Abstract
The rate-limiting step for skeletal muscle glucose uptake is transport from microcirculation to muscle interstitium. Capillary endothelium poses a barrier that delays the onset of muscle insulin action. Defining physiological barriers that control insulin access to interstitial space is difficult because of technical challenges that confront study of microscopic events in an integrated physiological system. Two physiological variables determine muscle insulin access. These are the number of perfused capillaries and the permeability of capillary walls to insulin. Disease states associated with capillary rarefaction are closely linked to insulin resistance. Insulin permeability through highly resistant capillary walls of muscle poses a significant barrier to insulin access. Insulin may traverse the endothelium through narrow intercellular junctions or vesicular trafficking across the endothelial cell. Insulin is large compared with intercellular junctions, making this an unlikely route. Transport by endothelial vesicular trafficking is likely the primary route of transit. Studies in vivo show movement of insulin is not insulin receptor dependent. This aligns with single-cell transcriptomics that show the insulin receptor is not expressed in muscle capillaries. Work in cultured endothelial cell lines suggest that insulin receptor activation is necessary for endothelial insulin transit. Controversies remain in the understanding of transendothelial insulin transit to muscle. These controversies closely align with experimental approaches. Control of circulating insulin accessibility to skeletal muscle is an area that remains ripe for discovery. Factors that impede insulin access to muscle may contribute to disease and factors that accelerate access may be of therapeutic value for insulin resistance.
Keywords: insulin, skeletal muscle, endothelial cell, capillary, transport, interstitium
Skeletal muscle comprises by mass the majority of insulin sensitive tissue based on mass. This review, therefore, will be on insulin access to skeletal muscle. Following an oral glucose load ~30% of ingested glucose is taken up by skeletal muscle (1). Muscle glucose uptake is ~85% of total whole body glucose uptake during a hyperinsulinemic-euglycemic clamp (2). Muscle insulin action is important for glucose homeostasis. Obesity-induced insulin resistance is a central defect in the progression of type 2 diabetes. Since obesity-induced skeletal muscle insulin resistance was first described (3), the mechanism by which muscle becomes resistant to insulin has been researched extensively (4). Microvascular insulin delivery to myocytes is rate limiting for the onset of insulin-stimulated muscle glucose uptake (5-10). A full understanding of microvascular regulation of insulin access to myocytes has been difficult because of technical challenges. The microcirculation is removed in model systems such as isolated tissues or cells in culture. This is important because movement of insulin from capillaries to myocyte require the interaction of physiological mechanisms.
In contrast, to the “perfusion-limited” movement of glucose from skeletal muscle capillaries to interstitial space, movement of insulin is primarily “dispersion” limited (11). The implications are that capillary glucose dispersion to the interstitial space is sensitively regulated by capillary blood flow, whereas insulin movement from capillaries to interstitial space is largely independent of capillary blood flow. Insulin dispersion from capillaries to interstitial space is instead determined by the permeability of capillaries to insulin and number of perfused capillaries that provide endothelial surface area for exchange.
Measurement of Insulin Delivery to Myocytes
Insulin delivery to skeletal muscle has been studied in vivo using microdialysis (12-14), lymph sampling (7-9), and an autoradiographic approach (15). These studies have added great insight into the microvascular barrier function of insulin access to muscle and potential impediments in muscle insulin delivery in disease states (9, 14, 16-18). From a mechanistic standpoint, these approaches cannot distinguish between the 2 components of insulin delivery. These are the (1) surface area for capillary insulin exchange and (2) capillary endothelial permeability to insulin. Techniques for interstitial sampling determine an index of insulin delivery that is dependent on both capillary surface area and capillary endothelial permeability. Lee and Klip (19) summarized the technical challenge of defining the mechanisms of insulin delivery: “…egress of insulin from the blood stream has been technically difficult to study, specifically because it has not been possible to study this step in isolation of changes in blood flow.” Recent efforts that will be described in this review have taken on the challenge of distinguishing determinants of insulin delivery to myocytes using sensitive intravital microscopic techniques (11, 20-22).
Muscle Capillary Surface Area Determines Insulin Movement to Interstitium
Movement of solutes from capillary blood to interstitial space may be dependent on the capillary surface area for exchange as well as blood flow velocity. The relative importance of capillary surface area and blood flow is largely dependent on the diameter of the solute. Insulin was first demonstrated to increase muscle blood flow in dogs (23) and subsequently in humans (24). This led to the hypothesis that vascular effects of insulin enhance its delivery to myocytes. Insulin movement from capillary lumen to interstitial space is not flow dependent, however. Instead, the rate of insulin delivery to muscle is dependent on the capillary surface area for exchange. The insulin-induced increase in blood flow is effective at increasing the movement of glucose, oxygen, and soluble nutrients from capillary to interstitial space. Various nutrients and oxygen are necessary for aspects of insulin action.
In recent years, contrast-enhanced ultrasound (CEU) has been a valuable tool to assess microvascular hemodynamics (25). CEU measures the intensity of sound waves reflected from 1 to 5 µm microbubbles injected IV. The application of CEU has been overextended as a means of measuring capillary recruitment (25-28). CEU is unable to measure recruitment of capillaries for reasons that have been discussed in detail extensively (11, 20, 29). Because CEU is so often used in humans and rodents, we will briefly summarize why we believe results using this technology have been misinterpreted. Results from CEU experiments conclude that < 50% of capillaries are perfused at rest. The weakness of this approach is that the number of microbubbles that can be injected populate only a small percentage of total capillaries. Moreover, microbubbles, as particulates, do not distribute evenly but rather are inclined to enter the capillaries that offer the least resistance (eg, central capillaries). Theoretical constructs support empirical data that show results obtained using CEU are not related to capillary recruitment (20, 30, 31). Direct capillary visualization in a variety of anesthetized rodent preparations conducted over the past 30 years show that > 90% of capillaries are perfused at rest (11, 32-38). A corollary to this is that there is little margin for capillary recruitment by insulin or any other vasoactive compound.
Surface area for capillary insulin movement to the interstitial space is an important determinant of insulin accessibility to myocytes. Diseases resulting in capillary rarefaction or conditions that provoke angiogenesis will predictably decrease and increase insulin access to myocytes, respectively. Indeed, skeletal muscle capillary density is closely correlated to skeletal muscle insulin sensitivity (39-41).
Genetic mouse models have been used to assess the dependence of endothelial function on insulin action. Kahn and colleagues generated 2 endothelial insulin receptor knockout mice created by breeding mice with insulin receptor lox-p flanked genes bred with Cre recombinase expressing mice driven by the Tie-2 or VE-cadherin promoters (42, 43). Endothelial insulin receptor knockout mice created using the Tie-2 Cre recombinase endothelial receptor had no effect on glucose homeostasis (42), whereas in the VE-cadherin-driven knockout mice insulin signaling was delayed (43). The investigators postulate that the divergent results are due to greater VE-cadherin-Cre expression in endothelial cells and the higher specificity of the VE-cadherin transgene compared with the Tie2-Cre (43). Another possible contributor to these phenotypic differences may be differences in genetic background. Endothelial insulin receptor knockout mice on the VE-cadherin-Cre recombinase mice were on the more insulin-resistant susceptible C57Bl6 background, whereas those bred to Tie-2 mice were on a hybrid background (42). The investigators conclude that the more complete and specific endothelial insulin receptor knockout mice in VE-cadherin-driven Cre mice on the C57Bl6 background reveals evidence of a role for endothelial insulin receptors in glucoregulation. Studies of mice lacking the endothelial insulin receptor substrate 2 supported this conclusion by demonstrating a role for endothelial cell insulin signaling. Endothelial insulin receptor substrate 2 knockout mice were characterized by a reduction in interstitial insulin during a hyperinsulinemic-euglycemic clamp (12), suggesting that insulin signaling maybe required for the number of perfused capillaries, endothelial insulin transport, cardiac function, or broad circulatory events that impair insulin delivery.
Trans-endothelial Insulin Transport in Skeletal Muscle Capillaries
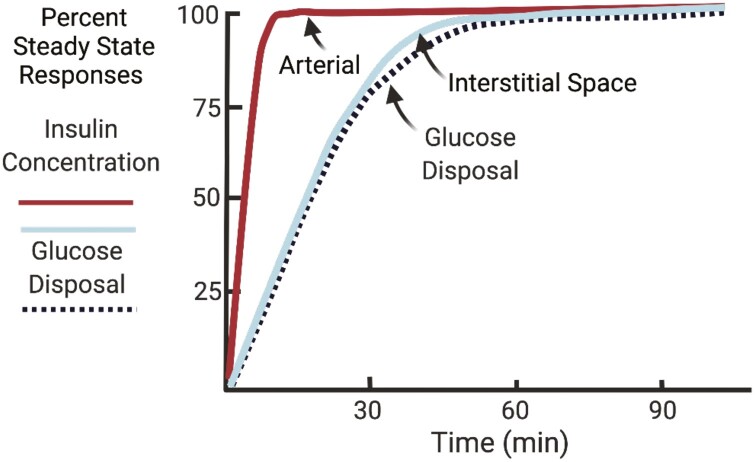
The hypothesis that endothelial insulin transport limits the onset of peripheral insulin action was first proposed by Andres and colleagues (10). They observed that plasma insulin levels peaked almost instantaneously with an insulin infusion, whereas it took considerably longer for glucose disposal to reach its maximum. These findings suggested the existence of a barrier between circulating insulin and insulin action. Using compartmental modeling, an interstitial compartment that equilibrates slowly with plasma insulin and was critical for insulin action was proposed (Fig. 1). They hypothesized that lower permeability of some vascular beds may impede insulin delivery to this interstitial compartment (10). Insulin appears rapidly in the hepatic lymph, but slowly in the lymph draining the limb following IV glucose load in the dog (44). This was consistent with a marked gradient between plasma and thoracic duct insulin following an IV glucose bolus in the dog (8). Muscle interstitial insulin concentrations measured using microdialysis in humans show that levels of insulin in the plasma are roughly 2-fold higher than in the muscle interstitial space (13, 14). The dynamics of interstitial insulin appearance is closely associated with insulin-stimulated muscle insulin receptor kinase activity (45) and glucose disposal (7, 8). The importance of the endothelial insulin barrier was tested by bypassing the endothelial barrier by insulin injection directly into the muscle interstitial space (5). Direct IM insulin injection stimulated maximal glucose uptake almost immediately. This provided direct support for the hypothesis that the rate-limiting step for muscle insulin action is trans-endothelial insulin transport.
Figure 1.
Arterial insulin concentrations reach a steady state rapidly after the onset of an hyperinsulinemic-euglycemic clamp, whereas interstitial insulin concentrations have delayed kinetics that are closely coupled to interstitial insulin. Data are expressed as percent of steady-state responses.
Capillary Endothelial Barrier
Capillary networks provide a vast surface area for fluid and solute exchange with the underlying tissue parenchyma. The endothelium lining capillaries forms a semipermeable barrier that provides a level of control of trans-capillary exchange. There are 3 main types of capillary endothelia: continuous, fenestrated, and sinusoidal (46). Sinusoidal endothelia are characterized by large distances between endothelial cells permitting free exchange of circulating blood cells and plasma constituents with the tissue parenchyma (eg, liver sinusoids). Fenestrated endothelia, as found in the liver and kidney, contain pores that permit exchange of large plasma proteins. Continuous endothelia form high resistance barriers in the muscle, adipose tissue, and brain. Endothelial cells are adjoined by adherens junctions in adipose and muscle. The capillary inter-endothelial junctions in these tissues range from ~2 to 5 nm. The characteristics of continuous capillary endothelial junctions are the basis of the high resistance. Given that the radius of insulin is ~1.34 nm, it is feasible that insulin may access inter-endothelial junctions. Several factors predict this would not be a favorable passage. Insulin can aggregate as a dimer or hexamer with a much larger radius. The layer of negatively charged glycocalyx that extrudes from the luminal endothelial surface, may hinder insulin’s access to the inter-endothelial junction. Furthermore, because insulin is negatively charged at physiological pH, the glycocalyx may impede trans-endothelial insulin movement through charge selectivity. The muscle capillary endothelium presents a formidable barrier to trans-endothelial insulin transport. Insulin may to some extent be able to access inter-endothelial junctions. However, the underlying structural and chemical interactions will create biophysical resistance and size restriction for insulin transit.
The movement of insulin from capillaries to interstitial space is thought to be primarily by transport across the endothelial cells. Electron microscopy shows that the very thin endothelium of muscle capillaries contains vesicles that span roughly 70 nm in diameter (47). These vesicles have been shown to be caveolae (48, 49), but clathrin-coated pits have also been implicated in the insulin transport process (50). These vesicles contribute to molecular exchange by either (1) fusing to form transient trans-endothelial channels or pores which allow for passive movement of plasma constituents to the interstitial space (51, 52) or (2) transport of macromolecules through transcytosis (53). Transcytosis begins with endocytosis of vesicles that are fused with the luminal endothelial plasma membrane. Clathrin vesicles are internalized through a mechanism that involves dynamin-mediated scission (54). Subsequently, the free vesicle is translocated across endothelial microtubules with assistance from motor proteins such as kinesin and dynein (54). Finally, the free vesicle uses soluble N-ethylmaleimide-sensitive factor attachment protein receptor machinery to dock with the abluminal plasma membrane and release its contents into the interstitial space (54).
Insulin Transport Across the Skeletal Muscle Capillary
There has been careful examination in recent years of the molecular mechanisms of trans-endothelial insulin transport. Insulin has been postulated to be actively transported across the endothelium by saturable insulin receptor-mediated transcytosis, whereas others have shown that insulin leaves the capillary lumen and crosses the endothelium by insulin receptor-independent nonsaturable mechanisms. The notion that insulin may be trafficked across the endothelium by a receptor-mediated process began with the demonstration that cultured human umbilical vein endothelial cells contain insulin receptors (15). The expression of insulin receptors in various cultured endothelial cell lines has been demonstrated (55, 56). Antibodies against the insulin receptor inhibited the movement of 125I-insulin across a monolayer of bovine retinal capillary endothelial cells in vitro (57). This suggested that insulin was trafficked across this cultured endothelial cell line by an insulin receptor-mediated process. Studies using immunohistochemistry reported that fluorescein isothiocyanate-labeled insulin can be detected in the endothelium of arterioles and venules, as well as capillaries (55).
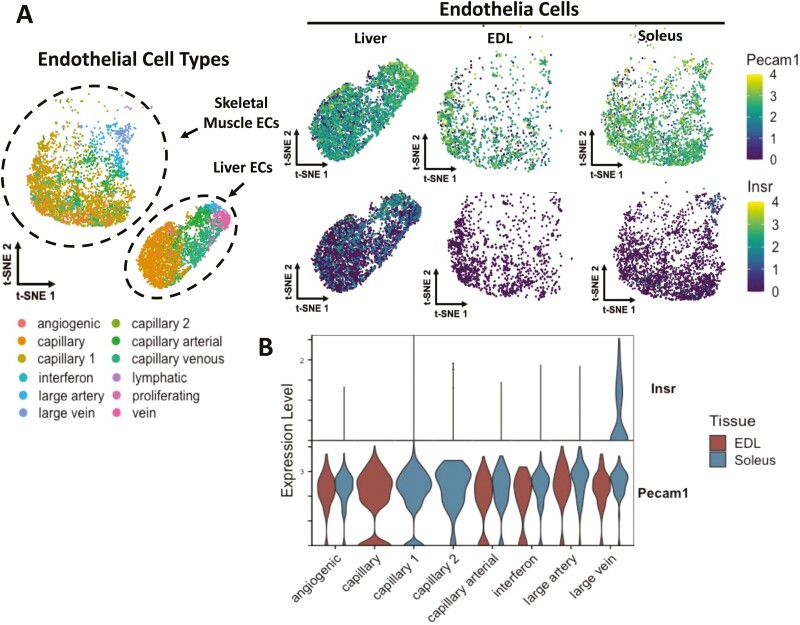
Studies conducted in the whole organism differ from those in endothelial cell lines. A site-specific conjugate of Alexa Fluor 647 to human insulin (Ins-647) that is fully bioactive was developed to study insulin transport in vivo (22). Ins-647 was administered to anesthetized mice intravenously and imaged using intravital laser scanning microscopy. There was no Ins-647 fluorescence in the capillary endothelium even at very high insulin doses. However, Ins-647 fluorescence in the endothelium of venules was extremely high (21). The presence of insulin accumulation in venule walls but not in capillary walls is consistent with the single-cell transcriptomic data that show that the insulin receptor is expressed in veins and not the capillaries of the muscle microcirculation (58). These studies emphasize the need to clearly differentiate endothelial cell populations within the microcirculation. The insulin receptor has been shown to colocalize with caveolin-1, the main structural component of caveolae in endothelial cells (49). Whether the insulin receptor colocalizes with capillary endothelial caveolae or clathrin-coated pits, however, is highly unlikely in vivo. Two independent single-cell transcriptomic studies (58, 59) generated open access datasets that both show that the insulin receptor is not expressed in skeletal muscle capillaries (Fig. 2 and Williams et al (21).). Figure 2A and 2B shows Insr and Pecam1 gene expression in the skeletal muscle microvasculature from the data generated by Kalucka and colleagues (https://endotheliomics.shinyapps.io/ec_atlas/). Although insulin receptor transcript is not present in skeletal muscle capillary endothelial cells, insulin transport may still be mediated by a distinct receptor that has yet to be defined as previously proposed by Rhea and colleagues for transit across the blood–brain barrier (60).
Figure 2.
Single-cell transcriptomics of endothelial cells in liver, extensor digitorum longus, and soleus for vessels of the microcirculation. Pecam1 was used as an endothelial cell (EC) marker. The insulin receptor (Insr) was expressed in liver endothelial cells but in neither the extensor digitorum longus (EDL) nor the soleus muscles (A). Violin plots for single cell transcripts of Insr and Pecam1 show that the insulin receptor is expressed in only the large venules of the microcirculation (B). Figure courtesy of Matthew A. Cottam, Vanderbilt University.
The insulin receptor independence of capillary endothelial insulin transport in vivo has been confirmed by functional measurements in the dog and mouse in vivo. To test whether trans-endothelial insulin transport is saturable, Steil et al (61) infused physiological and pharmacological doses of insulin in dogs and measured its appearance in the lymph. The results of this study showed the appearance of insulin was not saturable even at very high doses of insulin. These findings indicated that muscle insulin delivery was not saturable in vivo, as would be expected for a receptor-mediated process.
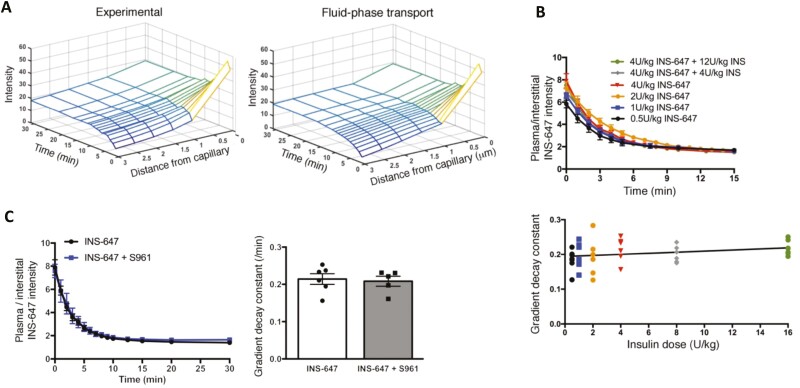
Studies by Williams et al (22) derived at a similar conclusion and expanded previous findings using intravital laser scanning microscopy and Ins-647 in anesthetized mice. The technique used permitted direct measurement of the kinetics of insulin movement in vivo from individual capillaries to perivascular interstitial space in real time. The approach was a breakthrough in that it allowed for measurement of plasma, perivascular interstitial, and total muscle interstitial insulin to be measured as a function of time and by doing so was able to distinguish between perfused capillary surface area and capillary permeability. This technique has high sensitivity for measurement of Ins-647, sufficient spatial resolution to differentiate between plasma and interstitial space, and high temporal resolution to track rapid insulin dynamics. Experiments using this technique show in vivo that data best fit the insulin-independent fluid phase transport model where movement of insulin from the capillaries is determined by Starling forces (balance between oncotic and hydrostatic pressures) and the permeability of the cell wall to insulin (Fig. 3A). Capillary trans-endothelial insulin transport is unsaturable over a 32-fold range of concentrations and the capillary Ins-647 to perivascular interstitial Ins-647 gradient decay constant (Ke), a kinetic measure of insulin movement across the endothelium, is unchanged (Fig. 3B). Detection limits of fluorescence microscopy and the relatively low levels of circulating insulin required that even the lowest Ins-647 dose used in these studies was supraphysiological. It has been proposed that a saturable endothelial insulin transport system that operates at physiological insulin concentrations and a nonsaturable transport process that exists at supraphysiological insulin concentrations could exist (56). Studies requiring high insulin does may obscure saturable kinetics that may be critical at physiologic levels. Diffusion is a nonsaturable process that could be increased if elevated insulin concentrations or resulting conditions regulate the adherens junctions leading to expansion of intercellular channels. On the surface, this concept is not supported by the data of Williams et al (22). If different transport processes were in play, Ke would be expected to change as insulin concentrations increase from physiological to supraphysiological concentrations. However, the constant Ke suggests the existence of a single capillary insulin transport process. The insulin receptor independence of the endothelial insulin transport process was validated using S961, a peptide insulin receptor antagonist that has nearly the same affinity for the insulin receptor (62). This insulin receptor antagonist does not affect the kinetics with which Ins-647 crosses the capillary endothelium (22) (Fig. 3C). Consistent with studies in muscle capillary endothelium, insulin receptor antagonism with S961 also does not impede capillary endothelial insulin transport at the blood–brain barrier in vivo (60).
Figure 3.
Experimental data for capillary insulin efflux into the interstitial space best fits the model for fluid phase transport across the capillary endothelium (A). Insulin movement across the capillary endothelium is unsaturable. Ins-647 and insulin have equal bioactivity and the total insulin dose is the sum of both. Kinetics for the gradient decay constant across for insulin movement from capillary to interstitial space is constant over a range (0.5 to 16 U/kg body weight) of insulin doses (B). Kinetics for the insulin movement from the capillary to interstitial space is unaffected by the insulin receptor blocker, S961 (C).
The controversy as to whether endothelial transport is dependent on the insulin receptor can be most clearly divided based on whether studies were conducted in the whole organism or in cultured cell lines. Studies conducted in the whole organism show that insulin delivery (22, 61) and direct transport of insulin across the capillary endothelium (22) are nonsaturable. Moreover, insulin receptor antagonism does not impede capillary endothelial insulin transport in vivo (22). These results are strongly supported by single-cell transcriptomic studies performed on cells isolated from mice (58, 59) that demonstrate that capillary endothelial cells do not express the insulin receptor (Fig. 2A and 2B).
On the other hand, studies performed in cultured endothelial cells generally show a reliance on the insulin receptor and insulin action (19, 63, 64). Although much has been learned from these studies, the use of endothelial cells in culture is not without major limitations. Macrovascular endothelial cells are often used (15, 49). Given the heterogeneity between endothelial cells at different levels of the vasculature (65, 66), drawing conclusions on capillaries in vivo from cultured macrovascular endothelial cells is problematic. Studies have also used “microvascular” endothelial cells (48, 50, 57). The microvasculature includes arterioles, venules, and capillaries, all of which have different properties (66). Microvascular endothelial cells in total do not strictly represent capillaries. Moreover, the organ from which endothelial cells are isolated is a critical factor. Studies of trans-endothelial insulin transport have used endothelial cells from retina (57), aorta (49, 55), adipose tissue (50), or lung (48). Endothelial cells in different tissue beds can have different properties (65). The extent to which endothelial insulin transport in other organs is representative of muscle is unresolved. Cultured endothelial cells can be problematic for other reasons. Immediately upon isolation, the endothelial proteome changes dramatically. A comparison of the proteome of freshly cultured endothelial cells and endothelial membranes isolated in vivo show 40% of proteins expressed in vitro are not expressed in vivo (67). Especially relevant is that endothelial monolayers can be up to 100-fold more permeable to proteins than intact vessels (68). Finally, endothelial cells in static culture are absent the in vivo environment. Endothelial cells in vivo are in close contact, attached to a complex basement membrane, exposed to shear stress from capillary plasma and red blood cells, and receive a myriad of biochemical signals from both the circulation and underlying tissue parenchyma. The environment of endothelial cells in vivo is not simple to replicate in vitro.
Trans-endothelial Insulin Transport in the Pathogenesis of Insulin Resistance
Given that obese and insulin-resistant subjects display endothelial dysfunction, it is reasonable to hypothesize that they also have impaired muscle insulin delivery. Indeed, several investigators have demonstrated that the delivery of insulin to the muscle interstitial space is reduced in humans and animal models of insulin resistance. The appearance of insulin in the interstitial space assessed using microdialysis during a hyperinsulinemic-euglycemic clamp (69) or following oral glucose ingestion (70) is delayed in obese, insulin-resistant humans. During hyperinsulinemic-euglycemic clamps in a cohort of obese humans, the gradient of arterial to lymph insulin gradient was increased relative to nonobese subjects, suggesting an impairment in the capillary insulin delivery (71). Despite the increased arterial to lymph insulin gradient, a prevailing hyperinsulinemia in the obese humans compensated for the impediment and showed that insulin resistance at the myocyte itself was the predominant defect. These findings were supported by experiments using animal models of obesity and insulin resistance (12, 16). Acute induction of insulin resistance due to lipid infusion in lean human subjects (72) and dogs (73) do not appear to impair the access of insulin to skeletal muscle.
Impairments in skeletal muscle insulin delivery in insulin-resistant and diabetic states may indeed contribute to decreased muscle capillary surface area, reduced endothelial cell insulin transit, or both. A study using intravital microscopy measured the movement of bioactive fluorescent Ins-647 across the endothelium of skeletal muscle capillaries in anesthetized mice that were resistant to insulin by a high-fat diet or in insulin-sensitive lean mice (21). These studies were the first to directly show that skeletal muscle trans-endothelial insulin transport is impaired in a model of insulin resistance. Transmission electron microscopy showed that capillary endothelial cell vesicles were fewer and smaller in obese, insulin-resistant mice. These findings demonstrate that capillary endothelium may pose a greater barrier to insulin-resistant skeletal muscle. Treatments that increase trans-endothelial insulin transport may be useful in enhancing the delivery of insulin to skeletal muscle for the treatment of insulin resistance or in generating fast-acting insulin analogs for diabetics. Studies of microcirculation make it clear, however, that defects in muscle insulin delivery alone are not sufficient to explain the entirety of muscle insulin resistance (21, 71). The well-established insulin resistance at the myocyte itself is likely to be the predominant defect in most cases.
Endothelial dysfunction is common in obesity and insulin-resistant states. This is evident from the impaired endothelial-dependent insulin stimulation of total limb blood flow in obese, insulin-resistant, and diabetic humans (74, 75). Endothelial dysfunction is characterized by reduced endothelial nitric oxide (NO) production due to a variety of factors including reduced endothelial NO synthase (eNOS) expression and activity, eNOS uncoupling, and enhanced scavenging of NO by oxidant species such as superoxide (76). In murine models of obesity, aortic NO production was shown to be reduced before muscle insulin resistance, suggesting that endothelial dysfunction may play a causative role in the development of muscle insulin (77, 78). An interesting finding using CEU in monkeys showed that as NO bioavailability declines during the development of obesity-induced insulin resistance, the primary muscle vasodilatory mechanisms switch to eicosanoid-based vasodilation (79). This study suggests that obesity results in alterations to the endothelial production of and response to vasoactive compounds. Importantly, insulin-stimulated microvascular perfusion is impaired in obese, insulin-resistant humans, and animal models (80, 81). At a structural level, muscle capillary density has been shown to be lower in obese and insulin-resistant humans (39, 82). Obesity, insulin resistance, and diabetes are strongly associated with both structural and hemodynamic vascular dysfunction. These vascular defects may contribute to muscle insulin resistance insofar as they decrease the capillary surface areas for exchange with myocytes.
Studies conducted in rodents have shown that pharmacological inhibition or genetic deletion of NO synthase increases microvascular permeability (83-86). Pharmacological blockade of eNOS, but not neuronal nitric oxide synthase increased capillary permeability using intravital microscopy in combination with the insulin fluorescent probe, Ins-647 (85). Studies conducted in rodent models were in direct contrast with results in a bovine aortic endothelial cell line that showed NO promotes the uptake of fluorescein isothiocyanate-labeled insulin (87). This study should be interpreted with caution given the various limitations of studying endothelial transport in cell lines discussed previously. Moreover, the internalization of insulin in cultured cells may reflect endocytotic-exocytotic receptor recycling rather than insulin transport. The consensus of in vivo studies is that decreased NO increases capillary permeability, including specific muscle capillary insulin permeability. Thus, although impaired eNOS at the arterioles may contribute to insulin resistance by effects on microvascular hemodynamics, it would not appear to contribute to insulin resistance at the capillary level.
Summary
The rate-limiting step for skeletal muscle glucose uptake in vivo is the transport from the microcirculation to the interstitial space of the muscle. This is evident from the close association between interstitial insulin and insulin action. A small solute like glucose equilibrates with the interstitial space in seconds and is limited by capillary blood flow (11). The skeletal muscle capillary wall is high resistance. A protein such as insulin is limited by the dispersion rate from the capillary. Dispersion rate is determined by capillary surface area for exchange and the permeability of the capillary endothelial wall to insulin. Defining the importance of these physiological barriers that control insulin access to the interstitial space has been difficult because of technical challenges that confront the study of microscopic events in an integrated physiological system. Controversy exists in the mechanisms that control capillary trans-endothelial insulin movement. The controversies closely align with experimental approaches that may lead to discrepant conclusions as to how insulin moves from the capillary to interstitial space. The use of indirect approaches to study the microcirculation or cell lines to study endothelial insulin transit has resulted in different results from studies that rely on direct visualization conducted in the whole organism. Capillary surface area for insulin movement into skeletal muscle interstitium is decreased in some disease states, but its control by circulating insulin is controversial. The conclusions from research designed to understand muscle capillary endothelial insulin permeability are highly dependent on methodology. Studies in vivo show that movement of insulin is not mediated by the insulin receptor. This would seem indisputable because single-cell transcriptomic data show that the insulin receptor is not expressed in skeletal muscle capillaries. However, a considerable body of work has been performed in cultured endothelial cell lines showing that insulin receptor activation can lead to the formation of transport vesicles. Control of circulating insulin accessibility to skeletal muscle is an area that remains ripe for discovery. Skeletal muscles are by mass the bulk of insulin sensitive tissues. Any factor that impedes insulin access to this tissue is a potential contributor to disease.
Glossary
Abbreviations
- CEU
contrast-enhanced ultrasound
- eNOS
endothelial nitric oxide synthase
- IM
intramuscular
- Ins-647
conjugate of Alexa Fluor 647 to human insulin
- IV
intravenous
- Ke
gradient decay constant
- NO
nitric oxide
Funding
This work was supported by National Institutes of Health grants R01 DK050277 and R01 DK054902, American Heart Association SFRN on Peripheral Artery Disease, U24 DK059637, and P30 DK020593. The authors are grateful to Matthew A. Cottam for the data analysis illustrated in Fig. 2A and 2B.
Disclosures
The authors have nothing to disclose.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated in this review.
References
- 1. Kelley D, Mitrakou A, Marsh H, et al. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Invest. 1988;81(5):1563-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000-1007. [DOI] [PubMed] [Google Scholar]
- 3. Rabinowitz D, Zierler KL. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962;41:2173-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S157-S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu JD, Richey JM, Harrison LN, et al. Direct administration of insulin into skeletal muscle reveals that the transport of insulin across the capillary endothelium limits the time course of insulin to activate glucose disposal. Diabetes. 2008;57(4):828-835. [DOI] [PubMed] [Google Scholar]
- 6. Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol. 2011;214(Pt 2):254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84(5):1620-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang YJ, Hope ID, Ader M, Bergman RN. Importance of transcapillary insulin transport to dynamics of insulin action after intravenous glucose. Am J Physiol. 1994;266(1 Pt 1):E17-E25. [DOI] [PubMed] [Google Scholar]
- 9. Kolka CM, Bergman RN. The barrier within: endothelial transport of hormones. Physiology (Bethesda). 2012;27(4):237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sherwin RS, Kramer KJ, Tobin JD, et al. A model of the kinetics of insulin in man. J Clin Invest. 1974;53(5):1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McClatchey PM, Williams IM, Xu Z, et al. Perfusion controls muscle glucose uptake by altering the rate of glucose dispersion in vivo. Am J Physiol Endocrinol Metab. 2019;317(6):E1022-E1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13(3):294-307. [DOI] [PubMed] [Google Scholar]
- 13. Sjöstrand M, Holmäng A, Lönnroth P. Measurement of interstitial insulin in human muscle. Am J Physiol. 1999;276(1):E151-E154. [DOI] [PubMed] [Google Scholar]
- 14. Sjöstrand M, Holmäng A, Strindberg L, Lönnroth P. Estimations of muscle interstitial insulin, glucose, and lactate in type 2 diabetic subjects. Am J Physiol Endocrinol Metab. 2000;279(5):E1097-E1103. [DOI] [PubMed] [Google Scholar]
- 15. Bar RS, Hoak JC, Peacock ML. Insulin receptors in human endothelial cells: identification and characterization. J Clin Endocrinol Metab. 1978;47(3):699-702. [DOI] [PubMed] [Google Scholar]
- 16. Broussard JL, Castro AV, Iyer M, et al. Insulin access to skeletal muscle is impaired during the early stages of diet-induced obesity. Obesity (Silver Spring). 2016;24(9):1922-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kolka CM, Bergman RN. The endothelium in diabetes: its role in insulin access and diabetic complications. Rev Endocr Metab Disord. 2013;14(1):13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolka CM, Harrison LN, Lottati M, Chiu JD, Kirkman EL, Bergman RN. Diet-induced obesity prevents interstitial dispersion of insulin in skeletal muscle. Diabetes. 2010;59(3):619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee WL, Klip A. Endothelial transcytosis of insulin: does it contribute to insulin resistance? Physiology (Bethesda). 2016;31(5):336-345. [DOI] [PubMed] [Google Scholar]
- 20. Akerstrom T, Goldman D, Nilsson F, et al. Hyperinsulinemia does not cause de novo capillary recruitment in rat skeletal muscle. Microcirculation. 2020;27(2):e12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams IM, McClatchey PM, Bracy DP, Bonner JS, Valenzuela FA, Wasserman DH. Transendothelial insulin transport is impaired in skeletal muscle capillaries of obese male mice. Obesity (Silver Spring). 2020;28(2):303-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams IM, Valenzuela FA, Kahl SD, et al. Insulin exits skeletal muscle capillaries by fluid-phase transport. J Clin Invest. 2018;128(2):699-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang C, Doherty JU, Faillace R, et al. Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest. 1982;69(6):1321-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267(2 Pt 1):E187-E202. [DOI] [PubMed] [Google Scholar]
- 25. Barrett EJ, Rattigan S. Muscle perfusion: its measurement and role in metabolic regulation. Diabetes. 2012;61(11):2661-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keske MA, Barrett EJ, Lindner JR, et al. Perfusion controls muscle glucose uptake by altering the rate of glucose dispersion in vivo. Am J Physiol Endocrinol Metab. 2020;318(3):E311-E312. [DOI] [PubMed] [Google Scholar]
- 27. Coggins M, Lindner J, Rattigan S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50(12):2682-2690. [DOI] [PubMed] [Google Scholar]
- 28. Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53(6):1418-1423. [DOI] [PubMed] [Google Scholar]
- 29. McClatchey PM, Williams IM, Xu Z, et al. Reply to Letter to the editor: perfusion controls muscle glucose uptake by altering the rate of glucose dispersion in vivo. Am J Physiol Endocrinol Metab. 2020;318(3):E313-E317. [DOI] [PubMed] [Google Scholar]
- 30. McClatchey PM, Frisbee JC, Reusch JEB. A conceptual framework for predicting and addressing the consequences of disease-related microvascular dysfunction. Microcirculation. 2017;24(6):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McClatchey PM, Schafer M, Hunter KS, Reusch JE. The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am J Physiol Heart Circ Physiol. 2016;311(1):H168-H176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cabrales P, Vázquez BY, Tsai AG, Intaglietta M. Microvascular and capillary perfusion following glycocalyx degradation. J Appl Physiol (1985). 2007;102(6):2251-2259. [DOI] [PubMed] [Google Scholar]
- 33. Kindig CA, Poole DC. A comparison of the microcirculation in the rat spinotrapezius and diaphragm muscles. Microvasc Res. 1998;55(3):249-259. [DOI] [PubMed] [Google Scholar]
- 34. Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol (1985). 2002;92(6):2513-2520. [DOI] [PubMed] [Google Scholar]
- 35. Padilla DJ, McDonough P, Behnke BJ, et al. Effects of type II diabetes on muscle microvascular oxygen pressures. Respir Physiol Neurobiol. 2007;156(2):187-195. [DOI] [PubMed] [Google Scholar]
- 36. Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol. 2013;98(12):1645-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol (1985). 2003;95(3):1055-1062. [DOI] [PubMed] [Google Scholar]
- 38. Delashaw JB, Duling BR. A study of the functional elements regulating capillary perfusion in striated muscle. Microvasc Res. 1988;36(2):162-171. [DOI] [PubMed] [Google Scholar]
- 39. Lillioja S, Young AA, Culter CL, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80(2):415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prior SJ, Goldberg AP, Ortmeyer HK, et al. Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes. 2015;64(10):3386-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes. 2013;62(2):572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vicent D, Ilany J, Kondo T, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111(9):1373-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Konishi M, Sakaguchi M, Lockhart SM, et al. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proc Natl Acad Sci U S A. 2017;114(40):E8478-E8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rasio EA, Mack E, Egdahl RH, Herrera MG. Passage of insulin and inulin across vascular membranes in the dog. Diabetes. 1968;17(11):668-672. [DOI] [PubMed] [Google Scholar]
- 45. Miles PD, Levisetti M, Reichart D, Khoursheed M, Moossa AR, Olefsky JM. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes. 1995;44(8):947-953. [DOI] [PubMed] [Google Scholar]
- 46. Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruns RR, Palade GE. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968;37(2):244-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127(5):1217-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang H, Wang AX, Barrett EJ. Caveolin-1 is required for vascular endothelial insulin uptake. Am J Physiol Endocrinol Metab. 2011;300(1):E134-E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Azizi PM, Zyla RE, Guan S, et al. Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells. Mol Biol Cell. 2015;26(4):740-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simionescu N. Cellular aspects of transcapillary exchange. Physiol Rev. 1983;63(4):1536-1579. [DOI] [PubMed] [Google Scholar]
- 52. Clough G, Michel CC. The role of vesicles in the transport of ferritin through frog endothelium. J Physiol. 1981;315:127-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simionescu M, Simionescu N. Endothelial transport of macromolecules: transcytosis and endocytosis. A look from cell biology. Cell Biol Rev. 1991;25(1):1-78. [PubMed] [Google Scholar]
- 54. Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2007;293(4):L823-L842. [DOI] [PubMed] [Google Scholar]
- 55. Wang H, Liu Z, Li G, Barrett EJ. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291(2):E323-E332. [DOI] [PubMed] [Google Scholar]
- 56. Jaldin-Fincati JR, Pereira RVS, Bilan PJ, Klip A. Insulin uptake and action in microvascular endothelial cells of lymphatic and blood origin. Am J Physiol Endocrinol Metab. 2018;315(2): E204-E217. [DOI] [PubMed] [Google Scholar]
- 57. King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science. 1985;227(4694):1583-1586. [DOI] [PubMed] [Google Scholar]
- 58. Kalucka J, de Rooij LPMH, Goveia J, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180(4):764-779.e20. [DOI] [PubMed] [Google Scholar]
- 59. Tabula Muris Consortium; Overall coordination; Logistical coordination; Organ collection and processing; Library preparation and sequencing; Computational data analysis; Cell type annotation; Writing group; Supplemental text writing group; Principal investigators. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562(7727):367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rhea EM, Rask-Madsen C, Banks WA. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol. 2018;596(19):4753-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest. 1996;97(6):1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schäffer L, Brand CL, Hansen BF, et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun. 2008;376(2):380-383. [DOI] [PubMed] [Google Scholar]
- 63. Yazdani S, Jaldin-Fincati JR, Pereira RVS, Klip A. Endothelial cell barriers: transport of molecules between blood and tissues. Traffic. 2019;20(6):390-403. [DOI] [PubMed] [Google Scholar]
- 64. Kusters YH, Barrett EJ. Muscle microvasculature’s structural and functional specializations facilitate muscle metabolism. Am J Physiol Endocrinol Metab. 2016;310(6):E379-E387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174-190. [DOI] [PubMed] [Google Scholar]
- 66. Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158-173. [DOI] [PubMed] [Google Scholar]
- 67. Durr E, Yu J, Krasinska KM, et al. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol. 2004;22(8):985-992. [DOI] [PubMed] [Google Scholar]
- 68. Albelda SM, Sampson PM, Haselton FR, et al. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol (1985). 1988;64(1):308-322. [DOI] [PubMed] [Google Scholar]
- 69. Sjöstrand M, Gudbjörnsdottir S, Holmäng A, Lönn L, Strindberg L, Lönnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002;51(9):2742-2748. [DOI] [PubMed] [Google Scholar]
- 70. Sandqvist M, Strindberg L, Schmelz M, Lönnroth P, Jansson PA. Impaired delivery of insulin to adipose tissue and skeletal muscle in obese women with postprandial hyperglycemia. J Clin Endocrinol Metab. 2011;96(8):E1320-E1324. [DOI] [PubMed] [Google Scholar]
- 71. Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93(1):10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Szendroedi J, Frossard M, Klein N, et al. Lipid-induced insulin resistance is not mediated by impaired transcapillary transport of insulin and glucose in humans. Diabetes. 2012;61(12):3176-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kolka CM, Richey JM, Castro AV, Broussard JL, Ionut V, Bergman RN. Lipid-induced insulin resistance does not impair insulin access to skeletal muscle. Am J Physiol Endocrinol Metab. 2015;308(11):E1001-E1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab. 1991;73(3):637-643. [DOI] [PubMed] [Google Scholar]
- 75. Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41(9):1076-1083. [DOI] [PubMed] [Google Scholar]
- 76. Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med. 2014;73:383-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim F, Pham M, Maloney E, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28(11):1982-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhao L, Fu Z, Wu J, et al. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin Sci (Lond). 2015;129(12):1025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chadderdon SM, Belcik JT, Bader L, Kievit P, Grove KL, Lindner JR. Vasoconstrictor eicosanoids and impaired microvascular function in inactive and insulin-resistant primates. Int J Obes (Lond). 2016;40(10):1600-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. 2008;295(4):E732-E750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55(5):1436-1442. [DOI] [PubMed] [Google Scholar]
- 82. Gavin TP, Stallings HW 3rd, Zwetsloot KA, et al. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol (1985). 2005;98(1):315-321. [DOI] [PubMed] [Google Scholar]
- 83. Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289(3):L371-L381. [DOI] [PubMed] [Google Scholar]
- 84. Kurose I, Kubes P, Wolf R, et al. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73(1):164-171. [DOI] [PubMed] [Google Scholar]
- 85. Williams IM, McClatchey PM, Bracy DP, Valenzuela FA, Wasserman DH. Acute nitric oxide synthase inhibition accelerates transendothelial insulin efflux in vivo. Diabetes. 2018;67(10):1962-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262(2 Pt 2):H611-H615. [DOI] [PubMed] [Google Scholar]
- 87. Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 2013;62(12):4030-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated in this review.