased odds of presenting at a progressively higher TxN0M0 PCa risk group compared with White patients. These findings underscore that, although the incidence of PCa may be lower among AANHPI men compared with White and Black men on average,8 AANHPI men with PCa may be more likely to present with higher-risk disease at diagnosis. Despite these trends of worse risk group on presentation, several racial and ethnic groups were associated with lower odds of receiving treatment or active surveillance compared with White patients, although these results were not statistically significant. In a secondary analysis, we found that Japanese, Filipino, Hmong, Native Hawaiian, and Pacific Islander race or ethnicity were associated with increased odds of higher risk localized PCa compared with Chinese patients.
These results corroborate other studies suggesting that there are differences in rates of high-risk PCa by race or ethnicity among Asian American subgroups. Some studies suggest a role of genetic ancestry in predisposing Asian American men toward high-risk PCa, such as differential prevalence of ERG oncoprotein, low rate of PTEN loss, high CHD1 enrichment, FOXA1 alterations, and deletions in ZNF292 and CHD1.27,28 However, it is likely that sociodemographic factors play a role as well, such as variable accessibility to PSA testing and urology clinics, paired with environmental influences such as diet.8 For example, McCracken et al29 suggest that the finding that Filipino men are at higher risk of PCa incidence compared with other Asian American men may be due in part to dietary risk. The interaction between genetic ancestry and sociodemographic factors is complex and requires further investigation. These disparities suggest the need to further personalize screening and treatment regimens in ways that may take into consideration race and ethnicity, as well as sociodemographic factors associated with AANHPI subgroups.
In addition to these established disaggregated Asian American subgroup disparities, Native Hawaiian and Pacific Islander race or ethnicity was associated with worse localized PCa risk group than White patients. Native Hawaiians and Pacific Islanders in the United States, including Samoans, Chamorros, Fijians, Palauans, and Tongans, experience complex cancer disparities and suffer from disproportionately high cancer morbidity and mortality.30 These disparities are likely due to poor social determinants of health and access to preventive services; low rates of routine cancer screening and financial toxicity post-treatment persist in the Native Hawaiian and Pacific Islander populations.31-34 Although our study aggregates Pacific Islanders, it is important to recognize the considerable heterogeneity inherent to these groups; there are more than 39 different Pacific Island languages spoken as a second language in the United States alone and more than 1,300 languages across Melanesia, Micronesia, and Polynesia.35,36 More work is needed to characterize and address the specific needs of these groups.
Considering this context, the role of barriers to PCa care in Asian Americans, Native Hawaiians, and Pacific Islanders merits further exploration. Asian Americans are generally studied as a homogenous group that is physically healthier and economically more successful than White patients in the literature, but significant within-group differences and unique health care needs exist between the several ethnic groups comprising Asian Americans.37 Although subgroups such as Laotian, Hmong, and Kampuchean patients, who are more likely to be of lower socioeconomic status than other AANHPI groups,11 have small sample sizes (n < 100) in the NCDB over a 14-year period, these groups are nonetheless associated with high rates of high-risk PCa at presentation. Despite presenting with higher risk PCa, the current study highlights that Laotian, Hmong, and Kampuchean patients were not more likely to receive treatment than White patients.
Barriers in access to care, such as language, health literacy, and socioeconomic differences, may explain these phenomena. At 35%, Asian Americans and Hispanic Americans exhibit the highest rates of limited English proficiency; however, disaggregated statistics indicate that rates of disfluency are significantly higher for specific groups, such as Vietnamese (53%), Chinese (46%), Korean (45%), Thai (45%), Kampuchean (44%), Bangladeshi (43%), Laotian (42%), and Hmong (41%) Americans.38 Many of these groups exhibit linguistic isolation as well, in which no member of a given household is proficient in English. As a result, Asian American patients report obstacles in scheduling doctor appointments, locating health clinics, communicating with health care providers, and obtaining knowledge about medical conditions because of lack of proficiency in English. Additionally, patients may feel hesitant when using interpreter services or asking questions regarding their health, potentially because of fear that confidentiality will be breached within the broader Asian American community.37,39,40 A direct consequence of these language barriers is deficits in health literacy, which entails the ability to understand health literature and use analytic decision-making skills in health care settings. Health literacy deficits have the effect of limiting the care that patients would otherwise receive, and certain cultural beliefs regarding disease may be associated with worse health outcomes. Such literacy deficits are particularly exacerbated in elderly Asian American populations, who may neglect preventative medicine and seek care when symptoms are severe enough; for example, one study found that in women age 65 years or older, 14.8% of Korean immigrants had heard of mammography screenings, compared with 40.9% of Whites.41 Likewise, another study found that lifetime rates of cancer screening among Chicago-area older Chinese Americans were low and higher health literacy correlated with an increased likelihood of cancer screening in this cohort.42 With regard to socioeconomic differences, Asian Americans exhibit considerable variation across subgroups. For instance, rates of non-insurance were low among Japanese (7%), South Asian (11%), Filipino (11%), and Chinese (15%) Americans, and high amongst Vietnamese (21%) and Korean (36%) Americans.43 Moreover, only 55% of Vietnamese Americans have completed some college or above, compared with 73% of all Asian Americans.44 On the basis of these examples, language, literacy, and socioeconomic barriers may also influence Asian Americans in seeking prostate care treatment at a more advanced stage than White patients, further complicating the role of genetics and environment in racial disparities. Given these and other barriers to care among Asian Americans, it is important to address these in culturally sensitive and culturally aware ways rather than assuming that the underlying causes are biologic, given the close association between sustained inequitable access to resources and poor social determinants.45
Indeed, barriers to treatment by race are reflected in cancer treatment uptake and outcomes in the literature. Dee et al found that, for example, Black and Asian patients are more likely to refuse or choose to not complete potentially survival-prolonging locoregional treatment, such as radiotherapy and surgery, for localized prostate adenocarcinoma despite provider recommendation.26,46 Alty et al47 similarly showed that Black patients are more likely to refuse surgery for colon cancer and greater refusal correlates with worse overall survival. Although there was no statistically significant difference in treatment uptake for most subgroups, the findings in the present study are slightly more complex because of the disaggregation of Asian Americans, as Chinese, Filipino, Korean, Vietnamese, Laotian, Kampuchean, Asian Indian or Pakistani, and Asian Indian patients received treatment less frequently on average than White patients (with a subset of these having significant ORs). However, Japanese, Pakistani, Native Hawaiian, and Pacific Islander patients received treatment more frequently on average than White patients. This split in treatment uptake by subgroup likely has complex underpinnings and may reflect differential barriers to care and data availability in the NCDB.
In future studies, it will be critical to separate out the role of genetic variants by race or ethnicity with sociodemographic factors that affect stage of presentation and access to treatment for PCa. For example, one factor that may elucidate these differences upon further investigation is screening rate of PCa by race or ethnicity during primary care appointments. This is motivated by data that suggest that many Asian countries may have lower incidences of PCa, but screening for PCa across the continent has been rapidly rising over the past two decades.48 Although some evidence indicates that Asian men display lower rates of PCa diagnosis in a controlled setting with equal rates of PSA-independent biopsies as White men over a four-year time period,49 longer-term studies are required to fully understand the relationship between screening and diagnosis. Indeed, studies from the United States offer conflicting results. On the one hand, Asian Americans have reported PSA screening less frequently than White men in California and there is an association between Asian and Pacific Islander race or ethnicity and higher PSA at diagnosis in the NCDB.50,51 Earlier studies revealed that, consistent with decreased screening behavior, Asian Americans were more likely than Whites to be diagnosed at a more advanced stage.52-54 However, more recent studies have concluded that Asian Americans were more likely to present at an earlier stage of disease than Whites, consistent with findings from another study on a population with mandatory PSA screening.51,55 Regardless, the higher association between Gleason score and Asian American race or ethnicity reported in recent studies is unlikely to be driven solely by screening behavior, since higher Gleason score has not been explicitly linked with timing of diagnosis.52,53,56 Finally, qualitative assessment is important to probe the reasons that different Asian American subgroups have for not receiving or pursuing treatment, despite tending to present at higher risk group of PCa.
Our study must be viewed in terms of its limitations. First, we could not completely ascertain the reasons for disparate risk group at presentation or access to treatment, which will be an important area for future qualitative studies. Second, our retrospective approach suffers from lack of quality control in data collection and potential for selection bias in the choice of patients represented by the NCDB and therefore carries the risk of misclassification. There are also other limitations within the NCDB data set—for instance, the database only covers the first course of cancer treatment, with no information about subsequent treatments. Confounders not included in the data set may partially explain the identified disparities. However, the NCDB is one of the largest available data sources on Asian Americans and PCa, underscoring the need for greater representation of minority racial and ethnic groups in data sources. Third, the use of discrete race and ethnic groups likely misses the nuances of cultural factors that may influence disparities; for example, the database does not specifically include patients who identified with multiple AANHPI identities and provides no information on cultural preferences other than the name of the group with which people identify. Additionally, our approach involved aggregating Pacific Islanders because of sample size, despite the considerable heterogeneity among the many diverse Pacific Islander cultures.35,36 Furthermore, groups such as Burmese and Malay are not included in the NCDB, limiting our understanding of the disparities affecting these populations. Therefore, our findings are hypothesis-generating and should be used to guide the study of disparities in patient- and provider-level biases in PCa care, as well as efforts to address and mitigate these disparities.
In conclusion, consistent with recommendations from cancer epidemiologists and oncology providers, our study uses a disaggregated approach to understand the risk group and treatment disparities in the vast spectrum of AANHPI men.17 Disparities exist in localized PCa risk group upon presentation and access to treatment in Thai, Asian Indian or Pakistani, Chinese, Vietnamese, Korean, Japanese, Filipino, Hawaiian, Pacific Islander, Laotian, Kampuchean, and Hmong men relative to White men, highlighting the need for further investigation into varying genetic and environmental influences, as well as barriers to care differentially affecting Asian American men with PCa. Assessing and addressing reasons for these disparities may help mitigate disparities by ensuring that all individuals have access to treatment. Furthermore, understanding cultural, sociodemographic, and clinical factors associated with presentation and treatment disparities may promote improved and shared decision making.
APPENDIX
TABLE A1.
Disaggregated Asian American, Native Hawaiian, and Pacific Islander Subgroups and Regions of Origin
TABLE A2.
ORs and 95% CIs Comparing the Odds of Presenting at Progressively Higher Risk Group on the Basis of Race or Ethnicity and Age
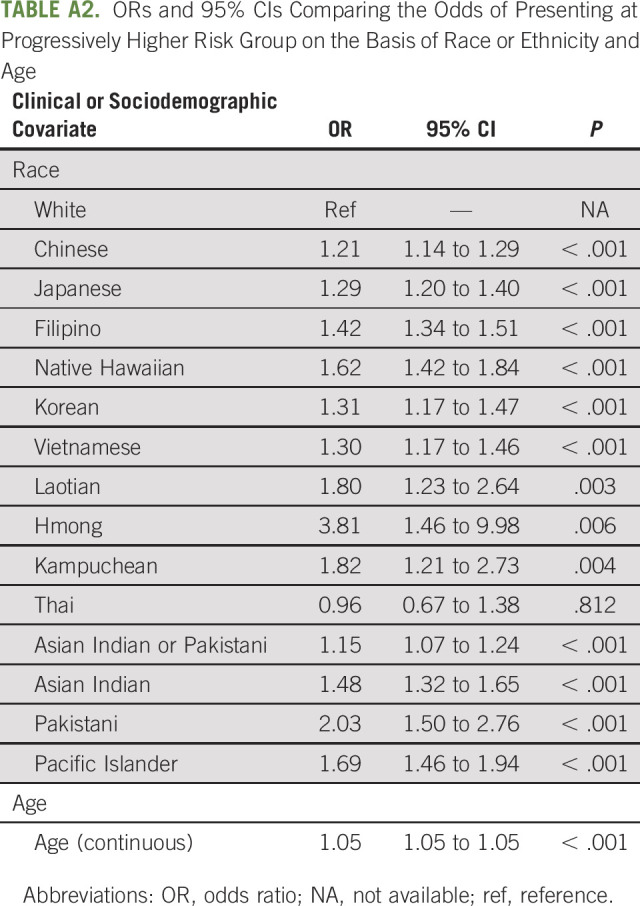
TABLE A3.
ORs and 95% CIs Comparing the Odds of Receiving Treatment or Active Surveillance on the Basis of Race or Ethnicity, Age, and Risk Group
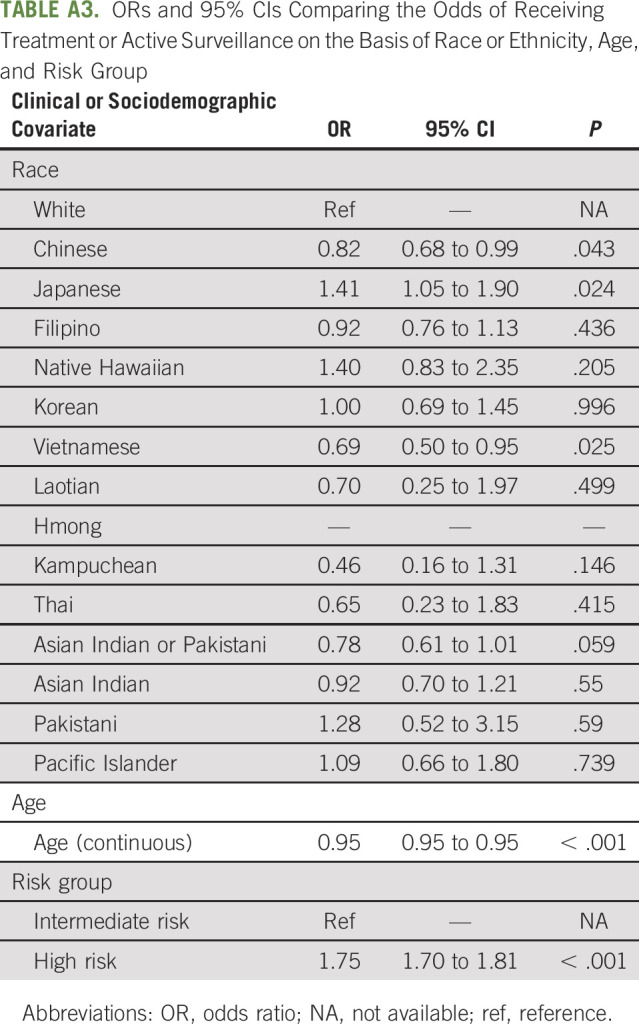
TABLE A4.
AORs and 95% CIs for Asian American, Native Hawaiian, and Pacific Islander Patients Only Comparing the Odds of Presenting at Progressively Higher Risk Group on the Basis of Race or Ethnicity, Age, Facility Type, Median Household Income for Each Patient's Area of Residence, Percentage of Adults in the Patient's Zip Code Who Did Not Graduate From High School, Charlson-Deyo Comorbidity Coefficient, and Insurance Status
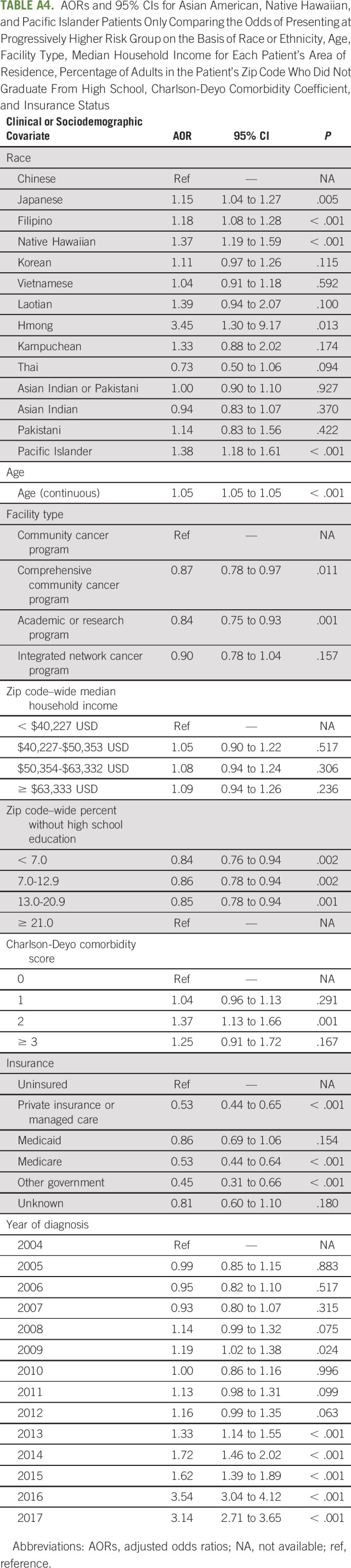
TABLE A5.
AORs and 95% CIs for Asian American, Native Hawaiian, and Pacific Islander Patients Only Comparing the Odds of Receiving Treatment or Active Surveillance on the Basis of Race or Ethnicity, Age, Risk Group, Facility Type, Median Household Income for Each Patient's Area of Residence, Percentage of Adults in the Patient's Zip Code Who Did Not Graduate From High School, Charlson-Deyo Comorbidity Coefficient, and Insurance Status
Kenrick Ng
Honoraria: Tesaro, Pfizer, Boehringer Ingelheim
Travel, Accommodations, Expenses: GlaxoSmithKline UK Ltd, Conquer Cancer Foundation,
Vinayak Muralidhar
Employment: Northwest Permanente
Brandon A. Mahal
Honoraria: Cancer Study Group
Speakers' Bureau: Myovant Sciences
Other Relationship: Prostate Cancer Foundation, Department of Defense-Prostate Cancer Research Program, American Society for Radiation Oncology
Quoc-Dien Trinh
Honoraria: Astellas Pharma, Bayer, Janssen
Research Funding: Intuitive Surgical
Paul L. Nguyen
Stock and Other Ownership Interests: Volatilyx (I), Augmenix
Consulting or Advisory Role: Medivation, GenomeDx, Ferring, Nanobiotix, Dendreon, Augmenix, GigaGen, Biogen (I), Bayer, Astellas Pharma, Blue Earth Diagnostics, Augmenix, Coda, Boston Scientific, Janssen Oncology, Myovant Sciences
Research Funding: Astellas Pharma, Nanobiotix, Janssen, Wako Diagnostics (I), Bayer
Patents, Royalties, Other Intellectual Property: Wife has a patent on volatile diagnostics of infections
Travel, Accommodations, Expenses: Ferring
No other potential conflicts of interest were reported.
SUPPORT
Funding grant No. R01-CA240582 (P.L.N.).
P.L.N. and E.C.D. contributed equally as co-corresponding authors.
DATA SHARING STATEMENT
Patient Deidentified National Cancer Database is available from the American College of Surgeons and is not owned by the authors of this work.
AUTHOR CONTRIBUTIONS
Conception and design: Bhav Jain, Edward Christopher Dee
Collection and assembly of data: Bhav Jain, Edward Christopher Dee
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prostate Cancer Disparities in Risk Group at Presentation and Access to Treatment for Asian Americans, Native Hawaiians, and Pacific Islanders: A Study With Disaggregated Ethnic Groups
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kenrick Ng
Honoraria: Tesaro, Pfizer, Boehringer Ingelheim
Travel, Accommodations, Expenses: GlaxoSmithKline UK Ltd, Conquer Cancer Foundation,
Vinayak Muralidhar
Employment: Northwest Permanente
Brandon A. Mahal
Honoraria: Cancer Study Group
Speakers' Bureau: Myovant Sciences
Other Relationship: Prostate Cancer Foundation, Department of Defense-Prostate Cancer Research Program, American Society for Radiation Oncology
Quoc-Dien Trinh
Honoraria: Astellas Pharma, Bayer, Janssen
Research Funding: Intuitive Surgical
Paul L. Nguyen
Stock and Other Ownership Interests: Volatilyx (I), Augmenix
Consulting or Advisory Role: Medivation, GenomeDx, Ferring, Nanobiotix, Dendreon, Augmenix, GigaGen, Biogen (I), Bayer, Astellas Pharma, Blue Earth Diagnostics, Augmenix, Coda, Boston Scientific, Janssen Oncology, Myovant Sciences
Research Funding: Astellas Pharma, Nanobiotix, Janssen, Wako Diagnostics (I), Bayer
Patents, Royalties, Other Intellectual Property: Wife has a patent on volatile diagnostics of infections
Travel, Accommodations, Expenses: Ferring
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society : Cancer Facts & Figures 2021, 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf [Google Scholar]
- 2.Siegel DA, O’Neil ME, Richards TB, et al. : Prostate cancer incidence and survival, by stage and race/ethnicity—United States, 2001–2017. MMWR Morb Mortal Wkly Rep 69:1473-1480, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohler JL, Antonarakis ES, Armstrong AJ, et al. : Prostate cancer, version 2.2019. J Natl Compr Cancer Netw 17:479-505, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Butler S, Muralidhar V, Chavez J, et al. : Active surveillance for low-risk prostate cancer in black patients. N Engl J Med 380:2070-2072, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahal BA, Alshalalfa M, Spratt DE, et al. : Prostate cancer genomic-risk differences between African-American and white men across Gleason scores. Eur Urol 75:1038-1040, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Krimphove MJ, Fletcher SA, Cole AP, et al. : Quality of care in the treatment of localized intermediate and high risk prostate cancer at minority serving hospitals. J Urol 201:735-741, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Dee EC, Nezolosky MD, Chipidza FE, et al. : Prostate cancer-specific mortality burden by risk group among men with localized disease: Implications for research and clinical trial priorities. Prostate 80:1128-1133, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Ito K: Prostate cancer in Asian men. Nat Rev Urol 11:197-212, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Ng K, Wilson P, Mutsvangwa K, et al. : Overall survival of black and white men with metastatic castration-resistant prostate cancer (mCRPC): A 20-year retrospective analysis in the largest healthcare trust in England. Prostate Cancer Prostatic Dis 24:718-724, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Lloyd T, Hounsome L, Mehay A, et al. : Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008-2010. BMC Med 13:171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochhar R, Cilluffo A: Income Inequality in the U.S. is Rising Most Rapidly Among Asians. Pew Research Center, Washington, DC. [Google Scholar]
- 12.López G, Ruiz N, Patten E: Key Facts about Asian Americans. Pew Research Center. 2017. https://www.pewresearch.org/fact-tank/2017/09/08/key-facts-about-asian-americans/ [Google Scholar]
- 13.Gordon NP, Lin TY, Rau J, et al. : Aggregation of Asian-American subgroups masks meaningful differences in health and health risks among Asian ethnicities: An electronic health record based cohort study. BMC Public Health 19:1551, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.8 Dead in Atlanta Spa Shootings, With Fears of Anti-Asian Bias. The New York Times. 2021. https://www.nytimes.com/live/2021/03/17/us/shooting-atlanta-acworth [Google Scholar]
- 15.Cowan J: A Tense Lunar New Year for the Bay Area After Attacks on Asian-Americans. The New York Times, 2021. https://www.nytimes.com/2021/02/12/us/asian-american-racism.html [Google Scholar]
- 16.Cabral S: Covid “Hate Crimes” against Asian Americans on Rise. BBC News, 2021. https://www.bbc.com/news/world-us-canada-56218684 [Google Scholar]
- 17.Dee EC, Chen S, Santos PMG, et al. : Anti-Asian American racism: A wake-up call for population-based cancer research. Cancer Epidemiol Biomarkers Prev 30:1455-1458, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Swanson DA. The number of native Hawaiians and part-Hawaiians in Hawaiʻi, 1778 to 1900: Demographic estimates by age. 2020. In: Jivetti B, Hoque MN (eds) Population Change and Public Policy. Applied Demography Series, vol 11. Springer, Cham. 10.1007/978-3-030-57069-9_17 [DOI]
- 19.Taparra K, Miller RC, Deville C: Navigating native Hawaiian and Pacific Islander cancer disparities from a cultural and historical perspective. JCO Oncol Pract 17:130-134, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Doàn LN, Takata Y, Sakuma KLK, et al. : Trends in clinical research including Asian American, Native Hawaiian, and Pacific Islander participants funded by the US National Institutes of Health, 1992 to 2018. JAMA Netw Open 2:e197432, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skalla KA, Bakitas M, Furstenberg CT, et al. : Patients’ need for information about cancer therapy. Oncol Nurs Forum 31:313-319, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Freedland SJ, Ye D: Prostate cancer and prostatic diseases best of Asia, 2019: Challenges and opportunities. Prostate Cancer Prostatic Dis 23:197-198, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Participant User Files. National Cancer Database. 2017. https://www.facs.org/quality-programs/cancer/ncdb/puf [Google Scholar]
- 24.D’Amico AV, Whittington R, Bruce Malkowicz S, et al. : Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc 280:969-974, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Sanda MG, Cadeddu JA, Kirkby E, et al. : Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Am Urol Assoc 199:683-690, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Dee EC, Arega MA, Yang DD, et al. : Disparities in refusal of locoregional treatment for prostate adenocarcinoma. JCO Oncol Pract 17:e1489-e1501, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Mo M, Wei Y, et al. : Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol 18:282-301, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Li J, Xu C, Lee HJ, et al. : A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature 580:93-99, 2020 [DOI] [PubMed] [Google Scholar]
- 29.McCracken M, Olsen M, Chen MS, et al. : Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin 57:190-205, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Goggins WB, Wong GKC: Poor survival for US Pacific Islander cancer patients: Evidence from the surveillance, epidemiology, and end results database: 1991 to 2004. J Clin Oncol 25:5738-5741, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Tanjasiri SP, Mouttapa M, Sablan-Santos L, et al. : Design and outcomes of a community trial to increase pap testing in pacific islander women. Cancer Epidemiol Biomarkers Prev 28:1435-1442, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Mishra SI, Bastani R, Huang D, et al. : Mammography screening and Pacific Islanders: Role of cultural and psychosocial factors. J Cancer Educ 22:32-36, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kaalekahi JM, Gandhi KR, Chen JJ, et al. : Colonoscopy screening among Native Hawaiians at Queen’s Medical Center between August 2011 and January 2013. Hawaii J Med Public Health 75:13-17, 2016 [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Narcisse MR, McElfish PA: Medical financial hardship reported by Native Hawaiian and Pacific Islander cancer survivors compared with non-Hispanic whites. Cancer 126:2900-2914, 2020 [DOI] [PubMed] [Google Scholar]
- 35.The White House Initiative on Asian Americans and Pacific Islanders. US Department of Commerce. https://www.commerce.gov/bureaus-and-offices/os/whiaapi [Google Scholar]
- 36.Jolly M Tcherkézoff S Tryon DT, et al. : Oceanic encounters: Exchange, desire, violence, ANU Press. http://www.jstor.org/stable/j.ctt24h8jn [Google Scholar]
- 37.Kim W, Keefe RH: Barriers to healthcare among Asian Americans. Soc Work Public Health 25:286-295, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Ramakrishnan K, Ahmad FZ: Language Diversity and English Proficiency: Part of the “State of Asian Americans and Pacific Islanders” Series, 2014. http://www.census.gov/acs/www/data_documentation/pums_data/ [Google Scholar]
- 39.Lee S, Martinez G, Ma GX, et al. : Barriers to health care access in 13 Asian American communities. Am J Health Behav 34:21-30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Lancet : Racism in the USA: Ensuring Asian American health equity. Lancet. 2021;397:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juon HS, Kim M, Shankar S, et al. : Predictors of adherence to screening mammography among Korean American women. Prev Med 39:474-481, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Li C-C, Matthews AK, Dong X: The influence of health literacy and acculturation on cancer screening behaviors among older Chinese Americans. Gerontol Geriatr Med 4:233372141877819, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kao D: Factors associated with ethnic differences in health insurance coverage and type among Asian Americans. J Community Health 35:142-155, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Budiman A: Vietnamese in the U.S. Fact Sheet. Pew Research Center, Washington, DC. [Google Scholar]
- 45.Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. : Race and genetic ancestry in medicine—A time for reckoning with racism. N Engl J Med 384:474-480, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dee EC, Muralidhar V, Arega MA, et al. : Factors influencing noncompletion of radiation therapy among men with localized prostate cancer. Int J Radiat Oncol Biol Phys 109:1279-1285, 2021 [DOI] [PubMed] [Google Scholar]
- 47.Alty IG, Dee EC, Cusack JC, et al. : Refusal of surgery for colon cancer: Sociodemographic disparities and survival implications among US patients with resectable disease. Am J Surg 221:39-45, 2021 [DOI] [PubMed] [Google Scholar]
- 48.Zhang K, Bangma CH, Roobol MJ: Prostate cancer screening in Europe and Asia. Asian J Urol 4:86-95, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidal AC, Oyekunle T, Feng T, et al. : Asian race and risk of prostate cancer: Results from the REDUCE study. Cancer Epidemiol Biomarkers Prev 29:2165-2170, 2020 [DOI] [PubMed] [Google Scholar]
- 50.California Health Interview Survey : UCLA Center for Health Policy Research. http://healthpolicy.ucla.edu/chis/Pages/default.aspx [Google Scholar]
- 51.Fedewa SA, Etzioni R, Flanders WD, et al. : Association of insurance and race/ethnicity with disease severity among men diagnosed with prostate cancer, National Cancer Database 2004-2006. Cancer Epidemiol Biomarkers Prev 19:2437-2444, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Robbins AS, Koppie TM, Gomez SL, et al. : Differences in prognostic factors and survival among white and Asian men with prostate cancer, California, 1995-2004. Cancer 110:1255-1263, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Man A, Pickles T, Chi KN, et al. : Asian race and impact on outcomes after radical radiotherapy for localized prostate cancer. J Urol 170:901-904, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Oakley-Girvan I, Kolonel LN, Gallagher RP, et al. : Stage at diagnosis and survival in a multiethnic cohort of prostate cancer patients. Am J Public Health 93:1753-1759, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raymundo EM, Rice KR, Chen Y, et al. : Prostate cancer in Asian Americans: Incidence, management and outcomes in an equal access healthcare system. BJU Int 107:1216-1222, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Lavery HJ, Droller MJ: Do Gleason patterns 3 and 4 prostate cancer represent separate disease states? J Urol 188:1667-1675, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient Deidentified National Cancer Database is available from the American College of Surgeons and is not owned by the authors of this work.