Abstract
Objective
COVID-19 infection is associated with peripheral neuropathy. However, subclinical neurological involvement may occur anytime, and diagnostic methods that reveal this subclinical involvement are not well established. We aimed to assess the subclinical neurological involvement by visual evoked potential (VEP) measurements and nerve conduction studies (NCS) and explore the relationship between neurological electrophysiological findings and the severity of COVID-19 infection.
Methods
Seventy-six patients recovered from COVID-19 infection, and 44 healthy controls were enrolled in the study. Patients were assessed for clinical and demographic parameters. NCS and VEP analyses were performed to detect any peripheral neuropathy or optic neuropathy in both groups.
Results
None of the COVID-19 patients had electrophysiological evidence of peripheral neuropathy. However, patients with COVID-19 pneumonia had significant abnormalities in several peripheral nerve measurements compared to patients without pneumonia. Although P100 parameters did not differ significantly between patients and controls, 12 patients with COVID-19 had prolonged P100 latencies.
Conclusions
We detected subclinical afferent visual pathway abnormality evaluated by VEP analysis. In addition, we found subtle electrophysiological features in the NCS of the patients presented with COVID-19 pneumonia. However, our findings did not fortify the diagnosis of peripheral neuropathy or optic neuropathy. Further studies are needed to determine the characteristics of COVID-19-related peripheral neuropathy/optic neuropathy whether it has distinct clinical features and disease course.
Keywords: COVID-19, Nerve conduction studies, Neuropathy, Optic neuropathy, SARS-COV-2, Visual evoked potential
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first emerged in Wuhan, China, in December 2019 and spread worldwide. In adults, COVID-19 can result in asymptomatic infection but may lead to severe respiratory failure or even death [1]. Although the primary clinical manifestation of COVID-19 is respiratory involvement, there is evidence suggesting that the neurological manifestations are directly associated with COVID-19. Encephalitis, cerebrovascular diseases, demyelinating diseases, and peripheral neuropathy are common neurological manifestations related to COVID-19. As a result of ACE-2 expressed in both neurons and glial cells, several mechanisms were proposed for direct viral invasion of the central nervous system and immune-mediated neurological syndromes [2].
Its transmission to the peripheral nervous system is not known. Molecular mimicry between COVID-19 proteins and peripheral nerve proteins (such as gangliosides) may trigger the immune response against peripheral nerves causing neuropathy [3]. Moreover, Guillain-Barré syndrome (GBS), mononeuropathy multiplex, and cranial neuropathies are seen in COVID-19 patients [2, 4–6]. The COVID-19 infection mainly causes peripheral facial paralysis and oculomotor paresis. However, there are rare cases with optic nerve involvement [4–6].
During the SARS-CoV-2 global pandemic, healthcare workers are at high risk for exposure to COVID-19 and surviving it. In this study, we examined the electrophysiological outcome of hospital staff recovered from COVID-19 disease, followed up in the Department of Infectious Diseases at our center. Thus, we aimed to determine the subclinical involvement of the peripheral nerves and the optic nerve in the course of COVID-19. Also, we examined the demographics and clinical and laboratory parameters associated with COVID-19 infection. We also compared the differences in patients with COVID-19 pneumonia and patients without lung involvement.
This study aimed to determine the presence of peripheral neuropathy, optic neuropathy, or both in patients affected by COVID-19. A secondary objective is to determine whether subtle electrophysiological abnormalities are associated with COVID-19 severity.
Material and methods
Participants
We included 76 participants aged between 18 and 60 years from a cohort of healthcare workers (physicians, nurses, laboratory technicians, emergency medical staff, medical administrative staff). They were admitted to the infectious disease department of our hospital with symptomatic COVID-19 infection between April 2020 and February 2021. The diagnosis of COVID-19 infection was confirmed by quantitative reverse transcription-polymerase chain reaction (PCR) assay by nasopharyngeal swab. Patients with respiratory failure, a high respiratory rate (≥ 24/min.), or low oxygen saturation (SpO2 < 93) underwent Chest CT (CCT). According to the severity of pneumonic infiltration in CCT, experienced radiologists classified COVID-19 disease as mild, moderate, and severe. Data on demographics, comorbidities, neurological symptoms, treatments, chest CT results, and clinical outcomes were retrieved from the electronic patient records.
We evaluated 76 healthcare workers with past COVID-19 infection and 44 healthy controls. We performed nerve conduction studies (NCS) and visual evoked potentials (VEPs). Subjects were questioned for any disease that could influence electrophysiological findings. These are subjects with a history of severe visual problems, any significant chronic ophthalmic disease, optic neuropathy, retinal disease, demyelinating disease, and glaucoma. Three patients with severe visual problems, two with chronic ophthalmic disease, and one with the retinal disease were excluded from the study. The subjects with a past medical history including the presence of factors which increase the risk for comorbid peripheral neuropathy or optic neuropathy were excluded from the study. These factors involved HIV infection, diabetes mellitus, hypertension, B12 deficiency, alcohol abuse, drug abuse, vasculitis, malignancy, malnutrition, family history of hereditary neuropathy, and previous exposure to neurotoxic agents (chemotherapeutics, heavy metals, solvents, pesticides). The participants were examined, and the data were recorded when available in the medical records. Three participants with hypertension and two with diabetes were also excluded. We examined the participants for recent eye medications with mydriatics and cycloplegics before the test, and we excluded one patient from the study.
Procedures
VEP analysis
Pattern reversal visual evoked potentials (VEPs) were recorded at the Electrophysiology Laboratory in the Neurology Department of Health Sciences University, Izmir Bozyaka Education and Research Hospital. The same experienced neurologist performed all VEP measurements. The VEP analysis was recorded with the two-channel electromyography, Neurosoft device (Neuron-Spectrum-5, Neurosoft Ltd., Russia). Before the recordings, the subjective refractive errors were corrected using contact lenses.
We recorded the pattern reversal VEPs in a darkened and silent room. We seated the participants in front of a television screen at a distance of 1 m at eye level. First, we cleaned the scalp (at the electrode location) to keep the impedance below five kΩ; we attached the electrodes to the skull with conductive paste. The recordings were performed after monocular full-field stimulation with the active scalp electrode being Oz, referenced to Cz based on the International Society for Clinical Electrophysiology of Vision (ISCEV) recommendations for electrode placement and pattern reversal VEP recordings [7]. We placed the ground electrode around the forearm. The visual stimulus is a high contrast black-and-white checkerboard spanning the central 20°–30° of the visual field whose black and white squares reversed each second. We instructed the participants to focus on the red mark placed in the center of the screen. VEP is the averaged response to this reversal pattern. We performed the recordings after monocular full-field stimulation with a frequency limit set at 2–100 Hz, and the analysis time was 500 ms. We recorded pattern reversal VEPs after a total of 256 responses averaged. The peak latencies of N75, P100, and N135 potentials were measured. We recorded peak-to-peak amplitude of P100 potential calculated as the amplitude from the N75 peak to the P100 peak. Peak P100 latencies and N75-P100 amplitudes were used in the statistical analysis of each participant.
Nerve conduction studies
Nerve conduction studies (NCS) were recorded at the Electrophysiology Laboratory in the Neurology Department of Health Sciences University, Izmir Bozyaka Education and Research Hospital. Every patient and control subject had a detailed physical and neurological examination. Nerve conduction studies were performed in two extremities of each subject. Thus, a total of 240 extremities were studied. All of the NCS measurements were performed by two experienced neurologists. This recording was done with a Nihon-Kohden device (Nihon Kohden-Neuropack®, S1MEB-9400 K). Motor NCS is composed of the median, ulnar, posterior tibial, and peroneal nerves. Sensory nerve conduction studies included the median, ulnar, and sural nerves. We used ring electrodes while recording the sensory nerve action potentials (SNAPs). We placed silver surface recording electrodes to the belly tendon of the related muscles for recording motor nerve conductions. Thus, we recorded the compound muscle action potentials (CMAPs) of related nerves. Also, we recorded F wave latencies of the median, ulnar, posterior tibial, and peroneal nerves.
Ethical approval
We conducted the present study according to the ethical principles suggested in the Declaration of Helsinki. The institutional review board (Ethical approval license reference number: 2021/30 Date 24.02.2021) and the Turkish Ministry of Health approved the study protocol (Reference number: 2021–02-17T21_40_53). Informed consent was obtained from all individuals who participated in the study.
Statistical analysis
We used the Statistical Package for the Social Sciences Version 21 (SPSS Version 21.0 Armonk, NY: IBM Corp) statistical program for statistical analysis. The normal distribution of the data was analyzed by examining the Shapiro–Wilk test and histogram graphs. Baseline demographic and clinical characteristics were summarized as count and percentage, mean with range, and standard deviation. The sample size was not calculated since there was no similar study in the literature. Categorical variables are expressed as percentages and were compared using the Chi-square test or Fisher’s exact test. We used the Mann–Whitney U test for the assessment of non-parametric variables. p < 0.05 was considered statistically significant for all comparisons.
Results
Seventy-six adults presented with SARS-CoV-2 infection before the study time frame, and forty-four healthy volunteers of similar age and gender participated in our study(p = 0.70, p = 0.70, respectively). The demographic and clinical characteristics of the patient and control groups are shown in Table 1. None of the patients who presented with COVID-19 infection had neurological involvement. A total of 27 patients (35.5%) had clinical and radiological features consistent with COVID-19 pneumonia. Of the 27 patients, 11.1% had severe (n = 3), 59.3% had moderate (n = 16), and 29.6% had mild pneumonia (n = 8). Five of the patients (6.6%) were hospitalized in the COVID-19 ward. Only two patients (2.6%) followed up in the intensive care unit and received noninvasive ventilation. Patients diagnosed with COVID-19 had no comorbidities as defined in the study protocol. Treatment modalities during COVID-19 infection are summarized in Table 1.
Table 1.
Baseline demographic and clinical characteristics of study participants
| COVID-19 patients (n = 76) | Healthy controls (n = 44) | p-value | |
|---|---|---|---|
| Age (years) | 37.5 ± 9.6 (18–58) | 38.8 ± 9.5 (20–57) | 0.70 |
| Gender (female/male) | 47/29 | 25/19 | 0.70 |
| Education (years) | 14.2 ± 3.6 (5–18) | 14.7 ± 3.1 (5–18) | 0.66 |
| Time to electrophysiological evaluation after COVID-19 infection (months) | 4.4 ± 2.2 (1–12) | - | |
| Severity of COVID-19 pneumonia | 27 (35.5%) | - | |
| Mild | 8 | ||
| Moderate | 16 | ||
| Severe | 3 | ||
| COVID-19 hospitalization | 6 (7.9%) | ||
| COVID-19 treatment | |||
| Favipiravir | 55 (72.4%) | ||
| Acetyl salicylic acid | 17 (22.4%) | ||
| Hydroxychloroquine | 10 (13.1%) | ||
| Vitamin C | 7 (9.2%) | ||
| Enoxaparin (SC) | 7 (9.2%) | ||
| Ampiric antibiotics | 3 (3.9%) | ||
| Oral prednisone | 1 (1.3%) | ||
Demographic data and clinical features of COVID-19 patients and controls are shown above
A p-value < 0.05 is significant
A questionnaire evaluated neurological symptoms associated with COVID-19 infection. The most common initial neurological symptoms in patients with pneumonia were myalgia, loss of taste, loss of smell, and headaches. In the patient group without pneumonia, this first common initial symptom was myalgia, and the headache was the second. Loss of smell and taste were the following symptoms. Besides, the rank of symptoms was similar in both groups (Table 2). However, patients suffering from paresthesia were all female. There was a significant difference in paresthesia symptoms regarding gender (p = 0.01). In addition, most of the symptoms were disappeared in the follow-up. Table 2 shows the symptoms that emerged with the disease onset and persisted after the COVID-19 infection. We found no significant differences in initial symptoms regarding the presence of pneumonia in patients with COVID-19. The initial and persistent symptoms associated with COVID-19 infection were shown in Figs. 1 and 2.
Table 2.
The frequency of initial and persistent symptoms associated with COVID-19 infection
| Symptoms | Initial symptoms of patients with pneumonia (n = 27) | Persistent symptoms of patients with pneumonia (n = 27) | Initial symptoms of patients without pneumonia (n = 49) | Persistent symptoms of patients without pneumonia (n = 49) | p-value (comparison of initial symptoms in groups with pneumonia and without pneumonia) |
|---|---|---|---|---|---|
| Myalgia | 22 (81.5%) | 3 (11.1%) | 42 (85.7%) | 5 (10.2%) | 0.43 |
| Loss of taste | 18 (66.7%) | 2 (7.4%) | 29 (59.2%) | 4 (8.2%) | 0.35 |
| Loss of smell | 16 (59.3%) | 1 (3.7%) | 30 (61.2%) | 2 (4.1%) | 0.53 |
| Headache | 16 (59.3%) | 4 (14.8%) | 36 (73.5%) | 9 (18.4%) | 0.15 |
| Limb weakness | 12 (44.4%) | 1 (3.7%) | 18 (36.7%) | 1 (2%) | 0.34 |
| Vertigo, dizziness | 10 (37%) | 2 (7.4%) | 10 (20.4%) | 1 (2%) | 0.09 |
| Paresthesia | 4 (14.8%) | 0 | 6 (12.2%) | 2 (4.1%) | 0.75 |
| Impaired consciousness, confusion | 3 (11.1%) | 0 | 1 (2%) | 0 | 0.09 |
| Neuropathic pain | 1 (3.7%) | 1 (3.7%) | 5 (10.2%) | 1 (2%) | 0.32 |
The distribution of symptoms and their frequency are summarized in details. The p-value is indicated for comparison of patients with COVID-19 according to the presence of pneumonia. A p-value < 0.05 is significant
Fig. 1.
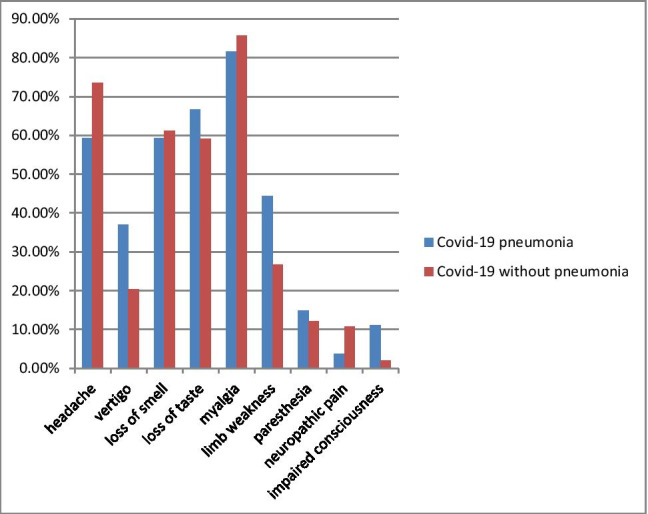
The frequency of initial symptoms that occurred with COVID-19 infection is compared among patients with COVID-19 pneumonia and without
Fig. 2.
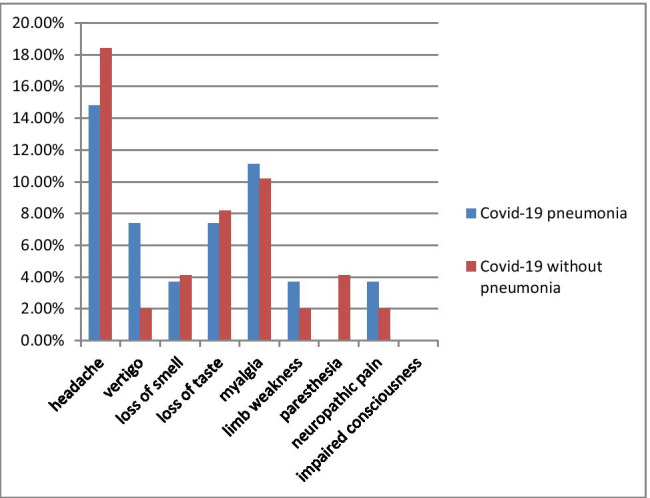
The frequency of persistent symptoms that occurred with COVID-19 infection is compared among patients with COVID-19 pneumonia and without
The electrophysiological examination was performed approximately 4.4 months after the COVID-19 infection. The nerve conduction study findings in patients and controls were shown in Table 3. Each nerve is evaluated individually. The nerve conduction study findings revealed no difference compared to healthy controls. Moreover, there were no electromyography findings consistent with peripheral neuropathy.
Table 3.
Nerve conduction study findings in patients and healthy controls
| COVID-19 patients (n = 76) Mean ± SD (min–max) |
Healthy controls (n = 44) Mean ± SD (min–max) |
p-value | |
|---|---|---|---|
| Median sensory first digit | |||
| Latency | 1.9 ± 0.3 (1.5–2.8) | 1.9 ± 0.2 (1.5–2.4) | 0.473 |
| SNAP amplitude | 34.5 ± 14.7 (14–73) | 36.5 ± 15.3 (15–70) | 0.350 |
| Conduction velocity | 52.9 ± 6.9 (37–69) | 52.5 ± 5.6 (42–68) | 0.946 |
| Ulnar sensory fifth digit | |||
| Latency | 1.9 ± 0.2 (1.5–2.6) | 1.9 ± 0.2 (1.5–2.4) | 0.974 |
| SNAP amplitude | 37.7 ± 16.4 (15–100) | 33.4 ± 12.7 (14–70) | 0.179 |
| Conduction velocity | 57.7 ± 5.9 (48–72) | 58.2 ± 5.7 (45–69) | 0.450 |
| Median motor nerve | |||
| Distal motor latency | 3.1 ± 0.5 (1.7–4.6) | 3.1 ± 0.5 (2.3–4.2) | 0.565 |
| CMAP amplitude | 14.9 ± 3.8 (8–25) | 13.4 ± 4.1 (7–22) | 0.096 |
| Conduction velocity | 57.3 ± 4 (50–69) | 55.9 ± 4.6 (48–69) | 0.172 |
| F wave latency | 26.1 ± 2.7 (21.4–41.7) | 25.6 ± 1.8 (22.6–30.9) | 0.344 |
| Ulnar motor nerve | |||
| Distal motor latency | 2.3 ± 0.3 (1.6–3) | 2.2 ± 0.4 (1.7–3.6) | 0.090 |
| CMAP amplitude | 14.9 ± 3.5 (8–28) | 13.8–2.8 (10–24) | 0.149 |
| Conduction velocity | 59.6 ± 5.2 (42–73) | 58.3 ± 5.6 (48–71) | 0.106 |
| F wave latency | 26.4 ± 3.0 (21.7–44.5) | 26.6 ± 4.7 (23.6–54) | 0.416 |
| Posterior Tibial motor nerve | |||
| Distal motor latency | 4.1 ± 0.8 (2.8–6.4) | 4.1 ± 0.6 (2.8–6) | 0.779 |
| CMAP amplitude | 13.7 ± 4.7 (4–26) | 12.6 ± 4.5 (5–24) | 0.345 |
| Conduction velocity | 44.8 ± 3.7 (38–59) | 45.9 ± 4.3 (40–56) | 0.368 |
| F wave latency | 48.5 ± 4.4 (35.8–59.6) | 48.1 ± 3.0 (41.1–54.7) | 0.544 |
| Peroneal motor nerve | |||
| Distal motor latency | 3.6 ± 0.7 (2.2–5.7) | 3.8 ± 0.9 (2.2–6.1) | 0.266 |
| CMAP amplitude | 7.7 ± 3.1 (2–20) | 7.1 ± 2.9 (2–15) | 0.272 |
| Conduction velocity | 49.5 ± 5.3 (40–68) | 48.4 ± 3.8 (40–59) | 0.540 |
| F wave latency | 47.5 ± 4.5 (38.5–57.9) | 46.8 ± 2.9 (40.8–53.5) | 0.642 |
| Sural sensory | |||
| Latency | 2.4 ± 0.5 (1.6–3.9) | 2.5 ± 0.6 (1.5–4.5) | 0.514 |
| SNAP amplitude | 19.6 ± 6.9 (5–40) | 21.7 ± 6.9 (6–37) | 0.423 |
| Conduction velocity | 51.6 ± 5.9 (42–67) | 51.2 ± 7.2 (42–74) | 0.076 |
Nerve conduction study results for the studied cases. Latency (in ms); CMAP amplitude (in mV); conduction velocity (in m/s); F wave latency (in ms). Abbreviations: CMAP compound muscle action potential, SNAP sensory nerve action potential. A p-value < 0.05 is significant
The patients were divided into two groups: those with COVID-19 pneumonia and those without lung involvement. We evaluated the demographics and nerve conduction study findings in both groups. We found that patients with pneumonia were older than patients without lung involvement (p = 0.013) Nevertheless, gender did not differ between the two groups (p = 0.06). Although within normal limits, peripheral nerve measurements revealed statistically significant differences in several electrophysiological parameters for patients with COVID-19 pneumonia (Table 4). We determined that the median nerve F wave latency was slightly prolonged in patients with pneumonia (p = 0.045). Ulnar sensory nerve conduction velocity was significantly lower in the pneumonia group (p = 0.001). Moreover, the ulnar sensory nerve amplitude was significantly lower in patients with pneumonia (p = 0.020). In addition, the conduction velocity of the posterior tibial motor nerve was significantly lower in the pneumonia group (p = 0.026). Moreover, the left p100 latency was significantly prolonged in patients with COVID-19 pneumonia (p = 0.026). The recordings of VEP analyses in two patients with COVID-19 pneumonia and two patients without pneumonia are shown in Figs. 3 and 4.
Table 4.
The abnormal nerve conduction study findings in patients with COVID-19 pneumonia
| Patients with pneumonia (n = 27) Mean ± SD (min–max) |
Patients without pneumonia (n = 45) Mean ± SD (min–max) |
p-value | |
|---|---|---|---|
| Age ( years) | 42.1 ± 9.4 (24–55) | 34.9 ± 8.8 (18–56) | 0.013* |
| Gender (F/M) | 13/14 | 34/15 | 0.06 |
| Median nerve F wave latency (ms) | 27.1 ± 3.5 (23.4–41.7) | 25.5 ± 1.9 (21.4–30.7) | 0.045* |
| Ulnar SNAP amplitude (μv) | 32.3 ± 15.4 (15–90) | 40.8 ± 16.4 (15–100) | 0.020* |
| Ulnar MCV (m/s) | 57.3 ± 4.5 (47–69) | 61.0 ± 5.2 (50–73) | 0.001* |
| Posterior tibial MCV (m/s) | 43.8 ± 3.7 (40–53) | 45.4 ± 3.7 (38–59) | 0.026* |
| Left p100 latency (ms) | 117.7 ± 12.0 (106–152) | 114.2 ± 11.1 (104–166) | 0.026* |
Nerve conduction study results of COVID-19 patients. Abbreviations: MCV motor conduction velocity, SNAP sensory nerve action potential. *Significant p values are presented in bold (p < 0.05)
Fig. 3.
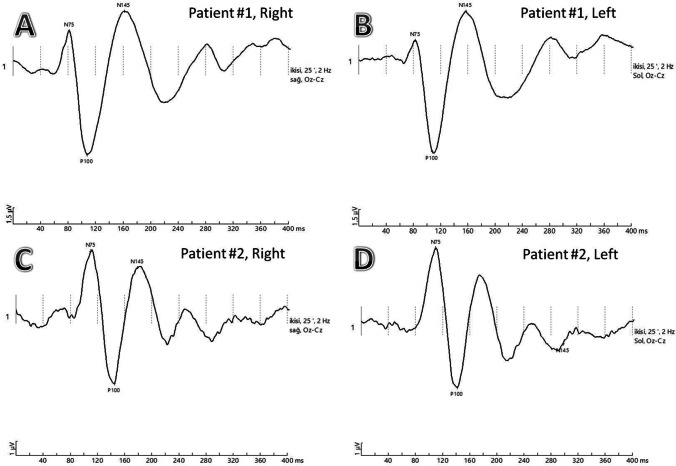
VEP recordings with N75, P100, and N145 waves are labeled A and B Patient #1, pattern reversal VEPs of a COVID-19 patient with pneumonia showing normal P100 latencies in both eyes. C and D Patient #2, pattern reversal VEPs of COVID-19 patient with pneumonia showing prolonged P100 latencies in both eyes
Fig. 4.
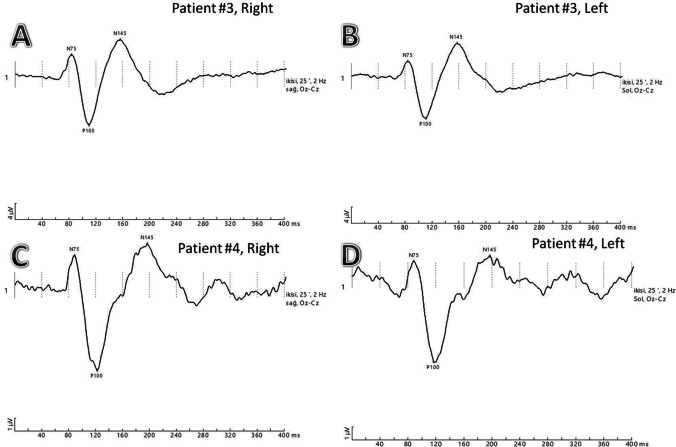
VEP recordings with N75, P100, and N145 waves are labeled A and B Patient #3, pattern reversal VEPs of a COVID-19 patient without pneumonia showing normal P100 latencies in both eyes. C and D Patient #4, pattern reversal VEPs of COVID-19 patient without pneumonia showing prolonged P100 latencies in both eyes
In Table 5, data regarding latency and amplitude of the P100 wave is shown. To conclude, the latency and amplitude of the P100 wave showed no significant differences between patients and controls. However, twelve patients had prolonged P100 latencies at least in one eye.
Table 5.
Visual evoked potential findings of study participants
| COVID-19 patients (n = 76) Mean ± SD (min–max) |
Healthy controls (n = 44) Mean ± SD (min–max) |
p-value | |
|---|---|---|---|
| Right P100 wave latency (ms) | 114.7 ± 11.8 (103–182) | 111.4 ± 4.9 (101–124) | 0.312 |
| Right P100 wave amplitude (μv) | 6.7 ± 3.4 (1–18.7) | 6.1 ± 2.9 (1.5–13.9) | 0.431 |
| Left P100 wave latency (ms) | 115.8 ± 12.1 (103–166) | 111.6 ± 5.1 (102–124) | 0.198 |
| Left P100 wave amplitude (μv) | 6.0 ± 2.8 (1.6–14.5) | 6.0 ± 2.8 (1.5–13.8) | 0.952 |
The parameters of visual evoked potentials are shown above. The latency and amplitude values are indicated in mean ± standard deviation, and minimum and maximum values are written in parenthesis
A p-value < 0.05 is significant
Discussion
No pathological electrophysiological findings supporting peripheral neuropathy were detected in any measurements performed in subjects with past COVID-19 infection. When the nerve conduction velocity, amplitude, and latency values of all examined nerves were compared, no significant difference was found between subjects with past COVID-19 infection and healthy controls. VEP analysis showed no differences in P100 wave parameters regarding latency and amplitude. However, some patients had prolonged P100 latencies, and subtle changes on peripheral nerves were detected.
Guerrero et al. reviewed peripheral neuropathies associated with COVID-19 reported up to now [8]. They conclude that 22 patients with GBS, 3 Miller Fisher syndrome, and one multiple cranial neuropathies infected with COVID-19. Most case reports described intensive care patients with severe respiratory symptoms. Among patients with GBS, nerve conduction studies revealed predominantly acute inflammatory demyelinating polyneuropathy and less frequently axonal sensory-motor axonal and axonal motor polyneuropathy [9–14]. Cabanes-Martinez et al. investigated the presence of neuropathy, myopathy, or both in intensive care unit patients [15]. The electrophysiological examination was performed on 12 patients who developed generalized weakness in a patient group hospitalized in the intensive care unit due to COVID-19. They detected sensory-motor axonal polyneuropathy in 4 out of 20 patients and myopathy in 7 patients.
In our study, we did not determine any cases with peripheral neuropathy. However, this finding could be related to our patients mainly presented with mild and moderate infection. Only two of the patients were hospitalized in the intensive care unit. Moreover, most patients had a mild clinical course and resolved immediately. The study of Stuart Neto et al. reported that 3 out of 45 COVID-19 patients with severe respiratory conditions developed peripheral neuropathy [16].
The transmission of the COVID-19 to the brain is thought to be via the olfactory nerve. It has been suggested that retrograde or anterograde spread from the olfactory nerve causes damage to the brain and cranial nerves [17, 18]. The mechanism of peripheral nerve involvement has not been fully elucidated. We found that almost 61.8% of patients with COVID-19 infection had a loss of taste and 60.5% had a loss of smell. Among our COVID-19 patients, we did not find any other cranial nerve involvement.
Rho et al. reported anterior ischemic nonarteritic optic neuropathy due to microvascular thrombosis caused by COVID-19 infection [5]. This patient who developed vision loss in one eye during COVID-19 infection, microembolism, and exudate optic pallor was observed in the eye socket. It was thought to occur due to hypoxia-related hypercoagulation and respiratory distress. Moreover, Yuksel et al. reported that a 72-year-old male patient who developed visual loss presented with a permanent inferior altitudinal defect due to progressive macular degeneration [6]. Also, optical coherence tomography revealed retinal thinning in the superior-temporal foveal area. Benito-Pascual et al. reported a case of unilateral panuveitis and optic neuritis as the first manifestation of COVID-19 infection [19]. They discussed this unusual manifestation presentation of ocular involvement preceded by COVID-19.
In patients with COVID-19 infection, the optic nerve might be involved due to the systemic inflammatory response. Except for a small number of case reports, we could not find any research investigating subclinical involvement of the optic nerve in the literature. Although VEP analysis did not show any difference among patients compared to controls, several patients had prolonged P100 latencies without any decrease in visual acuity. We could not explain the prolonged P100 latency, but the follow-up of these patients closely would determine whether this finding is permanent.
Our study did find subclinical partial involvement of peripheral nerves in patients with past COVID-19 infection. It is uncertain whether this subclinical involvement leads to any peripheral neuropathy in the future. This finding should be interpreted with further studies. No polyneuropathy was detected in our study, but we obtained subclinical peripheral nerve involvement in several cases. In our patient group, the number of patients with severe COVID-19 infection was low. It was remarkable that peripheral nerves were affected even in mild and moderate COVID cases. In a study of intensive care unit patients presented with quadriparesis, polyneuropathy, and myopathy, the electrophysiological examination was performed [15]. We have not come across any electrophysiological study carried on patients with past COVID-19 infection in the literature.
The critical limitation of this study is the small sample size. Further studies are required to confirm our observation and evaluate the electrophysiological abnormalities caused by COVID-19. Our study draws attention to the peripheral nerve and optic nerve involvement in patients with COVID-19. We suggest that symptoms such as numbness, tingling, and prickling should be assessed carefully after COVID-19 infection, and relevant electrophysiological examinations should be performed.
Conclusion
It can be considered that past COVID-19 infection has a mild effect on peripheral and optic nerves. Patients presenting neurological symptoms should be interpreted carefully in terms of both peripheral neuropathy and visual impairment.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Asli Koskderelioglu, Neslihan Eskut, and Pinar Ortan. The first draft of the manuscript was written by Asli Koskderelioglu, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics approval
The present study was performed under the ethical standards of the 1964 Declaration of Helsinki and its later amendments. The Ethical Committee of the University of Health Sciences, Izmir Bozyaka Education and Research Hospital (Ethical approval license reference number: 2021/30 Date 24.02.2021) and Turkish Ministry of Health approved the study protocol (Reference number: 2021–02-17T21_40_53). Informed consent was obtained from all individuals who participated in the study.
Conflict of interest
The authors declare no competing interests.
Informed consent
The informed consent for participation in the study and publication of their data in a journal article was obtained from all individuals.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andalib S, Biller J, Di Napoli M, Moghimi N, McCullough LD, Rubinos CA, O'Hana Nobleza C, Azarpazhooh MR, Catanese L, Elicer I, Jafari M, Liberati F, Camejo C, Torbey M, Divani AA. Peripheral nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2021;21(3):9. doi: 10.1007/s11910-021-01102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta M, Weaver DF. COVID-19 as a trigger of brain autoimmunity. ACS Chem Neurosci. 2021;12(14):2558–2561. doi: 10.1021/acschemneuro.1c00403. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigo-Armenteros P, Uterga-Valiente JM, Zabala-Del-Arco J, Taramundi-Argüeso S, Antón-Méndez L, Gómez-Muga JJ, Garcia-Monco JC (2021) Optic neuropathy in a patient with COVID-19 infection. Acta Neurol Belg. 1–3. 10.1007/s13760-021-01600-w [DOI] [PMC free article] [PubMed]
- 5.Rho J, Dryden SC, McGuffey CD, Fowler BT, Fleming J. A case of non-arteritic anterior ischemic optic neuropathy with COVID-19. Cureus. 2020;12(12):e11950. doi: 10.7759/cureus.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuksel B, Bicak F, Gumus F, Kusbeci T (2021) Non-arteritic anterior ischaemic optic neuropathy with progressive macular ganglion cell atrophy due to COVID-19. Neuro-Ophthalmol 1–5. 10.1080/01658107.2021.1909075 [DOI] [PMC free article] [PubMed]
- 7.Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Mizota A, Tormene AP. International society for clinical electrophysiology of vision ISCEV standard for clinical visual evoked potentials: (2016 update) Doc Ophthalmol. 2016;133(1):1–9. doi: 10.1007/s10633-016-9553-y. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero JI, Barragán LA, Martínez JD, Montoya JP, Peña A, Sobrino FE, Tovar-Spinoza Z, Ghotme KA. Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect Dis. 2021;21(1):515. doi: 10.1186/s12879-021-06185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigaut K, Mallaret M, Baloglu S, Nemoz B, Morand P, Baicry F, Godon A, Voulleminot P, Kremer L, Chanson JB, de Seze J (2020) Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 7(5):e785. 10.1212/NXI.0000000000000785. Erratum in: Neurol Neuroimmunol Neuroinflamm. 2020 Jul 9;7(5): PMID: 32461235; PMCID: PMC7286648 [DOI] [PMC free article] [PubMed]
- 10.Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25(2):204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sancho-Saldaña A, Lambea-Gil Á, Liesa JLC, Caballo MRB, Garay MH, Celada DR, Serrano-Ponz M. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med (Lond) 2020;20(4):e93–e94. doi: 10.7861/clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabañes-Martínez L, Villadóniga M, González-Rodríguez L, Araque L, Díaz-Cid A, Ruz-Caracuel I, Pian H, Sánchez-Alonso S, Fanjul S, Del Álamo M, Regidor I. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol. 2020;131(12):2809–2816. doi: 10.1016/j.clinph.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Studart-Neto A, Guedes BF, Tuma RLE, CameloFilho AE, Kubota GT, Iepsen BD, Moreira GP, Rodrigues JC, Ferrari MMH, Carra RB, Spera RR, Oku MHM, Terrim S, Lopes CCB, PassosNeto CEB, Fiorentino MD, DE Souza JCC, Baima JPS, DA Silva TFF, Moreno CAM, Silva AMS, Heise CO, MendonÇa RH, Fortini I, Smid J, Adoni T, GonÇalves MRR, Pereira SLA, Pinto LF, Gomes HR, Zanoteli E, Brucki SMD, Conforto AB, Castro LHM, Nitrini R. Neurological consultations and diagnoses in a large, dedicated COVID-19 university hospital. Arq Neuropsiquiatr. 2020;78(8):494–500. doi: 10.1590/0004-282x20200089. [DOI] [PubMed] [Google Scholar]
- 17.Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6(31):eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benito-Pascual B, Gegúndez JA, Díaz-Valle D, Arriola-Villalobos P, Carreño E, Culebras E, Rodríguez-Avial I, Benitez-Del-Castillo JM. Panuveitis and optic neuritis as a possible initial presentation of the novel coronavirus disease 2019 (COVID-19) Ocul Immunol Inflamm. 2020;28(6):922–925. doi: 10.1080/09273948.2020.1792512. [DOI] [PubMed] [Google Scholar]