Abstract
The concurrent prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Middle East respiratory syndrome coronavirus (MERS-CoV) raises the concern for the emergence of potential new β-CoV clades via genetic recombination, bearing high SARS-CoV-2-like transmissibility and high MERS-CoV-like mortality rates. Therefore, we argue that there is an urgent need to develop pan-β-CoV vaccines that can target not only current SARS-CoV-2 variants of concern, but also future putative SARS-CoV-3- or MERS-CoV-2-like coronavirus.
The development of effective vaccines was considered by some as potentially bringing a quick end to the economic and social ravages of the coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2. However, since the end of 2020, many SARS-CoV-2 variants of concern (VOCs), such as the Beta and Delta variants, have emerged. These VOCs have shown neutralization resistance to post-vaccination sera in humans and animal models and raised concerns over the apparent waning of protective immunity for the first-generation COVID-19 vaccines that were authorized for emergency use. This cast a shadow over previous expectations. A recent study showed that vaccine efficacy against infections of the Delta variant declined from 93% to 53% at only 4 months post-vaccination in humans [1]. Moreover, SARS-CoV-2 is feared to further mutate into escape variants under the selection pressure of antibodies in COVID-19 convalescents and vaccinees, such as the VOC Omicron (B.1.1.529), which harbors 32 mutations in the Spike protein, the latest SARS-CoV-2 VOC that was first reported to the World Health Organization (WHO) from South Africa on 24 November 2021 [2]. Many groups are investigating this rapid mutagenesis phenomenon by analyzing the impact of such mutants on viral entry and replication and neutralization by antibody or vaccine-induced immune responses, as well as cellular and tissue tropism. It is not hard to envision a case where SARS-CoV-2 or MERS-CoV may further evolve into a new clade, SARS-CoV-3 or MERS-CoV-2, respectively, by genetic recombination between SARS-CoV-2 and MERS-CoV. We know that SARS-CoV-2 and MERS-CoV are concurrently prevalent in the Arabian Peninsula and that genetic recombination can occur on the infection and replication of both SARS-CoV-2 and MERS-CoV in the same host, given that both viruses can infect the same cell (type-II alveolar) and they use identical transcription regulatory sequences, conserved sequences upstream of open reading frames that can mediate discontinuous transcription of the viral genome [3,4]. Moreover, the receptor-binding domain (RBD) of SARS-CoV-2 can putatively bind to dipeptidylpeptidase 4 (DPP4), the MERS-CoV receptor, based on bioinformatics analysis combining human–virus protein interaction prediction and protein docking [5]. Several cases of SARS-CoV-2 and MERS-CoV co-infection have been reported in Saudi Arabia [6], with a foreboding possibility of recombination between these two viruses.
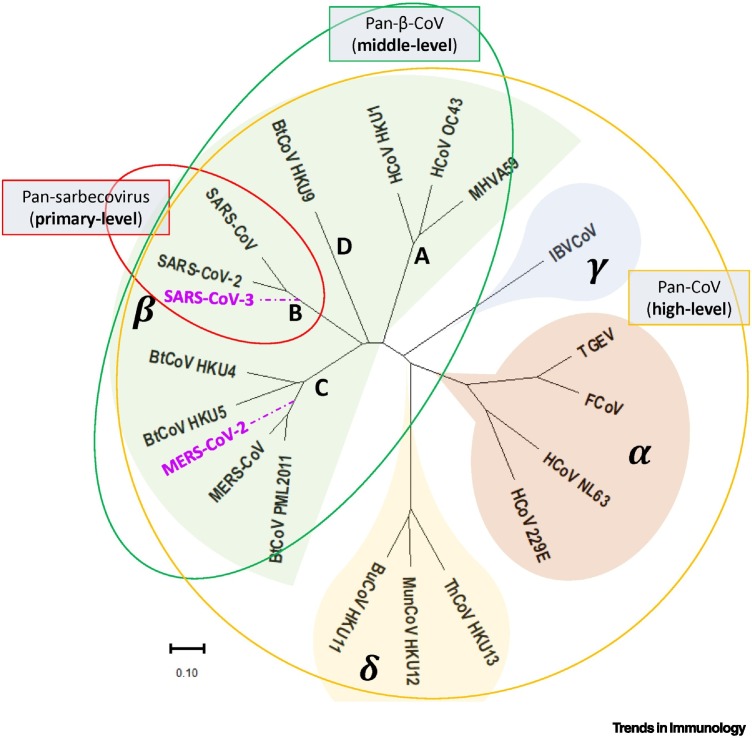
Historically, SARS-CoV-2 was thus named based on phylogenetic analysis, which suggests that it forms a sister clade with human SARS-CoV and bat SARS-related coronavirus (SARSr-CoV) prototypes (Figure 1 ) [7]. Following this logic, a novel SARS-CoV-3 could emerge if SARS-CoV-2 obtains a genomic segment from MERS-CoV. In addition, presumably, SARS-CoV-3 might preserve the high transmissibility potential of SARS-CoV-2 but acquire the high case-fatality rate (CFR) (35%) of MERS-CoV, whereas the global CFR of SARS-CoV-2 is about 2% based on the information reported by the WHO (https://covid19.who.int/). Similarly, a MERS-CoV-2 might preserve the high CFR of MERS-CoV, while potentially gaining the higher transmissibility rate of SARS-CoV-2. Such a hypothesis calls for the urgent development of pan-β-CoV vaccines that could combat any possible development of future SARS-CoV-3 or MERS-CoV-2.
Figure 1.
Phylogenetic tree of coronaviruses.
Viruses in Coronaviridae are divided into four genera: Alphacoronavirus (α-CoV), Betacoronavirus (β-CoV), Gammacoronavirus (γ-CoV), and Deltacoronavirus (δ-CoV). Sarbecovirus is the B lineage of the β-CoV that might emerge in the near future by genetic recombination and could hypothetically be termed severe acute respiratory syndrome coronavirus 3 (SARS-CoV-3) or Middle East respiratory syndrome coronavirus 2 (MERS-CoV-2).
Currently, many research groups are working on the development of CoV vaccines that are cross-reactive to sarbecovirus, lineage B of the Betacoronavirus genus (primary-level breadth), including SARS-CoV, SARS-CoV-2, and SARSr-CoVs. One study, for example, reported that mosaic nanoparticles comprising the RBDs of four to eight distinct zoonotic coronaviruses, including SARS-CoV, SARS-CoV-2, and some SARSr-CoVs from bats, elicited cross-reactive immune responses against these coronaviruses in mice [8]. Although the RBD of coronaviruses mutates frequently, recent work from our laboratory showed that the neutralizing antibodies (NAbs) induced by SARS-CoV-2 RBD linked to a human IgG Fc fragment (RBD-Fc) cross-neutralized infection by sarbecoviruses, including SARS-CoV, SARS-CoV-2, and some SARSr-CoVs from bats, in mice [9]. Moreover, another group demonstrated that the immunization of macaques with SARS-CoV-2 RBD-conjugated nanoparticles elicited cross-NAb responses against bat coronaviruses, SARS-CoV, and SARS-CoV-2 [10]. In addition, another study reported the development of a SARS-CoV-2 Spike protein ferritin nanoparticle (SpFN) vaccine, formulated with a liposomal adjuvant, that induced highly potent and broad NAb responses against the SARS-CoV-2 variants and SARS-CoV in nonhuman primates [11]. This SpFN vaccine entered a Phase I clinical trial in March 2021 (NCT04784767)i. Furthermore, others have documented the presence of potent cross-clade pan-sarbecovirus NAbs in the sera of SARS-CoV survivors immunized with an mRNA (BNT162b2) vaccine [12], indicating that sequential administration of SARS-CoV and SARS-CoV-2 vaccines might be a putative approach to achieving a pan-sarbecovirus vaccine – a possibility that will require robust assessment.
According to the protection range of a certain vaccine, we divided the coronavirus vaccines into three levels (Figure 1). The vaccines that can protect against infection with all sarbecoviruses, all Betacoronaviruses, and all coronaviruses fall into primary-level, middle-level, and high-level breadth, respectively. We argue that these efforts can result in successful pan-sarbecovirus (primary-level breadth) vaccines. However, as hypothesized in the preceding text, a pan-β-CoV vaccine (middle-level breadth) is urgently needed to curtail the potential pandemic tide of a likely SARS-CoV-3 or MERS-CoV-2. Of note, SARS-CoV-2-reactive CD4+ T cells have been detected in the blood of around 35% of unexposed healthy individuals; moreover, in vitro analyses have shown that the numbers of activated CD4+ T cells increase after the incubation of isolated SARS-CoV-2-specific CD4+ T cells with peptides derived from the Spike proteins of human endemic coronavirus 229E (belongs to α-CoV; Figure 1) and OC43 (belongs to β-CoV), indicating that SARS-CoV2-specific CD4+ T could also respond to coronaviruses from other genera [13]. This study suggests the presence of cross-reactive CD4+ T cells that might be induced by other coronaviruses in human blood. However, this does not mean that pan-coronavirus vaccine (high-level breadth) could become available based on the induction of cross-reactive CD4+ T cells.
First, rare evidence suggests that T cell responses can correlate with protection; however, many studies have shown that NAb titers induced by vaccines positively correlate with protection against symptomatic and asymptomatic SARS-CoV-2 infection in vaccinated subjects [14]. Second, T cell immune-based pan-CoV vaccines with low neutralization immunogenicity may induce a suboptimal concentration of NAbs that may enhance SARS-CoV-2 infection by antibody-dependent enhancement (ADE) of viral entry [15], despite the fact that researchers have reported findings in which non-NAbs that show ADE in vitro can still protect against SARS-CoV-2 replication in monkeys and mice [16]. Therefore, while chasing pan-β-CoV vaccines, taking into account both the titer of NAbs and the T cell responses induced by vaccines will be an important measure for efficacy and safety, moving forward.
We posit that it may be more realistic to develop a middle-level-breadth vaccine (i.e., pan-β-CoV vaccine) than a high-level vaccine (i.e., pan-CoV vaccine against the currently circulating SARS-CoV-2 and MERS-CoV as well as their variants and possible related new clades; e.g., SARS-CoV-3, MERS-CoV-2) to be able to equip ourselves for the possible emerging SARS-CoV-2 VOCs and SARS-CoV-3 or MERS-CoV-2 outbreaks.
Acknowledgments
Acknowledgments
The authors gratefully acknowledge funding received from the National Natural Science of China (82041025).
Author contributions
Conceptualization and funding: S.J. Writing – original draft: S.S. Writing – review and editing: S.J. and W.L.
Declaration of interests
The authors declare no interests.
Resources
ihttps://clinicaltrials.gov/ct2/show/NCT04784767References
- 1.Tartof S.Y., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2021. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern.https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern Published online November 26, 2021. [Google Scholar]
- 3.Van Boheemen S., et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3 doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., et al. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 Spike. iScience. 2020;23 doi: 10.1016/j.isci.2020.101400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elhazmi A., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Middle East respiratory syndrome coronavirus (MERS-CoV) coinfection: a unique case series. Travel Med. Infect. Dis. 2021;41 doi: 10.1016/j.tmaid.2021.102026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen A.A., et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science. 2021;371:735–741. doi: 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z., et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal. Transduct. Target Ther. 2020;5:282. doi: 10.1038/s41392-020-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders K.O., et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature. 2021;594:553–559. doi: 10.1038/s41586-021-03594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joyce M.G., et al. Efficacy of a broadly neutralizing SARS-CoV-2 ferritin nanoparticle vaccine in nonhuman primates. bioRxiv. 2021 doi: 10.1101/2021.03.24.436523. Published online March 25, 2021. [DOI] [Google Scholar]
- 12.Tan C.W., et al. Pan-sarbecovirus neutralizing antibodies in BNT162b2-immunized SARS-CoV-1 survivors. N. Engl. J. Med. 2021;385:1401–1406. doi: 10.1056/NEJMoa2108453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun J., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 14.Feng S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su S., et al. Learning from the past: development of safe and effective COVID-19 vaccines. Nat. Rev. Microbiol. 2021;19:211–219. doi: 10.1038/s41579-020-00462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D., et al. In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Cell. 2021;184:4203–4219.e4232. doi: 10.1016/j.cell.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]