Abstract
Coronavirus disease 2019 (COVID-19) has encompassed the globe since it was first observed just under 2 years ago. Although the disease is predominantly a respiratory illness, there have been observed complications throughout the various organ systems. Namely, cardiovascular complications, and, more specifically, arrhythmic complications have been described throughout the pandemic in patients with COVID-19. Management of atrial arrhythmias, ventricular arrhythmias, and bradyarrhythmias in patients with COVID-19 infection has been largely guided by our prior experience in the management of these arrhythmias in similar patient populations without infection. However, this review aims to highlight the specific considerations as they pertain to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the various arrhythmic manifestations observed with this disease.
Keywords: COVID-19, SARS-CoV-2, Arrhythmias, Atrial fibrillation, Ventricular tachycardia, Ventricular tachycardia storm, Atrioventricular block
Graphical abstract
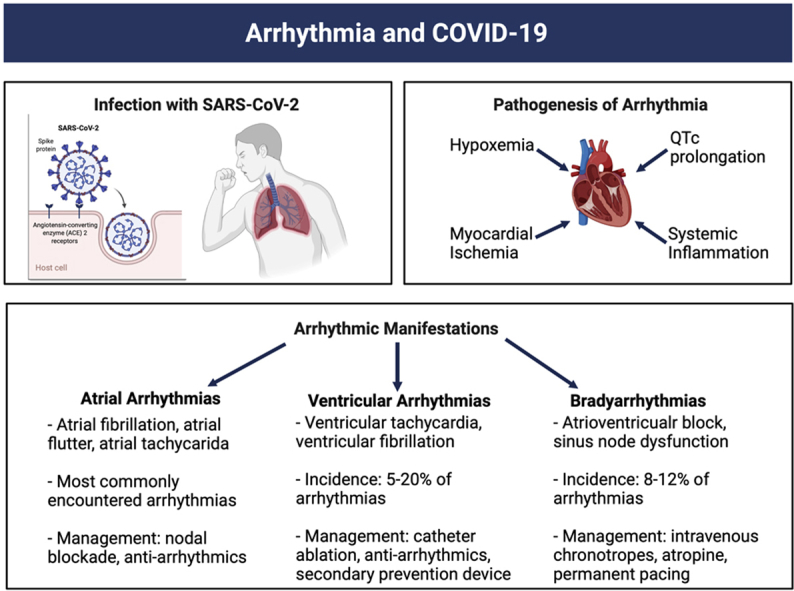
Key Findings.
-
▪
Infection with COVID-19 has been associated with many extrapulmonary manifestations; cardiac arrhythmias have become one of the more commonly reported. This review highlights the diagnosis and management of the most common arrhythmic manifestations associated with COVID-19.
-
▪
Managing arrhythmias in patients with COVID-19 can bring a unique set of challenges not experienced in other patients with the same arrhythmia. These unique challenges are addressed with proposed management strategies.
-
▪
In addition to the disease itself, there have been cardiovascular implications of the various therapeutics used to combat COVID-19, which are also highlighted in this manuscript.
Background
Coronavirus disease 2019 (COVID-19) is transmitted through the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is a significant and widespread international health threat. As of the time of this writing, there have been more than 250 million COVID-19 cases globally with over 5 million deaths.1 Significant differences in patient presentation have been observed in the disease, with significantly fewer hospitalizations and death recorded initially for patients under 50 years of age.2 While immediate symptoms of the disease are well known, such as anosmia, longer-term complications of COVID-19 are receiving increasing coverage under the umbrella of postacute sequelae of COVID-19 (PASC). These longer-term complications have been numerous, ranging from pulmonary to cardiovascular complications.3,4
While respiratory complications have seemed to be the most commonly reported among infected patients, cardiac arrhythmias have emerged as a well-documented complication of the virus, with a wide variety of arrhythmias reported with unique management considerations (Table 1).5 In a worldwide survey of 4526 patients admitted to the hospital with COVID-19, a cardiac arrhythmia was identified in 827 patients.6 Unsurprisingly, cardiovascular comorbidities such as hypertension (69%), diabetes mellitus (42%), coronary artery disease (24%), and congestive heart failure (30%) were common in the patients who developed cardiac arrhythmias.6 More generally, arrhythmia in this patient population is associated with older age, disease severity, history of congestive heart failure, and troponin levels.7 While the exact prevalence of cardiovascular complications in patients suffering from COVID-19 is not yet known, previous studies have suggested that those with preexisting cardiovascular disease might be most susceptible to these types of complications.8, 9, 10 One meta-analysis of 1527 patients found that patients with COVID-19 that required intensive care unit (ICU) treatment were 3 times as likely to have preexisting cardiovascular conditions compared to patients who did not require ICU treatment.11 Furthermore, this same analysis found that 8% of patients infected reported some type of cardiac-related injury. In addition to injury during the acute phase of the illness, PASC-related cardiac injury has also been frequently reported, often in conjunction with larger multiorgan manifestations.12 While dyspnea is the most commonly reported complaint of these PASC patients, with a likely cardiac component as part of its etiology, our understanding of the long-term frequency of arrhythmias associated with COVID-19 is still evolving.13 For instance, while atrial arrhythmias, including both bradyarrhythmias and tachyarrhythmias, are the most commonly reported arrhythmias in the setting of acute illness, their long-term prevalence remains an area of active investigation.14,15 Other arrhythmias such as ventricular tachycardia (VT), ventricular fibrillation (VF), atrioventricular block, inappropriate sinus tachycardia, and postural orthostatic tachycardia syndrome have all been reported in relation to the virus.5 Whether novel COVID-19 therapies and vaccines have any impact on the burden of arrhythmias is yet to be fully understood. Interestingly, in the RECOVERY trial, which demonstrated a mortality benefit with the use of dexamethasone in hospitalized patients with severe COVID-19, as published in the Supplemental Appendix (published as part of the RECOVERY trial), the use of steroids did not seem to have an impact on the rate of “major cardiac arrhythmias” (atrial fibrillation [AF]/flutter [AFl], supraventricular tachycardia, VT, VF, or atrioventricular block) with rates of 5.3% in the steroid group and 6.3% in the control arm.16 In this review, we aim to highlight the various arrhythmias experienced during COVID-19, potential mechanisms underlying them, and proposed management strategies (Table 1).
Table 1.
Management considerations for arrhythmias reported in COVID-19 infection
| Arrhythmia | Primary management considerations |
|---|---|
| Atrial fibrillation, atrial flutter |
|
| Supraventricular tachycardia |
|
| Ventricular tachycardia, ventricular fibrillation |
|
| Bradyarrhythmia |
|
| Postural orthostatic tachycardia syndrome (POTS), inappropriate sinus tachycardia (IST) |
|
| QT prolongation |
|
ECG = electrocardiogram; ICD = implantable cardioverter-defibrillator; IV = intravenous.
Pathogenesis and potential mechanisms of arrhythmia
Arrhythmic manifestations are just one aspect of the cardiac sequelae of COVID-19, and there have been several proposed mechanisms. The angiotensin-converting enzyme 2 (ACE2) receptor, the SARS-CoV-2 virus’s receptor of cell entry, has been notably demonstrated to be expressed in the heart at higher levels than in the lungs.17 This may explain the nature of cardiac complications of the disease, including cardiac arrhythmias.17,18 A well-established risk factor for the development of cardiac arrhythmias is myocardial ischemia.19 Ischemia in myocytes results in increases in extracellular potassium and increases in intracellular potassium, which results in significant alterations in action potentials and resultant arrhythmia.19 Infection with SARS-CoV-2 is associated with high rates of severe pulmonary illness and acute respiratory distress syndrome, which is associated with substantial hypoxemia, which can result in myocardial ischemia, particularly in those with underlying cardiovascular disease.20 SARS-CoV-2 infection has also been associated with severe systemic inflammation and cytokine storm.21 Several of these inflammatory markers, specifically interleukin (IL)-6, tumor necrosis factor α, and IL-1, have been shown to prolong the ventricular action potential, which also may be responsible for the arrhythmogenesis seen in critically ill patients with COVID-19.22 As a possible underlying mechanism for this observation, rat and stem cell–derived myocytes that were bathed in serum derived from critically ill patients with COVID-19 were shown to have perturbations of calcium homeostasis in vitro, which can be proarrhythmic.23 Interestingly, the addition of canakinumab, an IL-1β antagonist, to the serum had no impact on the observed proarrhythmogenic calcium release, suggesting alternate pathways may be responsible for this observation.
Myocardial damage may also result from mechanisms other than ischemia. Myocarditis has been a well-described clinical complication of patients with SARS-CoV-2 infection.24 As the spike protein of SARS-CoV-2 binds to ACE2 on the surface of cells to gain entry and ACE2 is present on cardiomyocytes, this is one possible mechanism through which SARS-CoV-2 can result in direct cardiomyocyte toxicity and cause myocarditis.24 Myocarditis of all causes has been associated with high rates of ventricular arrhythmias and is potentially responsible for some of the arrhythmia associated with COVID-19.25
A large cohort of patients admitted with COVID-19 infection compared to patients without COVID-19 showed that infection with COVID-19 was associated with a longer corrected QT (QTc) interval on electrocardiogram (mean QTc, 448.83 [95% CI, 440.53–457.13] ms vs 424.1 [95% CI, 409.41–437.78] ms; modeled mean difference, 24.73 [95% CI, 10.74–38.73]; P < .001), which was not impacted after adjustment for treatment with QT-prolonging medications.26 Thus, QTc can be prolonged and promote arrhythmia in patients with SARS-CoV-2 infection through infection alone, as well as through the use of QTc-prolonging medications that may be required during the management of patients hospitalized with COVID-19. These are a few of the proposed mechanisms for the arrhythmias that are observed in hospitalized patients with COVID-19.
Atrial arrhythmias
Atrial arrhythmias are the most commonly reported arrhythmias in patients with severe COVID-19.27,28 In a retrospective analysis of 3970 patients admitted with polymerase chain reaction (PCR)-confirmed COVID-19, the overall incidence of AF and AFl as assessed by manual chart review was 13% and 6.6% in those without a prior history of atrial arrhythmia.29 In this study, those with AF or AFl were more likely to be older and had higher inflammatory markers (C-reactive protein and IL-6), as well as higher levels of markers of myocardial dysfunction (peak troponin and B-type natriuretic peptide).29 The presence of in-hospital AF or AFl was also associated with increased mortality (46% vs 26%, relative risk 1.78, P < .01). Similarly, a large meta-analysis with a pooled total of 21,582 hospitalized patients with PCR-confirmed COVID-19 found a prevalence of AF of 11%.30 There was a 2.5-fold increase in the prevalence of AF in those over age 60 compared to those younger than 60 and a 6-fold increase in the prevalence in those with severe disease compared to those with milder disease.30 Of the portion of studies reporting mortality outcomes, the presence of AF was associated with an increase in all-cause mortality (50.6% vs 29.3%, OR 2.98, 95% CI 1.91–4.66).30
There is also a growing body of evidence that a history of prior AF is associated with worsened outcomes in patients hospitalized with COVID-19. In 696 consecutive patients admitted with COVID-19 in 13 cardiology centers across Italy, patients with a history of prior AF had a higher rate of death (38.7% vs 20.8%, P < .001) and this association remained after adjusting for confounders of clinical disease severity.31 Additionally, in a single-center retrospective study of 350 patients hospitalized with COVID-19, AF was independently associated with in-hospital death when adjusting for age and comorbidities through multivariate analysis (hazard ratio: 2.426, 95% CI 1.089–5.405, P = .032).32 In another study performed in Berlin, 15.9% of patients who required ICU treatment had a prior history of arrhythmia, with the most common being AF or AFl, consisting of 14.2% of those requiring ICU treatment. In this study, 44.2% of patients had sustained atrial arrhythmias, while 33.6% had nonsustained atrial arrhythmias, with AF being the most common atrial arrhythmia in both sustained and nonsustained categories. This high proportion of patients reporting arrhythmias also closely aligned with a high proportion (79.6%) of patients treated with mechanical ventilation.33
There are significant management considerations that must be considered for atrial arrhythmias from COVID-19 infection. AF is typically treated with a combination of rhythm control, rate control, and anticoagulation in patients without contraindications owing to bleeding risks.34 It is worth noting that consideration for therapeutic anticoagulation with heparin, regardless of AF, should be considered for all non–critically ill hospitalized patients with COVID-19 based on recent data reporting an improved probability of organ support–free survival to hospital discharge with therapeutic anticoagulation compared to prophylactic dose.35 Standard therapies for rate control consist of pharmacological management, typically with calcium channel blockers or beta blockers. Owing to possible concern of respiratory bronchoconstriction with beta-blocker usage for patients with acute respiratory illness such as COVID-19, calcium channel blockers are an alternative option as rate control agents.34,36 It is critical to be mindful of drug-drug interactions and the potential need to modify agents used for rhythm control, as patients with COVID-19 are often treated with many additional medications.37 On a case-by-case basis, rhythm control agents such as amiodarone, flecainide, ibutilide, and sotalol may be used as dictated by the comorbid conditions and drug-drug interactions as recommended in a summary statement providing guidance on short-term management of AF published in the Journal of the American Heart Association.37 In any hemodynamically unstable patient with AF, direct current cardioversion should be used.37 In a nonemergent setting, the American Society of Echocardiography provided recommendations suggesting alternate imaging modalities for clearance of the left atrial appendage prior to cardioversion, such as computed tomography or magnetic resonance imaging, owing to the increased risk of aerosolization associated with transesophageal echocardiography.38
The use of prophylactic antiarrhythmic therapy aimed to prevent atrial arrhythmias has been suggested for COVID-19, given the commonality of these atrial arrhythmias. However, caution has been recommended given possible QT-prolonging effects of many of these drugs and interactions with COVID-19 pharmacotherapy.26,39
Ventricular arrhythmias
Ventricular arrhythmias are typically not the predominant arrhythmia identified in hospitalized patients with COVID-19, with studies estimating nonsustained VT, VT, and VF comprising up to approximately 20% of all arrhythmias.6 In an inpatient cohort at a single center in New York City at the height of the pandemic, management of ventricular arrhythmias made up 7% of the electrophysiology consultations.40 One study examining the etiologies of 136 in-hospital cardiac arrests in patients admitted with severe COVID-19 pneumonia found that VT/VF was responsible for only 5.9% of cases.41
Overall rates of ventricular arrhythmia are low, so the use of prophylactic antiarrhythmic therapy does not have any clear role. Furthermore, the use of antiarrhythmic medications like amiodarone may contribute to QTc prolongation and increase the risk of torsades de pointes.5 Management of ventricular arrhythmias in patients with SARS-CoV-2 infection should follow a similar algorithm to other patients with ventricular arrhythmias: antiarrhythmics, such as amiodarone; beta blockers; and intubation and sedation as needed. Recommended practice guidelines from the Heart Rhythm Society published during the peak of the pandemic do not recommend delaying urgent, nonelective procedures such as ablation of medically refractory VT storm or implantation of a secondary prevention automatic implantable cardioverter-defibrillator, though decisions need to be individualized for each case.42 However, in certain situations, the use of a temporary wearable cardioverter-defibrillator may be reasonable if the ongoing risk of a permanent implantable device are outweighed by the benefits (eg, ongoing infection, thrombosis complicating access, potential improvement in ventricular function, etc).42 Mitacchione and colleagues43 report a case of successful ablation of VT in a patient with electrical storm through substrate modification in a critically ill patient with COVID-19 pneumonia, which supports the feasibility of performing such procedures in this patient population. One other patient population in which ventricular arrhythmias may be an important consideration is those with COVID-19-associated myocarditis. As has been reported in acute myocarditis from other causes, as high as 79% of patients have ventricular arrhythmia (nonsustained VT, VT, or VF) as part of their presentation.25 Data regarding the prevalence of ventricular arrhythmias specifically in COVID-19-related myocarditis is still developing, though proposed mechanisms include direct myocyte injury, microvascular ischemia, reentrant arrhythmia related to myocardial scar/fibrosis, and the proarrhythmogenic features previously described with cytokine storm.24
As previously discussed, infection with COVID-19 is associated with prolonged QTc interval, irrespective of treatment with known QTc-prolonging medications.26 Thus, monitoring the QTc interval regularly in these patients, particularly those receiving any additional medications known to prolong the QTc, is a critical consideration in this patient population. Torsades de pointes in the setting of severe COVID-19 pneumonia and treatment with QTc-prolonging medications has been described previously in the literature.15 Management with defibrillation, intravenous magnesium, repletion of electrolytes, and discontinuation of offending agents resulted in resolution of the ventricular arrhythmia in this case, which emphasizes the importance of careful monitoring of the QTc interval in these patients and using established management algorithms for management of ventricular arrhythmias that can result.15
Bradyarrhythmias
Arrhythmic complications observed during infection with COVID-19 also include bradyarrhythmias. In a worldwide survey reporting on arrhythmias observed in more than 800 hospitalized patients with SARS-CoV-2 infection, bradycardia and atrioventricular block accounted for 12.8% and 8.6% of arrhythmias, respectively.6 Single-center experience from a New York City hospital at the height of the pandemic revealed that bradyarrhythmia accounted for 16% of the cases of inpatient electrophysiology consultation requested.40 Because bradyarrhythmias account for a significant portion of the arrhythmias observed in inpatients with COVID-19, management of bradyarrhythmias is an important consideration in this patient population.
Given the observed association between heart block and myocarditis, as well as the association between infection with SARS-CoV-2 and myocarditis, myocarditis should be a clinical consideration in any patient who develops atrioventricular block with COVID-19.24,44 The majority of our knowledge about the management of bradyarrhythmias during the pandemic comes from small observational case series and case reports. In a case series of 7 patients with COVID-19 infection complicated by bradyarrhythmia, 5 patients had complete heart block and 2 patients had sinus node dysfunction.45 Given the possibility of recovery of the conduction system and the risks associated with an early invasive procedure and permanent implant, temporary pacing was used in the 5 symptomatic patients with complete heart block in this case series. Ultimately, 5 patients continued to have symptomatic bradycardia and conduction disease after 2 weeks, at which time permanent pacemaker implantation occurred. The 2 patients with sinus node dysfunction were managed medically and followed without permanent device implantation.45 Other case series have reported on the transient nature of high-grade block observed in crucially ill patients with COVID-19.46 In this series, 2 patients with critical illness related to COVID-19 developed high-grade atrioventricular block during their clinical course. In both cases, as the patients improved their conduction issues resolved, and neither required long-term permanent pacing. While bradycardia can be seen in critically ill patients for a variety of reasons, such as metabolic derangements, vagal tone due to pain and tracheal suctioning, and medication side-effects, it typically results in first- or second-degree block and not the high-grade block observed in these patients. Thus, likely there are myriad different mechanisms of conduction disease as a result of COVID-19, as has been observed with other infections (ie, Lyme with carditis).46 Given the complexity of critically ill patients with COVID-19, the risks associated with implantation of a permanent device, and the possible transient nature of the bradyarrhythmia, it is important to consider temporizing measures such as temporary transvenous pacemakers, atropine, and dopamine as viable options until a patient clinically improves. However, as has been recommended by the guidelines from the Heart Rhythm Society on guidance for cardiac electrophysiology during the COVID-19 pandemic, urgent procedures such as the implantation of permanent pacemakers for patients with symptomatic bradycardia should not be deferred.42
Autonomic dysfunction
Survivors of COVID-19, especially those with symptoms of PASC, have been reported to experience arrhythmias in the form of autonomic dysfunction, typically with features of postural orthostatic tachycardia syndrome (POTS) or inappropriate sinus tachycardia (IST).45,47,48 POTS is typically characterized by symptoms that are precipitated by changes in position or standing and heart rate increase of ≥30 beats per minute (or heart rate >120 beats/min) when moving from a supine to a standing position; IST is characterized by sinus tachycardia without an identifiable cause.49 A case series from Sweden reported by Johansson and colleagues48 reported on 3 patients who developed symptoms of PASC; all had confirmed symptomatic POTS in the months following infection with COVID-19. All patients were managed with the use of nonpharmacologic measures such as fluid and salt intake, avoidance of triggers, and compression stockings, as well as the use of beta blockers and ivabradine; only 1 of the 3 patients saw significant improvement in their symptoms.48 This finding in post-COVID-19 patients has been further supported with other reports from the literature. In a compilation of 6 case series (including the 3-patient series by Johansson and colleagues) put together by Bisaccia and colleagues,50 61 total patients were identified with evidence of cardiovascular autonomic dysfunction following COVID-19 infection. These patients had an average age of 42; 69% were women and 69% had a diagnosis of POTS, with orthostatic hypotension as the next most common diagnosis.50 When making this diagnosis of autonomic dysfunction, it is critical to be able to rule out other causes of tachycardia in the post-COVID-19 patient, including deconditioning, anemia, anxiety, heart failure, pulmonary disease, or ongoing hypoxia.50 Another case series of 11 patients referred to a cardiologist for management of unexplained tachycardia, palpitations, and orthostatic intolerance following PCR- or antibody-confirmed COVID-19 infection found that of the 9 patients managed with pharmacology, only 2 had resolution of their symptoms.51 These findings, coupled with the findings of the series reported by Johansson and colleagues, support that post-COVID-19 autonomic dysfunction carries a similar prognosis to POTS and IST with difficult-to-manage symptoms that require ongoing follow-up. While these presentations may be more common in patients with underlying autoimmune disease, this association has not yet been studied for PASC.48
Considerations in specific patient populations
As we have suggested throughout this review, individuals with previous cardiac complications are at an increased risk of arrhythmias following COVID-19 infection, and as such, history of structural heart disease or arrhythmias should raise concerns over the possibilities of worsened outcomes.8, 9, 10, 11 Additionally, certain high-risk patients, such as those with an inherited arrhythmia syndrome like long QT syndrome, Brugada syndrome, short QT syndrome, or catecholaminergic polymorphic ventricular tachycardia, often warrant special considerations and are at a heightened risk for arrhythmias.52
Management of fever is especially important in individuals predisposed to ventricular arrhythmias in the setting of viral illness or fever with underlying Brugada syndrome, a high-risk population for the development of arrhythmias during acute COVID-19 infection.53 Like other cases of Brugada syndrome, fever reduction with acetaminophen is typically suggested, with subsequent hospitalization if fever cannot be reduced, owing to high fever’s ability to precipitate arrhythmias.54,55 Slight changes in sympathetic or parasympathetic systems can often wreak havoc in these patients, so isolation and close monitoring is of utmost important, even among the COVID-19 population. Special management considerations in ICU settings should also be enacted owing to the possibility of episodes of arrhythmia being precipitated by anesthesia, and careful choice of drugs should be supplemented with constant cardiac monitoring for these patients.56
Patients with long QT syndrome are also particularly susceptible to arrhythmias from COVID-19. Importantly, Rubin and colleagues26 found that COVID-19 infection itself was independently associated with a significant increase in QTc and a QTc greater than 500 ms. Like other viral channelopathies, in which viral infection dysregulates ion channel function directly or through encoding their own ion channels (viroporins), it is likely that long QT syndrome–related arrhythmias likely manifest as a direct result of ion channel dysfunction owing to COVID-19 infection.57,58 Management of patients with these considerations should also make use of drugs without significant QT prolongation, diarrhea, or hypokalemia as side effects, as all of these are possible risk factors in the development of arrhythmias associated with COVID-19 for those with channel-related mutations.59 It is important to note, however, that management considerations are specific to certain channelopathies. For instance, short QT syndrome and catecholaminergic polymorphic VT do not have significant added concerns COVID-19 apart from avoiding electrolyte-related imbalances.52,60 Specific guidance for cardiomyopathies and other inherited conditions has also previously been described.61
As our experience with vaccination against SARS-CoV-2 continues to grow, the management of cardiovascular complications related to vaccination will continue to evolve. Rare cardiovascular adverse effects of COVID-19 vaccines, including myocarditis and pericarditis, have been reported, typically in young and healthy men. In 1 case series of 5 patients with cardiovascular complications following vaccination with the mRNA-based COVID-19 vaccines, 2 patients were diagnosed with myocarditis, 2 patients were diagnosed with pericarditis, and 1 patient was diagnosed with stress cardiomyopathy.62 The 2 patients diagnosed with myocarditis were young men (aged 18 and 19 years) and 1 of the 2 patients was noted to have episodes of nonsustained VT.62 Both of the patients diagnosed with pericarditis were women (aged 21 and 61 years); 1 was noted to have sinus tachycardia and the other new-onset atrial fibrillation.62 It should be noted that the extent of cardiovascular complications and arrhythmia due to vaccines are significantly lower than what is typically seen following SARS-CoV-2 viral infection.62
Conclusion and future directions
As we near the end of the pandemic phase of COVID-19, it is highly likely SARS-CoV-2 will remain endemic throughout the world. Thus, the lessons we learned during the acute phase must be carried forward as we continue to build on our understanding and knowledge of the presentation and management of COVID-19 disease. In particular, as outlined in this review, there have been a variety of arrhythmic complications observed in patients infected with SARS-CoV-2. Current data on the presentation and management of these patients are largely limited to small observational case series and case reports. Pooled data and larger registries would help better inform the incidence and optimal management strategies in this patient population. Additionally, as longer-term follow-up is accrued in larger patient populations, our understanding of both the acute and long-term arrhythmic complications of COVID-19 can be better understood.
Funding Sources
Dr Wan is supported by NIH R01 HL152236.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalantari H., Tabrizi A.H.H., Foroohi F. Determination of COVID-19 prevalence with regards to age range of patients referring to the hospitals located in western Tehran, Iran. Gene Rep. 2020;21:100910. doi: 10.1016/j.genrep.2020.100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramakrishnan R.K., Kashour T., Hamid Q., Halwani R., Tleyjeh I.M. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol. 2021;12:686029. doi: 10.3389/fimmu.2021.686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai A.D., Boursiquot B.C., Melki L., Wan E.Y. Management of arrhythmias associated with COVID-19. Curr Cardiol Rep. 2020;23:2. doi: 10.1007/s11886-020-01434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coromilas E.J., Kochav S.M., Goldenthal I., et al. Worldwide survey of COVID-19-Associated Arrhythmias. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rav-Acha M., Orlev A., Itzhaki I., et al. Cardiac arrhythmias amongst hospitalised Coronavirus 2019 (COVID-19) patients: prevalence, characterisation, and clinical algorithm to classify arrhythmic risk. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.13788. [DOI] [PubMed] [Google Scholar]
- 8.Driggin E., Madhavan M., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. 2020;323:1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 10.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C.C.E., Ali K., Connell D., et al. COVID-19-associated cardiovascular complications. Diseases. 2021;9:47. doi: 10.3390/diseases9030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satterfield B.A., Bhatt D.L., Gersh B.J. Publisher Correction: Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol. 2021;1:1. doi: 10.1038/s41569-021-00641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babapoor-Farrokhran S., Rasekhi R.T., Gill D., Babapoor S., Amanullah A. Arrhythmia in COVID-19. SN Compr Clin Med. 2020;1–6 doi: 10.1007/s42399-020-00454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochav S.M., Coromilas E.J., Nalbandian A., et al. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RECOVERY Collaborative Group, Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X., Chen P., Wang J., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolettis T.M. Coronary artery disease and ventricular tachyarrhythmia: pathophysiology and treatment. Curr Opin Pharmacol. 2013;13:210–217. doi: 10.1016/j.coph.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cron R.Q., Caricchio R., Chatham W.W. Calming the cytokine storm in COVID-19. Nat Med. 2021;27:1674–1675. doi: 10.1038/s41591-021-01500-9. [DOI] [PubMed] [Google Scholar]
- 22.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk, and inflammation: mind the gap. Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 23.Dimai S., Semmler L., Prabhu A., et al. COVID19-associated cardiomyocyte dysfunction, arrhythmias and the effect of Canakinumab. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peretto G., Sala S., Rizzo S., et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Rubin G.A., Desai A.D., Chai Z., et al. Cardiac corrected QT interval changes among patients treated for COVID-19 infection during the early phase of the pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gawalko M., Kaplon-Cieslicka A., Hohl M., Dobrev D., Linz D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han K.Y., Qiao Q., Zhu Y.Q., et al. Atrial arrhythmias in patients with severe COVID-19. Cardiol Res Pract. 2021;2021:8874450. doi: 10.1155/2021/8874450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musikantow D.R., Turagam M.K., Sartori S., et al. Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza. JACC Clin Electrophysiol. 2021;7:1120–1130. doi: 10.1016/j.jacep.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z., Shao W., Zhang J., et al. Prevalence of atrial fibrillation and associated mortality among hospitalized patients with COVID-19: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:720129. doi: 10.3389/fcvm.2021.720129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paris S., Inciardi R.M., Lombardi C.M., et al. Implications of atrial fibrillation on the clinical course and outcomes of hospitalized COVID-19 patients: results of the Cardio-COVID-Italy multicentre study. Europace. 2021;23:1603–1611. doi: 10.1093/europace/euab146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozdemir I.H., Ozlek B., Cetin N. Permanent atrial fibrillation portends poor outcomes in hospitalized patients with COVID-19: a retrospective observational study. J Electrocardiol. 2021;65:113–120. doi: 10.1016/j.jelectrocard.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parwani A.S., Haug M., Keller T., et al. Cardiac arrhythmias in patients with COVID-19: lessons from 2300 telemetric monitoring days on the intensive care unit. J Electrocardiol. 2021;66:102–107. doi: 10.1016/j.jelectrocard.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 35.ATTACC, ACTIV-4a, and REMAP-CAP Investigators Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattanawong P., Shen W., El Masry H., et al. Guidance on short-term management of atrial fibrillation in coronavirus disease 2019. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkpatrick J.N., Mitchell C., Taub C., et al. ASE Statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Soc Echocardiogr. 2020;33:648–653. doi: 10.1016/j.echo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manolis A.S., Manolis A.A., Manolis T.A., et al. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30:451–460. doi: 10.1016/j.tcm.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berman J.P., Abrams M.P., Kushnir A., et al. Cardiac electrophysiology consultative experience at the epicenter of the COVID-19 pandemic in the United States. Indian Pacing Electrophysiol J. 2020;20:250–256. doi: 10.1016/j.ipej.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao F., Shuang X., Ma X., et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakkireddy D.R., Chung M.K., Gopinathannair R., et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17:e233–e241. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitacchione G., Schiavone M., Gasperetti A., Forleo G.B. Ventricular tachycardia storm management in a COVID-19 patient: a case report. Eur Heart J Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagar S., Liu P.P., Cooper L.T., Jr. Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta M.D., Qamar A., MP G., et al. Bradyarrhythmias in patients with COVID-19: a case series. Indian Pacing Electrophysiol J. 2020;20:211–212. doi: 10.1016/j.ipej.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eneizat Mahdawi T., Wang H., Haddadin F.I., Al-Qaysi D., Wylie J.V. Heart block in patients with coronavirus disease 2019: a case series of 3 patients infected with SARS-CoV-2. HeartRhythm Case Rep. 2020;6:652–656. doi: 10.1016/j.hrcr.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shouman K., Vanichkachorn G., Cheshire W.P., et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johansson M., Stahlberg M., Runold M., et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal A.K., Garg R., Ritch A., Sarkar P. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478–480. doi: 10.1136/pgmj.2006.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bisaccia G., Ricci F., Recce V., et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desai A.D., Boursiquot B.C., Moore C.J., et al. Autonomic dysfunction post-acute COVID-19 infection. HeartRhythm Case Rep. Published online November. 2021;27 doi: 10.1016/j.hrcr.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu C.I., Postema P.G., Arbelo E., et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020;17:1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang D., Saleh M., Garcia-Bengo Y., et al. COVID-19 infection unmasking brugada syndrome. HeartRhythm Case Rep. 2020;6:237–240. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasquetto G., Conti G.B., Susana A., Leone L.A., Bertaglia E. Syncope, Brugada syndrome, and COVID-19 lung disease. J Arrhythm. 2020;36:768–770. doi: 10.1002/joa3.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sorgente A., Capulzini L., Brugada P. The known into the unknown: Brugada syndrome and COVID-19. JACC Case Rep. 2020;2:1250–1251. doi: 10.1016/j.jaccas.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dendramis G., Brugada P. Intensive care and anesthetic management of patients with Brugada syndrome and COVID-19 infection. Pacing Clin Electrophysiol. 2020;43:1184–1189. doi: 10.1111/pace.14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Etheridge S.P., Asaki S.Y. COVID-19 infection and corrected QT interval prolongation-collateral damage from our newest enemy. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.7192. [DOI] [PubMed] [Google Scholar]
- 58.Seebohm G., Strutz-Seebohm N., Ureche O.N., et al. Long QT syndrome-associated mutations in KCNQ1 and KCNE1 subunits disrupt normal endosomal recycling of IKs channels. Circ Res. 2008;103:1451–1457. doi: 10.1161/CIRCRESAHA.108.177360. [DOI] [PubMed] [Google Scholar]
- 59.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crotti L., Odening K.E., Sanguinetti M.C. Heritable arrhythmias associated with abnormal function of cardiac potassium channels. Cardiovasc Res. 2020;116:1542–1556. doi: 10.1093/cvr/cvaa068. [DOI] [PubMed] [Google Scholar]
- 61.Limongelli G., Crotti L. COVID-19 pandemia and inherited cardiomyopathies and channelopathies: a short term and long term perspective. Orphanet J Rare Dis. 2020;15:157. doi: 10.1186/s13023-020-01444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vidula M.K., Ambrose M., Glassberg H., et al. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus. 2021;13 doi: 10.7759/cureus.15576. [DOI] [PMC free article] [PubMed] [Google Scholar]


