Abstract
Gonadotropin-releasing hormone analogues (GnRHas) are an effective treatment to address the compromise in height potential seen in patients with central precocious puberty. There is no evidence in the literature of a single GnRHa used for longer than 2 years before being removed or replaced. We describe a patient who was on continuous gonadotropin suppression for 7 years and despite this, achieved a height potential within 1 SD of mid-parental height. A boy aged 10 years 3 months presented to the endocrine clinic with signs of precocious puberty and advanced bone age. Initial laboratory values were a random luteinizing hormone (LH) level of 9.4 mIU/mL, follicle-stimulating hormone (FSH) 16.3 mIU/mL, dehydroepiandrosterone sulfate 127 mcg/dL, and testosterone 628 ng/dL. The patient was initially started on Lupron injections before transitioning to a histrelin implant. Follow-up laboratory results 5 months post-suppression showed pre-pubertal random LH 0.2 mIU/mL, FSH 0.1 mIU/mL, and testosterone 5 ng/dL. The patient was lost to follow-up and returned 5 years later presenting with gynecomastia and delayed bone age. He had continuous gonadotropin suppression with random LH 0.10 mIU/mL, FSH 0.16 mIU/mL, and testosterone 8 ng/dL. The histrelin implant was removed, and 4 months later, his random pubertal hormone levels were LH 5.6 mIU/mL, FSH 4.3 mIU/mL, and testosterone 506 ng/dL. The patient’s mid-parental height was 175.3 cm and his near final height was 170.6 cm, which is within 1 SD of his genetic potential. Further studies are needed to explore continuous gonadotropin hormone suppression with a single histrelin implant beyond 2 years.
Keywords: central precocious puberty, gonadotropin-releasing hormone analogue, bone age delay, gynecomastia
Central precocious puberty (CPP) is defined as thelarche prior to the age of 8 years in females and testicular enlargement prior to age of 9 years in males [1]. A hallmark characteristic of CPP is bone age acceleration leading to early epiphyseal fusion of the growth plates [2]. Gonadotropin-releasing hormone analogues (GnRHas) are the mainstay of therapy to prevent compromise in growth potential by slowing down bone age maturation. Histrelin implant is a nonbiodegradable device inserted within the subcutaneous tissue that provides systemic distribution of the analogue [3]. The histrelin implant suppresses clinical and laboratory parameters of puberty over 1 year, providing an alternative to monthly GnRHa injections [4]. Six months after removal of the implant there is a return to pubertal levels of FSH and LH after discontinuation of long-term gonadotropin suppression [2]. A single histrelin implant has been reported to be effective for up to 24 months [5]. There is no evidence in the literature of a single histrelin implant being effective beyond 2 years. We describe a novel case of a histrelin implant for treatment of CPP showing continuous hormonal suppression 7 years after implantation.
Case Report
A boy aged 10 years and 3 months presented to the endocrine clinic with precocious puberty and advanced bone age. The family reported he had pubic hair development at 10 years. However, his pediatrician was concerned about an adult serum testosterone level. He denied vomiting, headaches, fatigue, blurry vision, polydipsia, polyuria, cold intolerance, heat intolerance, constipation, abdominal pain, diarrhea. His blood pressure was 115/65 mmHg, heart rate was 80 bpm, weight was 35.2 kg (66th percentile). His height was 141.5 cm (62nd percentile) and his height velocity was 10.7 cm/year. His mid-parental height was 175.3 cm. Body mass index (BMI) was 17.58 kg/m [2] (65th percentile). Bone age was read as 11 years 6 months at a chronological age of 9 years and 11 months. Physical exam showed Tanner 3 pubic hair, Tanner 4 genitalia, and testicular volume of 15 mL. Screening laboratory investigation revealed a random luteinizing hormone (LH) level of 9.4 mIU/mL, random follicle-stimulating hormone (FSH) of 16.3 mIU/mL, prolactin 10 ng/mL, dehydroepiandrosterone sulfate (DHEAS) 127 mcg/dL, 17-OHP 217 ng/dL, and testosterone 628 ng/dL. Brain magnetic resonance imaging was unremarkable and did not show any pituitary lesions. He was initially started on a once-monthly 11.25 mg Lupron-Depot injection for 4 months before receiving a single 50 mg histrelin implant. Laboratory results 5 months after gonadotropin-releasing hormone (GnRH) suppression showed a random LH level of 0.2 mIU/mL, random FSH 0.1 mIU/mL, and testosterone 5 ng/dL. Physical exam showed Tanner 3 pubic hair, Tanner 4 genitalia, and testicular volumes of 12 mL. Growth velocity at that time was 11.3 cm/year. Six months postimplant he had a growth velocity of 2.2 cm/year. At the age of 11 years, he was started on medical marijuana for treatment of refractory seizures. At 11 years and 10 months, his bone age was 13 years. The testicular volume at this time was 12 mL bilaterally. Laboratory results showed continued pubertal suppression with random LH of 0.16 mIU/mL, random FSH 0.16 mIU/mL, and testosterone 5 ng/dL.
The patient and family were lost to follow-up for 5 years, returning at the age 17 years and 6 months because the mother was concerned the patient had not yet developed facial or chest hair and noticed breast tissue. Height at this time was 167.8 cm (13th percentile), weight was 63.6 kg (40th percentile), BMI was 22.59 kg/m2 (63rd percentile), and growth velocity was 2.5 cm/year. Physical examination showed Tanner 3 to 4 glandular breasts, Tanner 3 pubic hair, 8 mL right testicle and 10 mL left testicle, Tanner 4 genitalia, and moderate axillary hair. Laboratory results at this time showed random LH 0.10 IU/mL, random FSH 0.34 IU/mL, testosterone 8 ng/dL. He has normal 46 XY karyotype. A bone age x-ray was done after the visit, which showed a bone age of 14 years at a chronological age 17 years and 7 months. The histrelin implant was removed during a same day procedure under general anesthesia. The implant was removed in portions and complete removal was confirmed with ultrasound. Laboratory results obtained 4 months after removal of the implant showed a random LH of 5.6 IU/mL, random FSH 4.3 IU/mL, and testosterone 506 ng/dL. At 4 months after removal of histrelin implant, the mother noted facial hair development. Physical exam showed Tanner 3 glandular breasts, Tanner 4 pubic hair, Tanner 4 genitalia, 10 mL right testicle and 12 mL left testicle. Height was 170.4 cm (21st percentile), weight 64.6 kg (39th percentile), BMI 22.25 (53rd percentile), and growth velocity was 5.5 cm/year. Gynecomastia remained significant 4 months after removal of implant and despite pubertal hormone levels.
Discussion
Our patient is the first described in the literature to show continuous suppression of gonadotropin hormones more than 3 years after a single subdermal GnRHa implant. Gonadotropins were suppressed for 7 years after a single subdermal histrelin implant. A summary of the timeline of the patient course is found in Table 1. There have been no studies that have observed a single histrelin implant showing continuous suppression for this length of time.
Table 1.
Baseline and follow-up laboratory results, growth, pubertal progression
| Chronological age | 9 y 11 mo | 10 y 6 mo | 11 y 0 mo | 11 y 4 mo | 11 y 10 mo | 17 y 7 mo | 17 y 11 mo | 18 y 2 mo |
|---|---|---|---|---|---|---|---|---|
| Bone age | 11 y 6 mo | 12 y 6 mo | 13 y 0 mo | 14 y 0 mo | ||||
| Height, cm | 141.5 | 146.4 | 147.2 | 149 | 152 | 167.8 | 170.4 | |
| Growth velocity, cm/year | 10.7 | 11.3 | 2.2 | 4.6 | 6.5 | 2.7 | 5.5 | |
| Mid-parental target height | 175.3 | |||||||
| Testicular volume, mL | 15 | 12 | 12 | 12 | 8 R 10 L | 10 R 12 L | ||
| Estradiol, pg/mL | 9.9 | |||||||
| Testosterone, ng/dL | 628 | 5 | 5 | 8.7 | 172 | 506 | ||
| LH, mIU/mL | 9.4 | 0.20 | 0.16 | 0.10 | 3.7 | 5.6 | ||
| FSH, μIU/mL | 16.3 | 0.10 | 0.16 | 0.34 | 1.8 | 4.3 | ||
| 17-OHP, ng/dL | 217 | |||||||
| DHEAS, mcg/dL | 127 | |||||||
| Prolactin, ng/mL | 10 | |||||||
| Treatment | Started Lupron 11.25 mg monthly for 4 mos | 5 mos post-suppression | 13-mos post-suppression | 87 mos post-suppression | 1-mo post-explantation | 4 mos post-explantation | ||
| 1-month post-histrelin | 84 mos post-histrelin |
Abbreviations: 17-OHP, 17-hydroxyprogresterone; DHEAS, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; L, left; LH, lutenizing-hormone; mo, month; R, right; y, year;
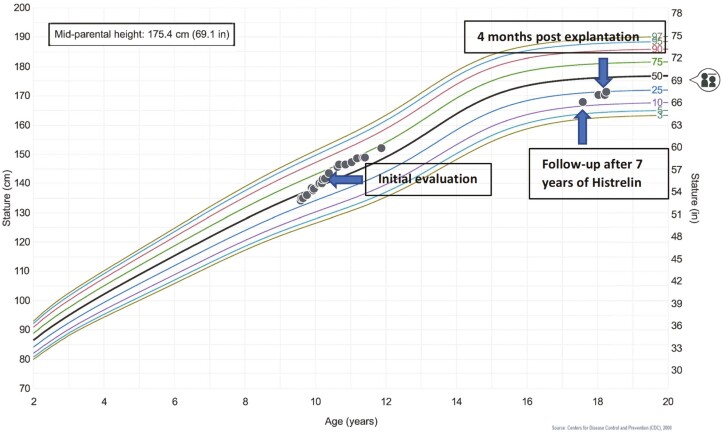
In a phase 3 prospective open-label study, Silverman et al observed biochemical suppression of the hypothalamic-pituitary-gonadal (HPG) axis 1 month after implantation of the subdermal GnRHa implant. Sustained gonadotropin suppression was seen over 72 months with annual replacement of the histrelin implant. Tanner breast stages in females were found to be unchanged after 60 months of treatment with histrelin implants; in addition, 1 boy had similar testicular size and Tanner stage of pubic hair after 60 months of treatment. The bone age advancement throughout the study decreased from 2.85 years to 1.28 years in the participants who received the histrelin implant without having received any other form of GnRHa treatment prior to enrollment into the study [2]. After explantation, there is a return of menarche in girls and testicular enlargement in boys within 1 year in those with CPP [6]. Besides the study from Lewis et al that demonstrated continuous suppression of a single histrelin implant over 2 years, the literature only shows suppression with yearly replacement of the histrelin implant [5]. Our case report argues that the duration of suppression from a single implant is possible beyond 2 years. Our patient’s bone age went from 2 years advanced to 3 years delayed after 7 years of treatment. Despite this, the patient still attained a height within 1 SD of his mid-parental height (171.5 cm vs mid-parental height of 175.3 cm; see Fig. 1) Linear growth is not yet complete as his growth velocity is 2.7 cm/year and bone age x-ray of the left hand was 14 years. In our patient iatrogenic hypogonadotropic hypogonadism from the retained subdermal GnRHa implant caused prolonged gonadotropin and sex steroid suppression. This allowed for continued bone growth beyond the normal time where closure of the epiphyseal growth plates would be seen. Prolonged growth due to delayed fusion of the growth plate can also be seen in aromatase deficiency and estrogen resistance. The patients do not have a pubertal growth spurt due to the absence of or resistance to sex steroids [7]. The 50 mg histrelin implant provides release of continuous GnRHa for treatment of CPP. Given a dose of 65 mcg/day in a single implant, a yearly device would theoretically last 2 years. Lewis et al found that a single histrelin implant is effective for up to 2 years [5]. However, in our patient, a single subdermal implant provided continuous gonadotropin suppression for 7 years. The mechanism for this suppression is not clear and pharmacokinetics studies need to be further elucidated by larger prospective trials.
Figure 1.
Height for age growth chart at initial presentation, after having the histrelin implant for 7 years, and then 4 months after the implant was removed. Each circle represents a growth measurement. Mid-parental height is marked with a symbol on the right side of the growth chart.
Gynecomastia is the growth of glandular breast tissue in males. It is a result of an imbalance between the availability of testosterone and estrogen. Due to the relatively increased estrogen level secreted by the testes during pubertal development, gynecomastia is a common temporal manifestation seen in adolescent males. Common causes of gynecomastia in males include testicular tumors such as Leydig-cell or Sertoli-cell tumors, adrenal neoplasms, and Klinefelter syndrome [8]. Drugs have also been implicated in cases of gynecomastia which may further impair the balance between estrogen and androgen production. Marijuana has been studied as a potential cause of gynecomastia; however, according to a review article in Andrology, there is insufficient evidence to make a significant association between marijuana use and presence of gynecomastia [9]. The patient in this case report had been taking medical marijuana based on a cannabidiol-dominant strain to manage epilepsy for 6 years prior to his presentation after being lost to follow-up with bilateral gynecomastia. Given that the literature has not been able to show marijuana’s association with gynecomastia, it is unlikely the medical marijuana contributed to his presentation. The gynecomastia in our patient could be due to hypogonadism/testosterone deficiency due to continuous gonadotropin hormone suppression with the histrelin implant for more than 7 years. Even though serum LH is suppressed in our patient, the sex steroids (testosterone 8.7 ng/dL and estradiol 9.9 pg/mL) are measurable. One possibility for our patient’s gynecomastia could be the peripheral conversion of adrenal androgens to estrogen. In addition, the karyotype analysis performed ruled out Klinefelter syndrome, another common cause of gynecomastia.
Conclusion
We present a patient with continuous gonadotropin suppression for 7 years after a single histrelin implant. Gonadotropin hormone analogues are effective treatments to delay compromise in height attainment in patients with central precocious puberty. Creation of a database that tracks the insertion and removal of histrelin implants can reduce the likelihood of an implant remaining longer than clinically indicated. With this, further studies are needed to demonstrate the effectiveness of long-term suppression of gonadotropin hormones beyond what is currently being reported in the literature. Further studies are also needed to elucidate the mechanism of the prolonged gonadotropin suppression beyond 2 years.
Glossary
Abbreviations
- BMI
body mass index
- CPP
central precocious puberty
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GnRHa
gonadotropin-releasing hormone analogue
- LH
luteinizing hormone
Financial Support
None.
Author Contributions
Dr. Quintos and Douglas Villalta conceptualized and designed the case report, collected, and analyzed the data, drafted the initial manuscript, and reviewed and revised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Disclosures
The authors have indicated they have no conflicts of interest relevant to this article to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Chen M, Eugster EA. Central precocious puberty: update on diagnosis and treatment. Paediatr Drugs. 2015;17(4):273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silverman LA, Neely EK, Kletter GB, et al. Long-term continuous suppression with once-yearly histrelin subcutaneous implants for the treatment of central precocious puberty: a final report of a phase 3 multicenter trial. J Clin Endocrinol Metab. 2015;100(6):2354-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahhal S, Clarke WL, Kletter GB, et al. Results of a second year of therapy with the 12-month histrelin implant for the treatment of central precocious puberty. Int J Pediatr Endocrinol. 2009;812517. doi:10.1155/2009/812517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirsch HJ, Gillis D, Strich D, et al. The histrelin implant: a novel treatment for central precocious puberty. Pediatrics. 2005;116(6):e798-e802. [DOI] [PubMed] [Google Scholar]
- 5. Lewis KA, Goldyn AK, West KW, Eugster EA. A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J Pediatr. 2013;163(4):1214-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher MM, Lemay D, Eugster EA. Resumption of puberty in girls and boys following removal of the histrelin implant. J Pediatr. 2014;164(4): 912-916.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albuquerque EVA, Scalco RC, Jorge AAL. Diagnostic and therapeutic approach of tall stature. Eur J Endocrinol. 2017;176(6):339-353. [DOI] [PubMed] [Google Scholar]
- 8. Braunstein GD. Clinical practice. Gynecomastia. New Engl J Med. 2007;357(12):1229-1237. [DOI] [PubMed] [Google Scholar]
- 9. Kanakis GA, Nordkap L, Bang AK, et al. EAA clinical practice guidelines-gynecomastia evaluation and management. Andrology. 2019;7(6):778-793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.