Abstract
Purpose
Individuals with primary progressive apraxia of speech (AOS) have AOS in which disruptions in articulation and prosody predominate the speech pattern. Many develop aphasia and/or dysarthria later in the disease course. The aim of this study was to describe the communication limitations in these patients, as measured by (a) the patient via the Communicative Participation Item Bank (CPIB) and (b) the speech-language pathologist via the American Speech-Language-Hearing Association's (ASHA) Functional Communication Measures (FCMs) and an adapted motor speech disorder (MSD) severity rating.
Method
Speech and language evaluations were completed for 24 patients with progressive AOS (n = 7 with isolated AOS; n = 17 with a combination of AOS and aphasia). Descriptive comparisons were utilized to evaluate differences in communication measures among patients with various combinations of MSDs and aphasia. Differences associated with phonetic predominant or prosodic predominant AOS were also examined. Across the entire cohort, correlations were calculated between the participation ratings and other clinical assessment measures.
Results
The CPIB reflected greater limitations for those with aphasia and AOS compared to isolated AOS, but was not notably different when dysarthria occurred with AOS (n = 9/24). Across the cohort, there were statistically significant correlations between the CPIB and ASHA FCM–Motor Speech and Language Expression ratings and the MSD severity rating. The CPIB did not correlate with the ASHA FCM–Language Comprehension or other speech-language measures.
Conclusions
Patients with neurodegenerative AOS experience reduced participation in communication that is further exacerbated by co-occurring language deficits. The study suggests measures of severity cannot be assumed to correlate with measures of participation restrictions and offers a foundation for further research examining the day-to-day sequela of progressive speech and language disorders.
Supplemental Material
Primary progressive apraxia of speech (PPAOS) is the diagnosis given when apraxia of speech (AOS) is insidious, progressive, and the first or only clinical complaint in the absence of stroke or other neurologic trauma to account for the onset. Additionally, there are no accompanying problems (e.g., memory, language, or motor difficulties) that meet criteria for a more specific neurodegenerative disease diagnosis (e.g., Alzheimer's disease dementia, corticobasal syndrome, or progressive supranuclear palsy syndrome) or account for limitations in activities of daily living (Botha & Josephs, 2019; Josephs et al., 2012). We have come to understand a great deal about the clinical presentation (Duffy et al., 2017; Josephs et al., 2013; Poole et al., 2017), underlying pathophysiology (Botha et al., 2015; Duffy et al., 2015; Josephs et al., 2005, 2010, 2012; Utianski, Duffy, Clark, Strand, Botha, et al., 2018; Utianski, Whitwell, et al., 2018; Whitwell et al., 2013), and evolving neurologic picture that is heralded by PPAOS (Josephs et al., 2014; Utianski, Duffy, Clark, Strand, Boland, et al., 2018; Whitwell, Duffy et al., 2017; Whitwell, Weigand, et al., 2017). However, the practical, day-to-day communication limitations of these patients have not been well detailed.
Across etiologies, AOS primarily reflects a disruption of articulation and prosody. In fact, at least in neurodegenerative AOS, disruptions in either articulation or prosody can predominate the speech pattern (Josephs et al., 2013; Utianski, Duffy, Clark, Strand, Botha, et al., 2018). Patients are referred to as having (articulatory) phonetic PPAOS if distorted sound substitutions or additions, often increasing in prominence with increased utterance length or syllable or word complexity, clearly dominate the speech pattern. On the other hand, prosodic PPAOS is used to describe patients in whom syllable segmentation or lengthened intersegment durations between syllables, words, or phrases clearly dominate the speech pattern. When neither phonetic nor prosodic features predominate, the term mixed PPAOS is applied.
With disease progression, the predominant characteristics of AOS can become difficult to parse. Verbal output may become limited to a small number of abnormally produced words or sounds. Some individuals elect to discontinue verbal communication due to increased effort and frustration, while others lose the ability to produce speech beyond undifferentiated sounds. Most patients ultimately become mute.
Patients who present with PPAOS usually develop aphasia at some point in the disease course. This often manifests as agrammatism in writing and in speaking, with omission of articles or function words or disrupted syntax; however, other aspects of language dysfunction may also be evident (e.g., anomia; impaired comprehension of syntactically complex sentences). If a patient is initially evaluated when AOS and aphasia are both present, it may be difficult or impossible to discern which came first or was the predominant communication disorder. Some patients may, in fact, have had aphasia first. It is important to note that, technically, all patients with PPAOS meet criteria for the nonfluent/agrammatic variant of primary progressive aphasia (PPA; Gorno-Tempini et al., 2011); however, this is due to the fact that the current diagnostic criteria consider motor speech as a component of language.
Patients with PPAOS may also develop dysarthria over time, most frequently a hypokinetic and/or spastic dysarthria, often associated with the developing neurological picture (e.g., progressive supranuclear palsy or corticobasal syndrome). The combined MSDs often yield further decrements in intelligibility. These additional clinical symptoms certainly inform treatment planning but it is not well documented how they impact communication in activities of daily living. Quantifying these changes in participation may help guide clinical decision making with regard to augmentative and alternative means of communication (AAC) in some or all communication situations. It may also guide counseling and education of care partners, as there may be different challenges in different communication situations (e.g., with family at home vs. novel communication partners in the community).
The aim of this study was to describe the practical, day-to-day communication limitations in this patient population, as measured by (a) the patient via the Communicative Participation Item Bank (CPIB) and (b) the examining speech-language pathologist (SLP) via the American Speech-Language-Hearing Association's (ASHA) Functional Communication Measures (FCMs) and an adapted motor speech disorders (MSD) severity rating. Given the frequent co-occurrence of aphasia and dysarthria, the relative contribution to each of those accompanying disorders was also explored. This study will allow us to better understand (a) the impact of progressive speech and language disorders on communication participation and (b) the relationship among measures of motor speech severity and subsequent participation restrictions.
Method
Participants
Between February 2018 and May 2019, 24 unique patients (12 men) with progressive AOS (n = 7 with isolated AOS [i.e., PPAOS, without aphasia]; n = 17 with a combination of AOS and aphasia, referred to as AOS + Aphasia) were enrolled in a National Institutes of Health–funded study. Although not an entry criterion, nine of the 24 patients also had dysarthria (six with AOS and aphasia). Aphasia and dysarthria were less prominent than AOS in all patients. With the current diagnostic criteria (Gorno-Tempini et al., 2011), all patients technically met root criteria for PPA and would be characterized as the nonfluent/agrammatic variant; however, it is not universally accepted that motor speech is an element of language. Because our research group considers motor speech to be separable from language, we do not consider patients with PPAOS (i.e., no aphasia) to have met root criteria for a nonfluent/agrammatic diagnosis. Given that we do not yet know the implications for the sequence of symptom onset (i.e., on prognosis or clinical evolution), we have used the term “PPAOS” to recognize it as the initial and sole problem at onset, and “AOS + Aphasia” to recognize the subsequent emergence of less prominent aphasia.
All patients were White, not Hispanic, per self-report. This study was approved by the Mayo Clinic Institutional Review Board and all patients provided written consent to participate.
Clinical Examination
A comprehensive communication evaluation was conducted, as previously described (Utianski, Duffy, Clark, Strand, Botha, et al., 2018). Briefly, full language and motor speech evaluations were conducted (by authors RLU, JRD, or HMC, all experienced clinicians in the differential diagnosis of neurologic speech and language disorders). Clinical judgments regarding the presence, nature (i.e., type), and severity of AOS, aphasia, and dysarthria were made by the examining clinician and subsequently confirmed by consensus agreement (among the other nonexamining SLP authors, RLU, JRD, or HMC). Severity ratings reflected gestalt clinical judgment on a 5-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = marked, 4 = severe). The Western Aphasia Battery–Revised (WAB-R; Kertesz, 2007) was administered and the WAB–Aphasia Quotient (AQ) served as an additional index of aphasia severity. With a maximum score of 100, a score greater than 93.8 is considered to be within normal limits. Clinical judgment regarding the presence and severity of aphasia was based on performance on the WAB-R, as well as other formal language measures and written and spoken picture descriptions. Raw scores from the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005) served as a proxy for general cognitive abilities.
A conversational speech sample, including narrative picture description, was collected as a part of the WAB-R. Additionally, supplementary speech and nonspeech tasks (alternating and sequential motion rates) were elicited. These speech samples were used to make a judgment regarding the predominance of phonetic or prosodic speech characteristics, determined by consensus agreement of the same two experienced SLPs. Following that subjective, gestalt judgment, the speech samples were also utilized to complete the Apraxia of Speech Rating Scale–Version 3 (ASRS-3; Strand et al., 2014; Utianski, Duffy, Clark, Strand, Botha, et al., 2018). The ASRS-3 rates the presence and severity of speech characteristics presumed to be specific to AOS, as well as characteristics that overlap with aphasia and dysarthria; it allows for quantification of (articulatory) phonetic and prosodic speech deficits. The total ASRS-3 score can range from 0 to 52 (where 0 indicates the absence of abnormal speech characteristics). There is no cutoff for the current version, but past research demonstrated a score of 8 was sensitive to the presence of AOS (Strand et al., 2014). An Articulatory Error Score (AES) was calculated from the proportion of incorrectly produced words on the supplementary speech tasks (repeated repetitions of 13 words of increasing length and complexity and single repetitions of three sentences). A score of 0 reflects the absence of the following features on all words: distorted or undistorted sound substitutions, additions, or repetitions; sound omissions; sound prolongations; false starts; and attempted self-correction of sound errors. The AES served as an index of articulation errors, which may result from any combination of AOS, aphasia, or dysarthria.
Communication Measures
The CPIB
The CPIB short form is a 10-question survey (0–3 points per question; 30 = disorder does not interfere with communication) intended to quantify communication participation, and, more specifically, interference of an unspecified “disorder” on the success of communication participation in a variety of situations (Baylor et al., 2013). Total scores and T-scores (Baylor et al., 2013) are recorded. On the T-scale, 50 is the mean of the calibration sample, which consisted of people with a range of communication disorders. The maximum T-score on the general short form is 71, reflecting no interference in communication in daily activities.
The generic nature of items on the CPIB (relative to the nature of any communication disorder) allows for its use across a variety of communication disorders. For example, one question is “Does your condition interfere with talking with people you know?” (rated: 3 = not at all, 2 = a little, 1 = quite a bit, and 0 = very much). In this case, patients answer the questions in relation to their difficulties without reference to the specific condition interfering with their success; in other words, they do not specify whether it is the AOS, aphasia, and/or dysarthria that is detrimental to communication. Patients are instructed to complete this independently, but in the event of aphasia, care partners may provide input to facilitate their understanding of the written questions. The CPIB first underwent psychometric evaluation in patients with spasmodic dysphonia (Baylor et al., 2009) and subsequently with patients with a variety of etiologies of communication disorders (e.g., multiple sclerosis, Parkinson's disease, amyotrophic lateral sclerosis [ALS], and head and neck cancer; Baylor et al., 2013); it has strong reliability and face validity.
MSD Severity Rating
The MSD Severity Rating (adapted from Hillel et al., 1989 and Yorkston et al., 1993), is a 1–10 rating of MSD severity which indexes the degree to which speech is understood, without regard to specific activities of daily living. This scale is derived from the ALS severity scale, originally designed and utilized for the judgment of speech symptom severity secondary to ALS (Hillel et al., 1989); here, it is utilized to index speech utility, resultant from a different disease. The ratings reflect normal speech processes (ratings of 10 or 9), detectable speech disturbances (ratings of 8 or 7), the use behavioral modifications (ratings of 6 or 5), use of AAC (ratings of 4 or 3), or loss of useful speech (ratings of 2 or 1). This judgment is made by the examining SLP. In its original form, high interrater reliability was demonstrated, as was strong concurrent validity with other measurements of speech functioning.
ASHA's Functional Communication Measures
ASHA's National Outcome Measurement System is intended to quantify challenges associated with different speech and language deficits (Mullen, 2004). The National Outcome Measurement System scales, referred to as Functional Communication Measures (FCMs), are 7-point rating scales (7 = independently successful) used to describe a patient's abilities specific to different domains of speech and language functioning; additional information regarding psychometric properties were not available. The examining SLP rated the FCMs specific to Motor Speech, Language Expression, and Language Comprehension.
Data Analyses
Descriptive Analysis
Given the subgroup sample sizes, descriptive statistics and box-plots were utilized to visually compare subgroups of patients with only AOS and subgroups with AOS plus aphasia and/or dysarthria. We expected greater reductions in communication participation when there were multiple communication disorders present. We also hypothesized differences associated with the predominance of AOS type (phonetic or prosodic), with greater impacts on communication expected in phonetic predominant AOS; these differences were also visually assessed.
Correlations
Spearman rank correlations were calculated among the measures of communication limitations (CPIB, FCMs) and quantitative clinical measures (WAB-AQ, MoCA, ASRS-3, and AES) across the entire cohort of patients. Statistical significance was assessed at p < .05, where the reported p value is false discovery rate corrected for multiple comparisons.
Reliability
Interrater reliability was calculated for judgments of MSD severity and ASHA FCM ratings for 20% (n = 5) of patients. A second rating was made (JRD or RLU), blinded to the first rater's (RLU or JRD) judgments. Given the ordinal data, a nonparametric Spearman rank correlation across the measures for the five patients yielded ρ = .937, p < .0001, suggesting good agreement. One hundred percent of reliability ratings fell within one point of the primary rater's judgments, which were used in subsequent analyses. Reliability was also calculated for ASRS and AES scores for 10 patients. The interrater intraclass correlation was .98 for the total ASRS score and .99 for the total AES score. The primary rater's scores were used in subsequent analyses.
Results
Clinical Groups
Across the whole cohort, mean age at evaluation was 66.4 years, with mean disease duration of 4.1 years. Mean education was 15.5 years. Average WAB-AQ was 91.4. Average ASRS-3 was 21.9, with an associated clinically rated mean severity of 2 (moderate AOS). Mean AES was 39.3% error across all items. On the overall gestalt clinical ratings, mean aphasia severity was 1.4 (mild–moderate), with mean dysarthria severity of .5 (equivocal). Mean CPIB was 6.6/30 and mean T-score was 35.6, reflecting interference with participation. Mean MSD severity rating was 5.7 (repeats message on occasion). Mean ASHA FCM–Motor Speech was 5.1 (intelligible for simple sentences), FCM–Language Expression was 5.8 (able to communicate, with self-initiated repair), and FCM–Language Comprehension was 6.4 (good comprehension in most activities, with self-initiated compensatory strategies). Clinical and demographic information are provided in Table 1 for two subgroups of patients: those with and without aphasia and those with and without dysarthria.
Table 1.
Demographics and speech and language data (mean [minimum, maximum]) of clinical groups.
| Progressive apraxia of speech (n = 24) |
||||
|---|---|---|---|---|
| PPAOS (Aphasia absent) (n = 7) |
AOS + aphasia (Aphasia present) (n = 17) |
Dysarthria absent (n = 15) |
Dysarthria present (n = 9) |
|
| Demographics (years) | ||||
| Age at evaluation | 70.86 (60, 80) | 64.59 (29, 88) | 66.33 (51, 79) | 66.56 (29, 88) |
| Disease duration | 3.5 (1.5, 8) | 4.27 (.5, 13) | 3.28 (.5, 8) | 5.71 (2, 13) |
| Education | 17.3 (15, 19) | 14.9 (11, 19) | 15 (11,19) | 16.63 (16,19) |
| Cognitive–language–speech measures | ||||
| MoCa (/30, unimpaired) | 27.67 (26, 30) | 21.19 (12, 26) | 22.93 (12, 29) | 23 (16, 30) |
| WAB-AQ (/100, unimpaired) | 98.11 (97.2, 99.2) | 88.64 (66.2, 98.9) | 93.7 (74.3, 99.2) | 87.58 (66.2, 99.2) |
| ASRS-3 total (/52, severe) | 20.71 (10, 39) | 22.44 (9, 49) | 17.79 (9, 26) | 28.33 (11, 49) |
| AES % error (/100, severe) | 31.61 (5.36, 89.09) | 42.64 (3.57, 87.3) | 31.60 (3.57, 54.55) | 53.68 (10.71, 89.09) |
| Patient rated measure | ||||
| CPIB (/30, no restrictions) | 12.29 (0, 28) | 4.29 (0, 24) | 7.6 (0, 28) | 5 (0, 18) |
| CPIB T-score (/71, no restrictions) | 43.13 (24.2, 64.2) | 32.46 (24.2, 57.8) | 37.27 (24.2, 64.2) | 32.9 (24.2, 50.3) |
| SLP rated measures | ||||
| Aphasia present | 0 | 1 | n = 11 | n = 6 |
| Aphasia severity (/4, severe) | 0 | 1.5 (1, 3) | 1.42 (0, 2.5) | 1.29 (0, 3) |
| Dysarthria present | n = 3 | n = 6 | 0 | 1 |
| Dysarthria severity (/4, severe) | 1.17 (.5, 2) | 1.42 (1, 2.5) | 0 | 1.33 (.5, 2.5) |
| AOS severity (/4, severe) | 2.07 (1, 4) | 2.18 (1, 4) | 1.7 (1, 3) | 2.89 (1, 4) |
| MSD severity (/10, normal) | 6.29 (3, 8) | 5.48 (3, 8) | 6.27 (4, 8) | 4.78 (3, 8) |
| FCM MS (/7, normal) | 5.7 (2, 7) | 4.9 (2, 7) | 5.9 (4, 7) | 3.9 (2, 7) |
| FMC LE (/7, normal) | 7 (7, 7) | 5.3 (2, 7) | 6 (4, 7) | 5.3 (2, 7) |
| FCM LC (/7, normal) | 7 (7, 7) | 6 (4, 7) | 6.6 (5, 7) | 5.9 (4, 7) |
Note. PPAOS = primary progressive apraxia of speech; AOS = apraxia of speech ; MoCA = Montreal Cognitive Assessment; WAB-AQ = Western Aphasia Battery–Revised Aphasia Quotient; ASRS-3 = Apraxia of Speech Rating Scale–Version 3; AES = Articulatory Error Score; CPIB = Communicative Participation Item Bank; SLP = speech-language pathologist; MSD = motor speech disorder; FCM = Functional Communication Measure; MS = Motor Speech; LE = Language Expression; LC = Language Comprehension.
Measures of Communication Limitations
Descriptive Analysis
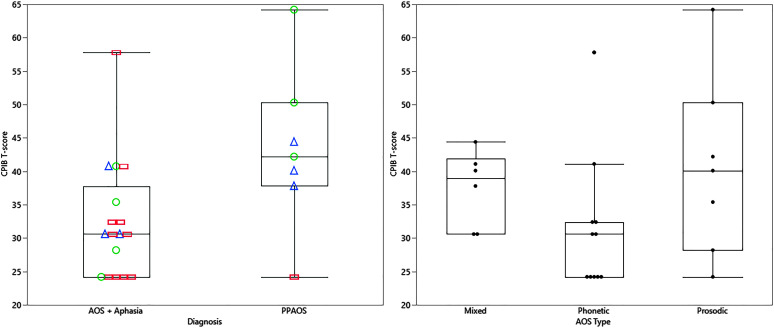
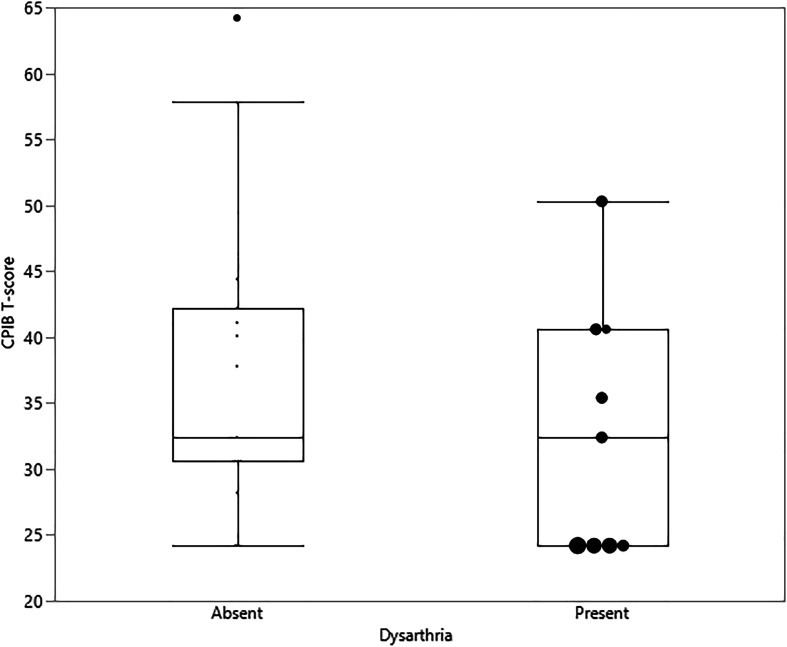
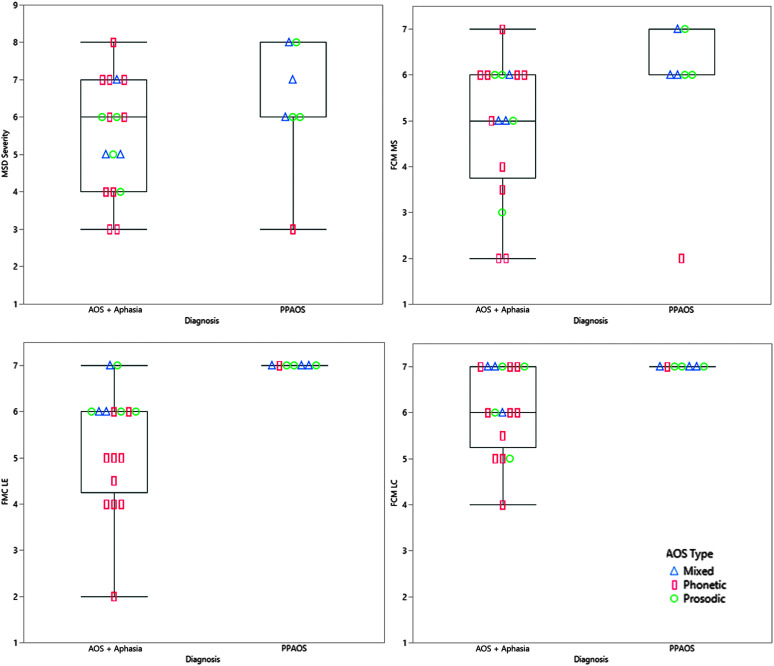
The CPIB reflected greater participation restrictions for those with AOS plus aphasia compared to PPAOS (see Figure 1), but was not notably different for those with concomitant dysarthria (n = 9; see Figure 2). A wider range of experience was noted on the CPIB for those with prosodic predominant AOS, with consistently greater limitations noted by those with phonetic predominant AOS type (see Figure 1). Additional visualization of data, using raw scores, is presented in Supplemental Materials S1 and S2. There was a wider range of MSD severity and all ASHA FCM ratings among patients with AOS plus aphasia, but this may reflect the larger sample in this group (see Figure 3). The FCM ratings for Language Expression and Language Comprehension were consistent with the same clinician's gestalt judgment of the absence of aphasia in the PPAOS group (see Figure 3).
Figure 1.
Box plots of CPIB T-scores for patients with PPAOS and those with isolated AOS (PPAOS) and combinations of AOS and aphasia (AOS + Aphasia; left panel). AOS type is indicated for each data point; mixed = triangle, phonetic = rectangle; prosodic = circle. Box plots of CPIB scores for all patients by AOS type, regardless of the presence of aphasia (right panel). PPAOS = primary progressive apraxia of speech; CPIB = Communicative Participation Item Bank; AOS = apraxia of speech.
Figure 2.
Box plots of Communicative Participation Item Bank (CPIB) T-scores for patients with (n = 9) and without dysarthria, regardless of co-occurring aphasia. When present, dysarthria severity is indicated by circle size, where the largest circle reflects severe and smallest circle reflects mild on a 4-point severity scale (mild, moderate, marked, severe). When dysarthria is absent, the larger circle indicates the data point is an outlier.
Figure 3.
Box plots of speech-language pathologist–rated measures, Motor Speech Disorder (MSD) Severity Rating and American Speech-Language-Hearing Association Functional Communication Measures (FCMs) of Motor Speech (MS), Language Expression (LE), and Language Comprehension (LC), for patients with isolated AOS (PPAOS) and AOS with aphasia (AOS + Aphasia). AOS type is indicated for each data point; mixed = triangle, phonetic = rectangle; prosodic = circle. PPAOS = primary progressive apraxia of speech; AOS = apraxia of speech.
Correlations
The correlations among measures of function (i.e., MSD severity rating, FCMs, and CPIB) and assessment measures (i.e., WAB-AQ, ASRS, AES) are summarized in Table 2. The CPIB significantly, but moderately, correlated with the ASHA FCM–Motor Speech (ρ = .53) and Language Expression (ρ = .40) ratings and the SLP-rated MSD severity rating (ρ = .48), in the direction of more severe deficits in each measure. The CPIB did not correlate with the ASHA FCM–Language Comprehension, other assessment measures (MoCA, WAB), or measures of speech production accuracy (ASRS-3, AES).
Table 2.
Spearman's correlations.
| Measure | MoCa | WAB-AQ | ASRS-3 | MSD Sev | AES | FCM MS | FCM LC | FMC LE | CPIB |
|---|---|---|---|---|---|---|---|---|---|
| MoCa | 1.0 | ||||||||
| WAB-AQ | .78* | 1.0 | |||||||
| ASRS-3 | −.06 | −.03 | 1.0 | ||||||
| MSD Sev | .15 | .20 | −.88* | 1.0 | |||||
| AES | −.35 | −.38 | .71* | −.68* | 1.0 | ||||
| FCM MS | .26 | .36 | −.81* | .91* | −.68* | 1.0 | |||
| FCM LC | .75* | .80* | −.27 | .32 | −.46* | .44* | 1.0 | ||
| FMC LE | .71* | .91* | −.03 | .16 | −.38 | .33 | .71* | 1.0 | |
| CPIB | .30 | .37 | −.31 | .48* | −.30 | .53* | .33 | .40* | 1.0 |
Note. * indicates statistically significant correlation, FDR, p < .05; MoCA = Montreal Cognitive Assessment; WAB-AQ = Western Aphasia Battery–Revised Aphasia Quotient; ASRS-3 = Apraxia of Speech Rating Scale–Version 3; MSD Sev = Motor Speech Disorder Severity Rating; AES = Articulatory Error Score; FCM = Functional Communication Measure; MS = Motor Speech; LC = Language Comprehension; LE = Language Expression; CPIB = Communicative Participation Item Bank.
The SLP-rated MSD severity ratings strongly and significantly correlated with the ASRS-3 (ρ = −.88), AES (ρ = −.68), and ASHA FCM–Motor Speech (ρ = .90), again in the direction of greater severity in all measures. The ASHA FCM–Motor Speech also strongly correlated with the ASRS-3 (ρ = −.81) and AES (ρ = −.68). Importantly, correlations between the ASHA FCM–Motor Speech rating and measures of language and cognitive functioning (WAB-AQ, MoCA) were not significant. The ASHA FCM–Language Expression, however, was strongly correlated with the MoCA (ρ = .71) and WAB-AQ (ρ = .91). Finally, the ASHA FCM–Language Comprehension rating showed significant strong, positive correlations with the MoCA (ρ = .75) and WAB-AQ (ρ = .80), and a moderate, negative correlation with the AES (ρ = −.46).
Discussion
This study described the communication limitations in patients with progressive AOS, including those with PPAOS (i.e., isolated AOS) and in those with less prominent concomitant aphasia and/or dysarthria. It included a number of indices of communication success or challenges, including ratings made by the patients (CPIB) and several made by the examining SLP (MSD severity rating and ASHA FCMs). Overall, these clinical ratings capture the impact of communication deficits in patients with AOS that are greater in severity when aphasia is also present.
The CPIB reflected greater impact on communication participation for those with AOS plus aphasia compared to those with PPAOS (see Figure 1). Given that CPIB scores were not notably different in those with AOS plus dysarthria (see Figure 2), there may be additive detriment to communication participation of combined language and MSDs, but not of combined MSDs, at least when AOS is the predominant MSD, as was the case in this study. The distribution of the data for participants with dysarthria suggests that more severe dysarthria is associated with greater communication limitation, per the CPIB; however, more severe dysarthria was also often associated with more severe AOS and longer disease duration. In this limited sample, it is thus difficult to parse out the relative contributions of each of these influences. While the patients in this study technically meet diagnostic criteria for nonfluent/agrammatic PPA, it is not a heterogeneous entity. Their experiences may mirror those patients with aphasia greater than AOS in a cross-sectional analysis. Systematic comparison with the experience of patients with language greater than motor speech difficulties will further refine our understanding of these similar neurologic syndromes. It may be that the perceived disability in patients with PPAOS and other presentations of nonfluent/agrammatic PPA worsens with disease progression and additional symptom development. This requires empirical evaluation.
While the subgroups examined in this study were small, viewing the self-reported participation restrictions on the CPIB relative to the predominance of phonetic versus prosodic speech disruptances suggests that greater deleterious effects are experienced by those with phonetic predominant AOS (see Figure 1). This distinction is less obvious when viewing the examiner-rated MSD severity rating and ASHA FCM–Motor Speech (see Figure 3). Identifying possible reasons for this discrepancy should be the focus of future work; two possible explanations are the overall reductions in intelligibility and quantity of verbal output on which the judgments are based. Another possible, albeit less likely, contributor is examiner experience with the severity continuum. There are possible confounds in fully interpreting the impact of the speech characteristics as patients with phonetic predominant AOS tend to have more severe aphasia (Utianski, Duffy, Clark, Strand, Boland, et al., 2018), which is also likely contributing to their restrictions in communication participation.
The CPIB was significantly correlated with the SLP-rated ASHA FCM–Motor Speech and Language Expression ratings and MSD severity rating, supporting concurrent validity among those rating scales as indices verbal expression abilities. The CPIB did not, however, correlate with the ASHA FCM–Language Comprehension rating or other measures of speech and language abilities (MoCA, WAB, ASRS-3, or AES). These relationships, or lack thereof, support the notion that measures of severity (ASRS, AES) cannot be assumed to correlate with measures of participation restrictions (CPIB). Of course, this may also reflect statistical power, the assessments not accurately capturing the severity continuum (mild or severe), or something else. For instance, the severity of communication challenges is not a direct reflection of the severity captured in any single assessment. The successful implementation of communication reflects the combination of different communication functions; success may also vary in different communication situations. It is also the case that AOS was the predominant problem in this sample, with little to no language or cognitive symptoms; thus, language comprehension, aphasia, and cognition would not be expected to have as much influence as the MSD on participation. Statistical modeling in a larger sample size might offer insight into the relative contributions of each of the speech-language assessments to the indexed communication limitations. Success in different communication situations should also be assessed.
Interestingly, the SLP-rated MSD severity and ASHA FCM–Motor Speech each strongly correlated with the ASRS-3 and AES, suggesting robust associations among focused ratings of motor speech capabilities. While these are all made by the same rater, they offer cross-validation of one another. Notably, there were no significant correlations between the ASHA FCM–Motor Speech rating and measures of language and cognitive functioning (WAB-AQ, MoCA), while there were between the ASHA FCM–Language Expression and the language measures. This is exactly as expected and reflects concurrent validity among measures of language functioning.
The ASHA FCM–Language Comprehension rating was positively correlated with the MoCA and WAB-AQ, reflecting aphasia. The ASHA FCM–Language Comprehension was also negatively correlated with the AES, with more severe comprehension restrictions associated with more errors on the AES; this may be mediated by overall aphasia severity and the combined effects of aphasic phonological errors and AOS, both of which are captured by the AES. Importantly, there is limited correlation between the CPIB and FCM–Language Comprehension. One possible explanation for this is reduced insight for comprehension deficits. This is compatible with clinical experience that many patients do not endorse difficulty understanding others, despite evidence of reduced comprehension on examination. Or perhaps their communication partners adequately modify the length and density of content to be processed, such that they do not experience such difficulties day-to-day. Likely further contributing to the observed lack of correlation was the limited range of ratings obtained on the FCM–Language Comprehension and the relatively mild comprehension challenges observed in this sample. Finally, a recent study also suggested that some items on the CPIB were not sufficient to capture the participation limitations of patients with hearing loss; perhaps a different set of items is needed to reflect difficulties stemming from auditory perception or comprehension deficits (Miller et al., 2017). While hearing loss and auditory comprehension are not equivalent, it is important to acknowledge the interplay of sensation and perception, and speech production and perception, as they contribute to the overall communication experience.
Overall, it appears the CPIB captures the amalgamated restrictions in participation from a number of sources (e.g., language and motor speech); however, the level of interference does not necessarily reflect the severity of any single speech or language disorder. The ASHA FCMs, however, appear to reflect the intended domain, given the respective correlations with speech and language measures. A strength of this analysis is that the examiners' judgments are made based on a number of tasks, including picture description, repetition, and other motor speech tasks, rather than a single task.
General Discussion: Relationship to Other Communication Disorders
Overall, these findings are consistent with studies that have used the CPIB to describe communication restrictions associated with speech disorders due to other diseases. Here, the CPIB reflected greater communication complications for those with AOS plus aphasia compared to PPAOS. This is not unlike results from a recent study that concluded that the CPIB reflected the combined influence of dysarthria and cognitive deficits in patients with multiple sclerosis (Feenaughty et al., 2018). In both of these patient populations, multifaceted communication disorders yielded more apparent restrictions in participation than speech production challenges alone.
Yorkston and colleagues suggested the severity and usage of speech greatly influenced communication participation in patients with ALS (Yorkston et al., 2017), recently corroborated in another study (Sixt Börjesson et al., 2020). Further discussion of Yorkston and colleagues findings, however, suggested other variables, such as cognition, mood, or fatigue, may also influence participation. This was similarly true in a study exploring communication participation of patients who stuttered (Boyle et al., 2018). Variables such as self-esteem and social support may also influence communication participation. Importantly, other research suggests that speech severity, self-perceived and otherwise, along with other factors, may influence decisions regarding participation in speech therapy (Yorkston et al., 2017). This study, and the other cited literature, support the notion that the experience of communication restrictions can be similar, regardless of the source (e.g., stroke or degenerative disease, speech or language; Baylor et al., 2011).
Given the importance of incorporating the patient's experience into treatment planning, this patient reported outcome (i.e., CPIB) serves to index the experienced restrictions in participation, beyond the severity of the MSD itself, and should be incorporated into clinical practice (de Riesthal & Ross, 2015; Donovan, 2012; Yorkston & Baylor, 2019). In the context of progressive conditions, the CPIB can document increasing limitations. When treatment is provided, it may document outcomes to which other measures may not be sensitive. As mentioned, quantifying participation limitations may help guide clinical decision making with regard to AAC. Assistance from AAC, ideally with support from insurance coverage, should be available to maximize independence and maintain participation in activities of daily living, as soon as those restrictions become evident. Many patients report positive experiences with AAC to facilitate more effective and efficient communication (Botha & Utianski, 2020; Utianski, Duffy, Clark, Strand, Boland, et al., 2018). Of course, there are a few key issues to consider when planning for AAC. This includes, but is not limited to, concomitant motoric limitations, aphasia, and other nonaphasic cognitive communication deficits. An individual's desire and willigness to use nonverbal means of communication, especially advanced technology, also dictate recommendations and success of high- or low-tech AAC.
Limitations and Future Directions
There are limitations to this study. A larger sample, with a greater range of severity of AOS, dysarthria, and aphasia would allow for the data to be stratified to determine if and how the relationships among measures and between severity and participation restrictions are different along the severity continuum. Such a study could explore the potential interaction of speech and language disorders and compare the experience of patients with PPAOS and other presentations of nonfluent/ agrammatic PPA. Furthermore, more patient report measures are needed, particularly ones that minimize possible confounds imposed by aphasia. Here, we allowed caregivers to facilitate completion of the survey if patients requested it, but that imposes other biases on the findings (Baylor et al., 2017); the surveys were completed in private and we did not monitor this assistance. As the clinician was not present, there was no opportunity to assist patients with comprehension or to observe the care partner facilitating communication. As such, the degree to which the patient understood the questions on the survey is not entirely known. Future studies should consider having the clinician administer a survey such as the CPIB to ensure as optimal and valid use of the questionnaire as possible.
While it is outside the scope of the current study to explore fully, the MoCA is a speech- and language-mediated assessment, and may mispresent aphasia or AOS as “cognitive” deficits. There is therefore a need for measures of cognitive abilities that are minimally influenced by speech or language disorders. Future studies should expand the current analyses to include statistical analyses and predictive modeling in a larger sample longitudinally to assess possible relationships with disease duration or severity and explore disease acclimation. Correlations between patient reported outcomes and additional measures of cognitive–linguistic and motor speech deficits (e.g., intelligibility) are also recommended.
Conclusions
To our knowledge, this is the first study to detail the patient- and examiner-perceived communication challenges in patients who present with a combination of progressive speech and language disorders (e.g., PPAOS and those who have also developed aphasia and/or dysarthria). Overall, patients with PPAOS experience reduced participation in communication that is further exacerbated by co-occurring aphasia. The patient reported CPIB is well aligned with the clinician-rated ASHA FCM measures of motor speech functioning and language expression and offers a complementary, quantitative measure of communication limitations in these patients. However, the study also suggests measures of severity (AES, ASRS) cannot be assumed to correlate with measures of disability (CPIB) and offers a foundation for further research examining the day-to-day sequela of progressive speech and language disorders.
Supplementary Material
Acknowledgments
We extend our gratitude to these patients and their families for their time and dedication to our research program. This study was funded by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grants R01 DC014942 (PI: Josephs) and R01 DC012519 (PI: Whitwell).
Funding Statement
We extend our gratitude to these patients and their families for their time and dedication to our research program. This study was funded by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grants R01 DC014942 (PI: Josephs) and R01 DC012519 (PI: Whitwell).
References
- Baylor, C. , Burns, M. , Eadie, T. , Britton, D. , & Yorkston, K. (2011). A qualitative study of interference with communicative participation across communication disorders in adults. American Journal of Speech-Language Pathology, 20(4), 269–287. https://doi.org/10.1044/1058-0360(2011/10-0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor, C. , Oelke, M. , Bamer, A. , Hunsaker, E. , Off, C. , Wallace, S. E. , Pennington, S. , Kendall, D. , & Yorkston, K. (2017). Validating the Communicative Participation Item Bank (CPIB) for use with people with aphasia: An analysis of differential item function (DIF). Aphasiology, 31(8), 861–878. https://doi.org/10.1080/02687038.2016.1225274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor, C. , Yorkston, K. , Eadie, T. , Kim, J. , Chung, H. , & Amtmann, D. (2013, August). The Communicative Participation Item Bank (CPIB): Item bank calibration and development of a disorder-generic short form. Joural of Speech, Language, and Hearing Research, 56(4), 1190–1208. https://doi.org/10.1044/1092-4388(2012/12-0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor, C. , Yorkston, K. M. , Eadie, T. L. , Miller, R. M. , & Amtmann, D. (2009). Developing the Communicative Participation Item Bank: Rasch analysis results from a spasmodic dysphonia sample. Journal of Speech, Language, and Hearing Research, 52(5), 1302–1320. https://doi.org/10.1044/1092-4388(2009/07-0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha, H. , Duffy, J. R. , Whitwell, J. L. , Strand, E. A. , Machulda, M. M. , Schwarz, C. G. , Reid, R. I. , Spychalla, A. J. , Senjem, M. L. , Jones, D. T. , Lowe, V. , Jack, C. R. , & Josephs, K. A. (2015, August). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. https://doi.org/10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha, H. , & Josephs, K. A. (2019). Primary progressive aphasias and apraxia of speech. Continuum: Lifelong Learning in Neurology, 25(1), 101–127. https://doi.org/10.1212/con.0000000000000699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha, H. , & Utianski, R. L. (2020). Primary progressive apraxia of speech. In Utianski R. L. (Ed.), Primary progressive aphasia and other frontotemporal dementias: Diagnosis and treatment of associated communication disorders (pp. 101–134). Plural. [Google Scholar]
- Boyle, M. P. , Beita-Ell, C. , Milewski, K. M. , & Fearon, A. N. (2018). Self-esteem, self-efficacy, and social support as predictors of communicative participation in adults who stutter. Journal of Speech, Language, and Hearing Research, 61(8), 1893–1906. https://doi.org/10.1044/2018_JSLHR-S-17-0443 [DOI] [PubMed] [Google Scholar]
- de Riesthal, M. , & Ross, K. B. (2015). Patient reported outcome measures in neurologic communication disorders: An update. Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 25(3), 114–120. https://doi.org/10.1044/nnsld25.3.114 [Google Scholar]
- Donovan, N. J. (2012). Patient-reported outcomes for acquired dysarthria. Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders, 22(4), 152–159. https://doi.org/10.1044/nnsld22.4.152 [Google Scholar]
- Duffy, J. R. , Hanley, H. , Utianski, R. L. , Clark, H. M. , Strand, E. A. , Josephs, K. A. , & Whitwell, J. L. (2017). Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain and Language, 168, 84–94. https://doi.org/10.1016/j.bandl.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, J. R. , Strand, E. A. , Clark, H. , Machulda, M. , Whitwell, J. L. , & Josephs, K. A. (2015, May). Primary progressive apraxia of speech: Clinical features and acoustic and neurologic correlates. American Journal of Speech-Language Pathology, 24(2), 88–100. https://doi.org/10.1044/2015_ajslp-14-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenaughty, L. , Tjaden, K. , Weinstock-Guttman, B. , & Benedict, R. H. B. (2018). Separate and combined influence of cognitive impairment and dysarthria on functional communication in multiple sclerosis. American Journal of Speech-Language Pathology, 27(3), 1051–1065. https://doi.org/10.1044/2018_AJSLP-17-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini, M. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , Ogar, J. M. , Rohrer, J. D. , Black, S. , Boeve, B. F. , Manes, F. , Dronkers, N. F. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. L. , Knopman, D. S. , Hodges, J. R. , Mesulam, M. M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillel, A. D. , Miller, R. M. , Yorkston, K. , McDonald, E. , Norris, F. H. , & Konikow, N. (1989). Amyotrophic lateral sclerosis severity scale. Neuroepidemiology, 8(3), 142–150. https://doi.org/10.1159/000110176 [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , Boeve, B. , Duffy, J. , Smith, G. , Knopman, D. , Parisi, J. , Petersen, R. , & Dickson, D. (2005). Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase, 11(4), 283–296. https://doi.org/10.1080/13554790590963004 [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Fossett, T. R. , Strand, E. A. , Claassen, D. O. , Whitwell, J. L. , & Peller, P. J. (2010). Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Archives of Neurology, 67(5), 596–605. https://doi.org/10.1001/archneurol.2010.78 [DOI] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Gunter, J. L. , Schwarz, C. G. , Reid, R. I. , Spychalla, A. J. , Lowe, V. J. , Jack, C. R., Jr. , & Whitwell, J. L. (2014). The evolution of primary progressive apraxia of speech. Brain, 137(10), 2783–2795. https://doi.org/10.1093/brain/awu223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Lowe, V. J. , Jack, C. R. , & Whitwell, J. L. (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81(4), 337–345. https://doi.org/10.1212/WNL.0b013e31829c5ed5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Master, A. V. , Lowe, V. J. , Jack, C. R., Jr. , & Whitwell, J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(5), 1522–1536. https://doi.org/10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz, A. (2007). Western Aphasia Battery–Revised. PsychCorp. [Google Scholar]
- Miller, C. W. , Baylor, C. R. , Birch, K. , & Yorkston, K. M. (2017). Exploring the relevance of items in the Communicative Participation Item Bank (CPIB) for individuals with hearing loss. American Journal of Audiology, 26(1), 27–37. https://doi.org/doi:10.1044/2016_AJA-16-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, R. (2004). Evidence for whom?: ASHA's National Outcomes Measurement System. Journal of Communication Disorders, 37(5), 413–417. https://doi.org/10.1016/j.jcomdis.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. , Phillips, N. A. , Bédirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , Cummings, J. , & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–669. https://doi.org/10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Poole, M. L. , Brodtmann, A. , Darby, D. , & Vogel, A. P. (2017). Motor speech phenotypes of frontotemporal dementia, primary progressive aphasia, and progressive apraxia of speech. Journal of Speech, Language, and Hearing Research, 60(4), 897–911. https://doi.org/10.1044/2016_JSLHR-S-16-0140 [DOI] [PubMed] [Google Scholar]
- Sixt Börjesson, M. , Hartelius, L. , & Laakso, K. (2020). Communicative participation in people with amyotrophic lateral sclerosis. Folia Phoniatrica et Logopaedica. Advance online publication. https://doi.org/10.1159/000505022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, E. A. , Duffy, J. R. , Clark, H. M. , & Josephs, K. A. (2014). The Apraxia of Speech Rating Scale: A new tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders, 51, 43–50. https://doi.org/10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Boland, S. M. , Machulda, M. M. , Whitwell, J. L. , & Josephs, K. A. (2018). Clinical progression in four cases of primary progressive apraxia of speech. American Journal of Speech-Language Pathology, 27(4), 1303–1318. https://doi.org/10.1044/2018_AJSLP-17-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Botha, H. , Schwarz, C. G. , Machulda, M. M. , Senjem, M. L. , Spychalla, A. J. , Jack, C. R., Jr. , Petersen, R. C. , Lowe, V. J. , Whitwell, J. L. , & Josephs, K. A. (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. https://doi.org/10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski, R. L. , Whitwell, J. L. , Schwarz, C. G. , Senjem, M. L. , Tosakulwong, N. , Duffy, J. R. , Clark, H. M. , Machulda, M. M. , Petersen, R. C. , Jack, C. R., Jr. , Lowe, V. J. , & Josephs, K. A. (2018). Tau-PET imaging with [18F]AV-1451 in primary progressive apraxia of speech. Cortex, 99, 358–374. https://doi.org/10.1016/j.cortex.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell, J. , Duffy, J. R. , Machulda, M. M. , Clark, H. M. , Strand, E. A. , Senjem, M. L. , Gunter, J. L. , Spychalla, A. J. , Petersen, R. C. , Jack, C. R., Jr. , & Josephs, K. A. (2017, May). Tracking the development of agrammatic aphasia: A tensor-based morphometry study. Cortex, 90, 138–148. https://doi.org/10.1016/j.cortex.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell, J. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Gunter, J. L. , Kantarci, K. , Eggers, S. D. , Jack, C. R., Jr. , & Josephs, K. A. (2013). Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. European Journal of Neurology, 20(4), 629–637. https://doi.org/10.1111/ene.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell, J. , Weigand, S. , Duffy, J. , Clark, H. , Strand, E. , Machulda, M. , Spychalla, A. , Senjem, M. , Jack, C. R., Jr. , & Josephs, K. A. (2017). Predicting clinical decline in progressive agrammatic aphasia and apraxia of speech. Neurology, 89(22), 2271–2279. https://doi.org/10.1212/WNL.0000000000004685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston, K. , & Baylor, C. (2019). Patient-reported outcomes measures: An introduction for clinicians. Perspectives of the ASHA Special Interest Groups, 4(1), 8–15. https://doi.org/10.1044/2018_PERS-ST-2018-0001 [Google Scholar]
- Yorkston, K. , Baylor, C. , & Britton, D. (2017). Speech versus speaking: The experiences of people with Parkinson's disease and implications for intervention. American Journal of Speech-Language Pathology, 26(2S), 561–568. https://doi.org/10.1044/2017_AJSLP-16-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston, K. , Baylor, C. , & Mach, H. (2017). Factors associated with communicative participation in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 60(6S), 1791–1797. https://doi.org/10.1044/2017_JSLHR-S-16-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston, K. , Strand, E. , Miller, R. , Hillel, A. , & Smith, K. (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology, 1(1), 35–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.