Abstract
Background
Although epidermal growth factor receptor‐tyrosine kinase inhibitors (EGFR‐TKIs) have been the standard treatment for advanced EGFR‐mutant adenocarcinoma, the effects of upfront EGFR‐TKI use in unresectable stage III EGFR‐mutant adenocarcinoma remain unexplored. Here, we conducted a retrospective study to compare different treatment strategies in these patients.
Methods
From October 2010 to June 2019, patients with unresectable stage III adenocarcinoma who received treatment at a tertiary referral center were enrolled. Patients were classified into three groups: EGFR‐mutant adenocarcinoma treated with concurrent chemoradiotherapy (group 1) or EGFR‐TKI (group 2) and EGFR wild‐type adenocarcinoma treated with concurrent chemoradiotherapy (group 3). Progression‐free survival, progression‐free survival‐2, and overall survival were estimated and compared using Kaplan–Meier and log‐rank tests.
Results
A total of 92 patients were enrolled; 10, 40, and 42 patients were assigned to groups 1, 2, and 3, respectively. Patients with EGFR mutations who received upfront EGFR‐TKIs had significantly longer progression‐free and overall survival than those who received upfront concurrent chemoradiotherapy (hazard ratio 0.33 vs. 0.34, p = 0.006 vs. 0.031) according to a Cox model adjusted for possible confounders. Moreover, upfront concurrent chemoradiotherapy did not lead to higher survival rates in patients with EGFR mutations than in those with EGFR wild‐type adenocarcinoma (progression‐free survival; hazard ratio 0.37, p = 0.036; overall survival; hazard ratio 0.35, p = 0.080) by Cox regression analysis.
Conclusion
This current study suggests that EGFR‐TKIs is a better choice for patients with unresectable stage III EGFR‐mutant adenocarcinoma. However, further randomized studies are required to validate the results.
Keywords: adenocarcinoma, chemoradiotherapy, epidermal growth factor receptor, stage III, tyrosine kinase inhibitors
The study regarding comparison of upfront EGFR‐TKI and upfront CCRT in unresectable stage III EGFR‐mutant adenocarcinoma remain limited. Our real‐world data suggest that upfront EGFR‐TKI monotherapy is a better treatment strategy than upfront CCRT in unresectable stage III EGFR‐mutant adenocarcinoma.

INTRODUCTION
Approximately one‐third of patients with lung cancer are initially diagnosed with stage III non‐small cell lung cancer (NSCLC), 1 for whom treatment remains a great challenge given its heterogeneity. 1 , 2 The standard treatment for unresectable stage III NSCLC is concurrent chemoradiotherapy (CCRT), whereas durvalumab was approved as consolidation immunotherapy for patients with disease control after CCRT. However, despite a recent study showing that patients with epidermal growth factor receptor (EGFR)‐mutant NSCLC will not benefit from consolidation treatment with durvalumab in subgroup analysis of PACIFIC study 3 the use of upfront EGFR‐tyrosine kinase inhibitors (TKI) instead of CCRT remains controversial.
A previous meta‐analysis including six studies of first‐generation EGFR‐TKIs revealed that EGFR‐TKIs provided a better objective response rate and progression‐free survival (PFS) than chemotherapy. 4 Subsequent studies have also shown that second‐generation EGFR‐TKIs, such as afatinib and dacomitinib, also have a better treatment response. 5 , 6 Further, the phase 3 FLAURA study demonstrated that the third‐generation EGFR‐TKI osimertinib provides better PFS and OS than first‐generation ones, 7 , 8 and it is the mainstay treatment in patients with EGFR mutations. Although the above studies recruited stage III patients, the staging system during trial enrollment only included patients with malignant pleural effusion (stage IIIB in the American Joint Committee on Cancer sixth edition). 4 , 5 , 6 Therefore, data regarding the treatment efficacy of EGFR‐TKIs in locally advanced stage III NSCLC remain limited.
Several studies have focused on the treatment efficacy of EGFR‐TKIs in patients with early‐stage EGFR‐mutant NSCLC. In the single‐group SELECT trial, adjuvant erlotinib provided longer disease‐free survival in patients with EGFR‐mutant stage IA to IIIA NSCLC than in historical genotype‐matched controls 9 ; however, in the post‐hoc analysis of the EGFR‐mutant subgroup in the randomized, placebo‐controlled RADIANT trial demonstrated longer disease‐free survival with adjuvant erlotinib in patients with stage IB to IIIA disease, although the difference was not statistically significant. 10 Two more randomized studies, the EVAN and ADJUVANT/CTONG1104 trials, also revealed longer disease‐free survival with adjuvant EGFR‐TKIs than with adjuvant chemotherapy in patients with resected EGFR‐mutant NSCLC. 11 , 12 Moreover, a recent phase 3 trial, the ADUARA study, demonstrated the clinical benefit of osimertinib in early‐stage EGFR‐mutant NSCLC. 13 Taken together, upfront EGFR‐TKI use seems to have good treatment efficacy among patients with early‐and late‐stage EGFR‐mutant NSCLC.
Although the recent LAURA trial (NCT03521154) evaluated the benefits and safety of maintenance therapy with osimertinib in patients with locally advanced EGFR‐mutant stage III NSCLC after CCRT but no disease progression, the study did not compare upfront osimertinib use with other treatments. Therefore, this study aimed to compare the treatment efficacy of upfront CCRT and upfront EGFR‐TKIs in patients with unresectable stage III EGFR‐mutant NSCLC.
METHODS
Patients
All patients with newly diagnosed stage III NSCLC at a tertiary referral center between October 2010 and June 2019 were enrolled. Since this study aimed to compare the efficacy of first‐line TKI together with CCRT, tumor subtypes for which TKI is not indicated, that is, squamous cell carcinoma or poorly differentiated carcinoma, were excluded. Patients receiving palliative care, with previous surgical intervention for NSCLC, or receiving other nonstandard treatments were excluded. Patients with incomplete data were excluded from the analysis. The remaining patients were classified into three groups: EGFR‐mutant patients receiving CCRT (group 1), EGFR‐mutant patients receiving TKI (group 2), and EGFR wild‐type patients receiving CCRT (group 3). For patients who received CCRT, six cycles of chemotherapy with weekly cisplatin and vinorelbine and definitive radiotherapy of 60 Gy were administered. The use of durvalumab was based on the discretion of the treating physician. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Review Board and Ethics Committee of National Cheng Kung University Hospital (NCKUH B‐ER‐109‐054) which waived the requirement for written consent due to the retrospective nature of the study.
All patients' electronic medical records were manually reviewed for eligibility and data were de‐identified. All patients underwent chest computed tomography, bone scan, and brain imaging (computed tomography with/without contrast or magnetic resonance imaging) and were staged according to the tumor, node, metastasis system proposed by the American Joint Committee on Cancer, eighth edition, at the time of diagnosis. Baseline characteristics, including age, sex, mutation subtype, performance status, tumor size, and nodal staging, were recorded. Follow‐up images to detect disease progression were arranged in the frequency suggested by the NCCN guidelines or when suspicious new symptoms developed.
EGFR mutation analysis
Tumor tissues were obtained from the primary lung mass for analysis. Tissue samples were selected if they had >80% tumor content, as examined with microscopy in hematoxylin and eosin staining. Subsequently, DNA was extracted through the QIAcube automated extractor (Qiagen) with the QIAamp DNA FFPE tissue kit (Qiagen), eluted in ATE (QIAmp Tissue Elution) buffer (Qiagen) according to the manufacturer's instructions. The EGFR polymerase chain reaction kit (EGFR RUO Kit) and Therascreen EGFR RGQ PCR Kit (EGFR IVD Kit, Qiagen) were used to detect EGFR mutations. Finally, scorpion and amplification‐refractory mutation system technologies were combined to disclose the mutations via real‐time quantitative polymerase chain reaction. 14
Statistical analysis
The comparison of demographics between groups was performed using the chi‐square test or Fisher's exact test for categorical variables and the Student's t‐test or the Wilcoxon rank‐sum test for continuous variables. The PFS and OS of patients with stage 3 NSCLC were analyzed using the Kaplan–Meier method and reported with 2‐sided 95% CI, groups compared using the log‐rank test. PFS was calculated from the date of initiation of first‐line stage III NSCLC treatment to disease progression 15 or death, censoring at the date of the last follow‐up in case of lack of progression. OS was calculated from the date of diagnosis to that of death. Because patients in group 1 received EGFR‐TKIs after disease progression from CCRT, PFS2 was also calculated from the date of initiation of definitive CCRT treatment to that of disease progression after EGFR‐TKI use in group 1. For patients in groups 2 and 3, PFS2 and PFS were the same (Figure S1).
Cox proportional hazards regression analysis was performed to determine the adjusted hazard ratio (HR) for PFS, PFS2, and OS. The possible determinants were selected based on previous studies investigating prognostic survival factors. 16 The prognostic factors selected were age, sex, tumor size, nodal stage, performance status, and treatment. Statistical analysis system (version 9.4; SAS Institute) was used to perform all analyses. All reported p‐values are two‐sided.
RESULTS
Patient characteristics
Overall, 366 patients with newly diagnosed NSCLC underwent screening from October 2010 to June 2019. Seventy‐eight patients (52 squamous cell carcinoma, 26 poorly differentiated carcinoma) were excluded because of nonadenocarcinoma histology and 196 patients because of inadequate treatment strategies or incomplete data. Finally, survival analysis included 92 patients with unresectable stage III adenocarcinoma. Detailed subject inclusion is shown in Figure 1.
FIGURE 1.
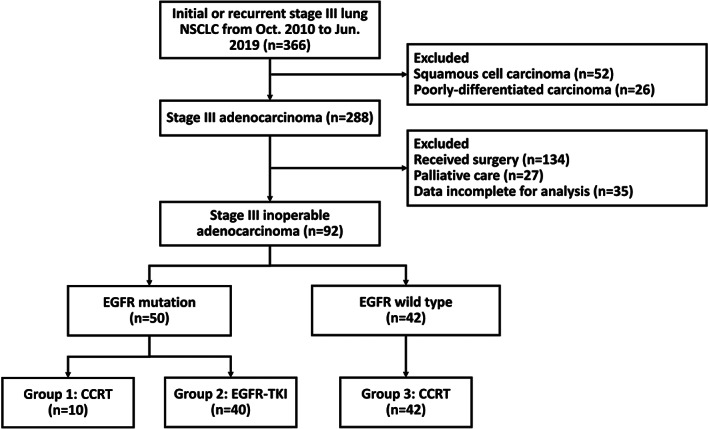
Study flowchart
Fifty‐one patients (51/93, 54.8%) were EGFR mutation‐positive, of whom 10 received definitive CCRT (group 1) and 40 EGFR‐TKI (group 2) as first‐line therapy; the remaining 42 patients were EGFR wild‐type‐positive and received definitive CCRT (group 3). Their baseline characteristics are summarized in Table 1. Group 2 was significantly older (median 70.8 years, interquartile range 62.3–76.2), whereas group 3 tended to have a higher male population (36/42, 85.7%) and had a larger tumor size (39/42, 92.6%, >30 mm). Most patients had an N stage of N3 status and had a good performance status (86/92, 93.5%, performance status 0–1) without group differences. In group 2, only two patients received palliative radiotherapy of 30 Gy because of a mechanical obstruction of the airway; none of these patients received residual tumor resection. Moreover, only two patients in group 3 received durvalumab consolidation therapy.
TABLE 1.
Baseline characteristics
| Characteristic | Group 1 (n = 10) | Group 2 (n = 40) | Group 3 (n = 42) | p‐value |
|---|---|---|---|---|
| Median (range), (years) | 64.3 (55.1–72.4) | 70.8 (62.3–76.2) | 61.0 (52.5–69.8) | 0.028 |
| <65 years | 5 | 13 | 26 | |
| ≥65 years | 5 | 27 | 16 | |
| Sex | <0.001 | |||
| Male | 6 | 17 | 36 | |
| Female | 4 | 23 | 6 | |
| Tumor size | 0.005 | |||
| <30 mm | 3 | 14 | 3 | |
| ≥30 mm | 6 | 26 | 39 | |
| Not available | 1 | 0 | 0 | |
| Lymph node | 0.160 | |||
| N0 | 0 | 0 | 1 a | |
| N1 | 0 | 0 | 0 | |
| N2 | 0 | 6 | 12 | |
| N3 | 10 | 34 | 29 | |
| Stage | 0.105 | |||
| IIIA | 0 | 2 | 3 | |
| IIIB | 5 | 25 | 14 | |
| IIIC | 5 | 13 | 25 | |
| Performance status | 0.065 | |||
| 0–1 | 10 | 37 | 39 | |
| ≥2 | 0 | 3 | 3 | |
| Mutation | 0.669 b | |||
| Exon 19 deletion | 5 | 17 | ||
| L858R | 5 | 23 | ||
| EGFR‐TKIs | 0.820 b | |||
| Gefitinib | 4 | 14 | ||
| Erlotinib | 4 | 14 | ||
| Afatinib | 2 | 12 |
Note: Group 1: Patients with EGFR‐mutant adenocarcinoma receiving CCRT as first‐line therapy. Group 2: Patients with EGFR‐mutant adenocarcinoma receiving TKIs as first‐line therapy. Group 3: Patients with EGFR wild‐type adenocarcinoma receiving CCRT as first‐line therapy.
Abbreviations: CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitors.
Pancoast tumor with vertebrae invasion.
Group 1 vs. Group 2.
PFS and OS
After a median follow‐up period of 22.3 months, the comparison of PFS, PFS2, and OS of all patients, stratified by EGFR status and therapies, is shown in Figure 2. The PFS of patients with EGFR mutation who received CCRT as first‐line therapy (group 1) was 7.1 months (interquartile range, 62.3–76.2), shorter than that of patients with EGFR wild‐type who received CCRT as first‐line therapy (group 3, 9.1 months, interquartile range 4.5–17.0). In contrast, patients with EGFR mutations who received EGFR‐TKI as first‐line therapy had significantly longer PFS than those in the other two groups (log‐rank test, p = 0.003; Figure 2(a)). After adjusting for possible confounders and using Cox proportional hazards regression analysis, we found that upfront EGFR‐TKIs was an independent good prognostic factor compared with upfront CCRT (HR 0.33, 95% confidence interval [CI] 0.15–0.73, p = 0.006) in patients with EGFR‐mutant adenocarcinoma. The absence of EGFR mutation was also an independent good prognostic factor (HR 0.37, 95% CI, 0.15–0.94, p = 0.036) in patients who received CCRT. Another independent good prognostic factor for PFS was female sex (Table 2). Upon disease progression, the performance status and recurrence pattern were summarized in Table S1. Among patients with EGFR mutations who received CCRT as first‐line therapy (group 1), there was a higher proportion of patients with a poor performance status (p = 0.003) and higher distant recurrence rate (p = 0.038) at disease progression than among patients with EGFR mutations who received EGFR‐TKIs as first‐line therapy (group 2).
FIGURE 2.
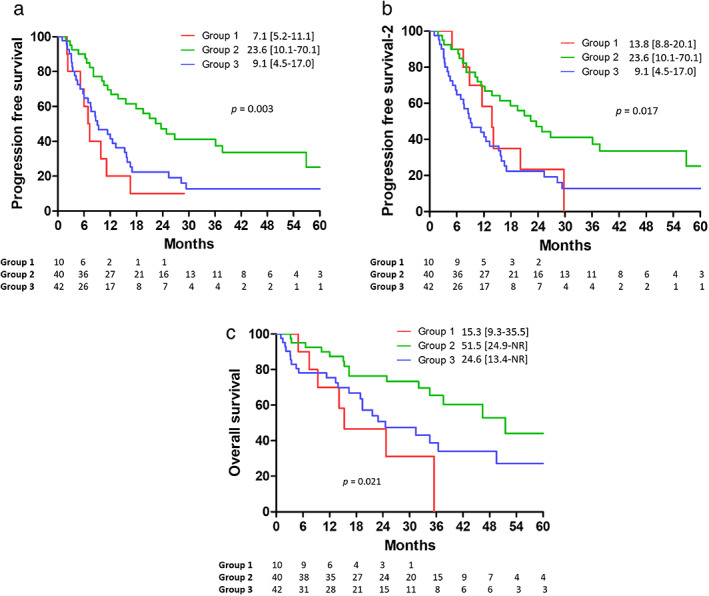
(a) PFS, (b) PFS2, and (c) OS among patients with EGFR‐mutant adenocarcinoma receiving CCRT (Group 1) or upfront EGFR‐TKI (Group 2), and patients with EGFR wild‐type adenocarcinoma receiving CCRT (Group 3). CCRT, concurrent chemoradiotherapy; EGFR, epidermal growth factor receptor; PFS, progression‐free survival; OS, overall survival; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; PFS2, progression‐free survival‐2
TABLE 2.
Cox proportional hazard regression analysis of PFS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKI
| Characteristic | PFS | ||
|---|---|---|---|
| Hazard ratio | p‐value | ||
| Age (years) | ≥65 vs. <65 | 0.79 (0.47–1.34) | 0.389 |
| Sex | Male vs. female | 1.88 (1.04–3.39) | 0.037 |
| Tumor size (cm) | ≥3 vs. <3 | 1.52 (0.75–3.05) | 0.244 |
| N stage | N3 vs. N0–2 | 1.03 (0.55–1.92) | 0.933 |
| Performance status | ECOG ≥2 vs. ECOG <1 | 1.01 (0.35–2.87) | 0.464 |
| Patient group a | Group 2 vs. Group 1 | 0.33 (0.15–0.73) | 0.006 |
| Group 3 vs. Group 1 | 0.37 (0.15–0.94) | 0.036 | |
Note: Group 1: Patients with EGFR‐mutant adenocarcinoma receiving upfront CCRT. Group 2: Patients with EGFR‐mutant adenocarcinoma receiving upfront EGFR‐TKIs. Group 3: Patients with EGFR wild‐type adenocarcinoma receiving upfront CCRT.
Abbreviations: CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PFS, progression‐free survival; TKI, tyrosine kinase inhibitors.
Treatment strategy for different patient groups.
In group 1, all 10 patients received EGFR‐TKIs after disease progression with CCRT. When considering the time to second objective disease progression, PFS2 was still significantly longer in group 2 (23.6 Â months, interquartile range 10.1–70.1) than in group 1 (13.8 Â months, interquartile range 8.8–20.1) and group 3 (9.1 Â months, interquartile range 4.5–17.0; log‐rank test, p = 0.016; Figure 2(b)). However, after adjusting for potential confounding factors in Cox proportional hazard regression analysis, the choice of first‐line therapy was not an independent predictor of PFS2 (Table 3).
TABLE 3.
Cox proportional hazard regression analysis of PFS2 among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKI
| Characteristic | PFS2 | ||
|---|---|---|---|
| Hazard ratio | p‐value | ||
| Age (years) | ≥65 vs. <65 | 0.87 (0.57–1.47) | 0.598 |
| Sex | Male vs. female | 1.82 (0.99–3.32) | 0.051 |
| Tumor size (cm) | ≥3 vs. <3 | 1.84 (0.91–3.71) | 0.090 |
| N stage | N3 vs. N0–2 | 1.01 (0.54–1.88) | 0.979 |
| Performance status | ECOG ≥2 vs. ECOG <1 | 0.98 (0.34–2.81) | 0.976 |
| Patient group a | Group 2 vs. Group 1 | 0.59 (0.26–1.33) | 0.200 |
| Group 3 vs. Group 1 | 0.59 (0.22–1.55) | 0.283 | |
Note: Group 1: Patients with EGFR‐mutant adenocarcinoma receiving upfront CCRT. Group 2: Patients with EGFR‐mutant adenocarcinoma receiving upfront EGFR‐TKIs. Group 3: Patients with EGFR wild‐type adenocarcinoma receiving upfront CCRT.
Abbreviations: CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PFS2, progression‐free survival‐2; TKI, tyrosine kinase inhibitors.
Treatment strategy for different patient groups.
Finally, OS was also significantly longer in group 2 (51.5 months, interquartile range 24.9–NR) than in those who received CCRT regardless of the EGFR mutation status (log‐rank test, p = 0.021; Figure 2(c)). After adjusting for possible confounders in Cox proportional hazards regression (Table 2), use of upfront EGFR‐TKIs in patients with EGFR‐mutation was an independent good prognostic factor compared with that of upfront CCRT (HR 0.34, 95% CI, 0.13–0.91, p = 0.031). Another independent good prognostic factor for OS was female sex (Table 4). However, the presence of EGFR mutations was not a significant prognostic factor for OS, nor were other determinants such as age, tumor size, N stage, and performance status. Although an advanced stage (stage IIIC), the presence of EGFR mutations, or the use of EGFR‐TKIs might affect treatment outcomes in these patients, the multivariate analysis revealed that these variables were not independent prognostic factors for PFS, PFS2, and OS (Tables S2–S4).
TABLE 4.
Cox proportional hazard regression analysis of OS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKI
| Characteristic | OS | ||
|---|---|---|---|
| Hazard ratio | p‐value | ||
| Age (years) | ≥65 vs. <65 | 0.80 (0.41–1.55) | 0.504 |
| Sex | Male vs. female | 3.40 (1.53–7.56) | 0.003 |
| Tumor size (cm) | ≥3 vs. <3 c | 1.16 (0.49–2.75) | 0.736 |
| N stage | N3 vs. N0–2 | 1.51 (0.65–3.53) | 0.337 |
| Performance status | ECOG ≥2 vs. ECOG <1 | 0.86 (0.20–3.65) | 0.834 |
| Patient group a | Group 2 vs. Group 1 | 0.34 (0.13–0.91) | 0.031 |
| Group 3 vs. Group 1 | 0.35 (0.11–1.13) | 0.080 | |
Note: Group 1: Patients with EGFR‐mutant adenocarcinoma receiving upfront CCRT. Group 2: Patients with EGFR‐mutant adenocarcinoma receiving upfront EGFR‐TKIs. Group 3: Patients with EGFR wild‐type adenocarcinoma receiving upfront CCRT.
Abbreviations: CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; OS, overall survival; TKI, tyrosine kinase inhibitors.
Treatment strategy for different patient groups.
DISCUSSION
In this retrospective, single‐center, real‐world study of survival outcomes in unresectable stage III adenocarcinoma, we found that patients with EGFR mutations treated with upfront EGFR‐TKIs (group 2) had significantly longer PFS, PFS2, and OS than those treated with definitive CCRT (group 1). When comparing EGFR mutation status among patients receiving definitive CCRT (group 1 vs. group 3), those with EGFR mutations had a shorter PFS but similar OS than those with EGFR wild‐type.
Stage III NSCLC comprises a heterogeneous patient population, with varying staging characteristics and multimodal treatment options, including chemotherapy, surgery, and targeted therapy. Approximately 70% of patients with stage III NSCLC have unresectable tumors, with the standard treatment being CCRT administration. 17 A previous randomized phase III PACIFIC study was conducted to evaluate the efficacy of consolidative therapy with durvalumab, an anti‐PD‐L1 immune checkpoint inhibitor, in patients with stage III NSCLC who achieved disease control after CCRT completion. The study demonstrated significant improvement in PFS (16.8 months vs. 5.6 months) 18 and OS (47.5 vs. 28.7 months) 19 compared with placebo. Although CCRT followed by durvalumab has become a mainstay treatment for patients with unresectable stage III NSCLC, not all patient subgroups benefit. Disease progression was similar at the 4‐year follow‐up (durvalumab vs. placebo, 72.4% vs. 78.6%) in the EGFR‐mutant patient subgroup. 19 As only 6.1% of patients in the PACIFIC study had an EGFR mutation, 19 the statistical analysis for OS benefits in this subgroup is limited. Recently, a retrospective analysis on patients with stage III EGFR‐ and HER2‐mutant NSCLC showed a PFS of only 7.5 months when consolidation therapy with durvalumab was administered. 20 Another multi‐institutional retrospective study of patients with unresectable stage III tumors and EGFR mutations also demonstrated a PFS of only 10.3 months in patients who received durvalumab as consolidation therapy, not significantly different from those who did not. 3 In conclusion, standard therapy with CCRT followed by durvalumab might not provide clinical benefit in patients with unresectable stage III EGFR‐mutant NSCLC.
In the current study, the patients with EGFR mutation had significantly shorter PFS when receiving CCRT than patients with EGFR wild‐type (7.1 vs. 9.1 months, HR 0.37, p = 0.036), consistent with findings of previous cohort studies showing that EGFR mutation is associated with significantly shorter PFS. 21 , 22 , 23 Some studies further showed that despite better local control, EGFR‐mutant patients receiving CCRT were prone to develop distant recurrence, 23 , 24 , 25 including brain metastasis. 26 However, these studies did not compare survival outcomes between patients with EGFR mutations receiving upfront EGFR‐TKI or CCRT. In the current study, of the 10 patients with EGFR mutations who received upfront CCRT, nine had disease progression at the time of data collection and eight presented with distant metastasis. Moreover, CCRT is associated with a higher rate of grades 3 and 4 toxicity, and treatment delay or interruption is not rare. 27 Given its better systemic response, lower distant recurrence rate, and lower toxicity profile, 28 , 29 upfront EGFR‐TKIs have the potential to be a better first‐line treatment strategy.
One population‐based retrospective study in Taiwan showed the comparative effectiveness of upfront TKI and CCRT (HR 0.71, 95% CI: 0.34–1.47) in unresectable stage III NSCLC. 30 Similar to our results, the TKI group was also significantly older (mean 70.2% vs. 60.1%, p < 0.001), implying a preference for prescription in older patients because of treatment toxicity. However, of the 566 525 potential study candidates, due to a large proportion of missing data, only 199 patients were included, which precludes a definitive conclusion. In a recent global real‐world study (the KINDLE study), patients with unresectable stage III EGFR‐mutant NSCLC who received upfront chemoradiotherapy had better OS than those receiving upfront EGFR‐TKIs. 31 However, the patients enrolled in the KINDLE study had to survive for ≥9 months based on study design. 31 In the PACIFIC study, patients without durvalumab consolidation had a 1‐year OS rate of 74.5%. 19 As patients enrolled in clinical trials usually have better performance status, the 1‐year OS rate in a real‐world study may be lower than 74.5%, which may imply that a certain proportion of patients were not enrolled in the KINDLE study. To the best of our knowledge, our study is the first real‐world study to demonstrate better treatment efficacy of upfront EGFR‐TKIs in patients with unresectable stage III EGFR‐mutant NSCLC, highlighting the importance of targeting the driving mutation for better control in EGFR‐mutant populations.
There are some limitations to our study. First, it was a single‐center, retrospective study, and treatment was chosen according to physician preference, possibly harboring many confounders. To control for this confounding effect, we used Cox proportional hazard regression analysis. Second, although EGFR‐TKI use provided longer PFS and OS than CCRT use in patients with unresectable stage III EGFR‐mutant NSCLC, the sample size was small and had an imbalanced distribution. Further prospective randomized controlled trials are warranted to validate the results of this study. Third, group 2 (EGFR‐mutant adenocarcinoma receiving upfront EGFR‐TKI) was significantly older (median age, 70.8 years) than groups 1 and 3. However, group 2 showed the longest PFS and OS among all groups. This could be viewed as the opposite of a lead time bias, wherein patients diagnosed later had a longer survival. This strengthens our conclusion that targeted therapy is beneficial in EGFR‐mutant patients, even in an older, apparently frail, population. Fourth, in the ADAURA study, the adjuvant osimertinib was proven to prolong disease‐free survival and lower the CNS recurrence rate. Whether consolidation therapy with EGFR‐TKIs could provide survival benefits remains unknown. The ongoing LAURA study (NCT03521154) may provide additional information on the role of consolidation osimertinib.
In conclsuion, our real‐world data suggest that upfront EGFR‐TKI monotherapy is a better treatment strategy than upfront CCRT in unresectable stage III EGFR‐mutant adenocarcinoma. Considering the limitation of our study size, prospective randomized controlled trials are needed to validate these findings.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
FIGURE S1 Diagram of survival analysis.
TABLE S1 The performance status, distant metastasis rate, and subsequent osimertinib use among group 1 and group 2 patients with disease progression at first‐line therapy
TABLE S2. Cox proportional hazard regression analysis, including the presence of EGFR mutations, of PFS, PFS2, and OS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKIs.
TABLE S3. Cox proportional hazard regression analysis, including the cancer stage, of PFS, PFS2, and OS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKIs.
TABLE S4. Cox proportional hazard regression analysis, including the use of EGFR‐TKIs, of PFS, PFS2, and OS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKIs.
ACKNOWLEDGMENTS
The present study was based in part on data sourced from the Cancer Data Bank of the National Cheng Kung University Hospital. We are grateful to Dr Sheng‐Hsiang Lin for providing the statistical consulting services from the Biostatistics Consulting Center, Clinical Medicine Research Center, National Cheng Kung University Hospital.
Wang S‐Y, Lai C‐H, Chen C‐W, Yang S‐C, Chang C‐C, Lin C‐Y, et al. Improved survival in patients with unresectable stage III EGFR ‐mutant adenocarcinoma with upfront EGFR‐tyrosine kinase inhibitors. Thorac Cancer. 2022;13:182–189. 10.1111/1759-7714.14237
Sheng‐Yuan Wang and Ching‐Han Lai contributed equally to this study
Funding information Ministry of Science and Technology, Taiwan, Grant/Award Numbers: 109‐2314‐B‐006 ‐083 ‐, 110‐2314‐B‐006 ‐102 ‐; National Cheng Kung University Hospital, Grant/Award Number: NCKUH‐11002003
Contributor Information
Po‐Lan Su, Email: polan.750317@gmail.com.
Chien‐Chung Lin, Email: joshcclin@gmail.com.
REFERENCES
- 1. Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in Taiwan: a population‐based cancer registry. J Thorac Oncol. 2013;8:1128–35. [DOI] [PubMed] [Google Scholar]
- 2. Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28:2181–90. [DOI] [PubMed] [Google Scholar]
- 3. Aredo JV, Mambetsariev I, Hellyer JA, Amini A, Neal JW, Padda SK, et al. Durvalumab for stage III EGFR‐mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 2021;16:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee CK, Davies L, Wu YL, Mitsudomi T, Inoue A, Rosell R, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation‐positive lung cancer: individual patient data meta‐analysis of overall survival. J Natl Cancer Inst. 2017;109:djw279. 10.1093/jnci/djw279 [DOI] [PubMed] [Google Scholar]
- 5. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–51. [DOI] [PubMed] [Google Scholar]
- 6. Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non‐small‐cell lung cancer and EGFR‐activating mutations. J Clin Oncol. 2018;36:2244–50. [DOI] [PubMed] [Google Scholar]
- 7. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 8. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 9. Pennell NA, Neal JW, Chaft JE, Azzoli CG, Jänne PA, Govindan R, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor‐mutant non‐small‐cell lung cancer. J Clin Oncol. 2019;37:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly K, Altorki NK, Eberhardt WE, O'Brien ME, Spigel DR, Crinò L, et al. Adjuvant erlotinib versus placebo in patients with stage IB‐IIIA non‐small‐cell lung cancer (RADIANT): a randomized, double‐blind, phase III trial. J Clin Oncol. 2015;33:4007–14. [DOI] [PubMed] [Google Scholar]
- 11. Yue D, Xu S, Wang Q, Li X, Shen Y, Zhao H, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation‐positive non‐small‐cell lung cancer (EVAN): a randomised, open‐label, phase 2 trial. Lancet Respir Med. 2018;6:863–73. [DOI] [PubMed] [Google Scholar]
- 12. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II‐IIIA (N1‐N2) EGFR‐mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open‐label, phase 3 study. Lancet Oncol. 2018;19:139–48. [DOI] [PubMed] [Google Scholar]
- 13. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med. 2020;383:1711–23. [DOI] [PubMed] [Google Scholar]
- 14. Chen YL, Lu CC, Yang SC, Su WP, Lin YL, Chen WL, et al. Verification of wild‐type EGFR status in non‐small cell lung carcinomas using a mutant‐enriched PCR on selected cases. J Mol Diagn. 2014;16:486–94. [DOI] [PubMed] [Google Scholar]
- 15. Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics. 2013;33:1323–41. [DOI] [PubMed] [Google Scholar]
- 16. Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non‐small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol. 2011;3:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non‐small‐cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28:iv1‐21. [DOI] [PubMed] [Google Scholar]
- 18. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 19. Faivre‐Finn C, Vicente D, Kurata T, Planchard D, Paz‐Ares L, Vansteenkiste JF, et al. Four‐year survival with durvalumab after chemoradiotherapy in stage III NSCLC‐an update from the PACIFIC trial. J Thorac Oncol. 2021;16:860–7. [DOI] [PubMed] [Google Scholar]
- 20. Hellyer JA, Aredo JV, Das M, Ramchandran K, Padda SK, Neal JW, et al. Role of consolidation durvalumab in patients with EGFR‐ and HER2‐mutant unresectable stage III NSCLC. J Thorac Oncol. 2021;16:868–72. [DOI] [PubMed] [Google Scholar]
- 21. Park SE, Noh JM, Kim YJ, Lee HS, Cho JH, Lim SW, et al. EGFR mutation is associated with short progression‐free survival in patients with stage III non‐squamous cell lung cancer treated with concurrent chemoradiotherapy. Cancer Res Treat. 2019;51:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishihara M, Igawa S, Sasaki J, Otani S, Fukui T, Ryuge S, et al. Evaluation of concurrent chemoradiotherapy for locally advanced NSCLC according to EGFR mutation status. Oncol Lett. 2017;14:885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka K, Hida T, Oya Y, Oguri T, Yoshida T, Shimizu J, et al. EGFR mutation impact on definitive concurrent chemoradiation therapy for inoperable stage III adenocarcinoma. J Thorac Oncol. 2015;10:1720–5. [DOI] [PubMed] [Google Scholar]
- 24. Nakamura M, Kageyama SI, Niho S, Okumura M, Hojo H, Motegi A, et al. Impact of EGFR mutation and ALK translocation on recurrence pattern after definitive chemoradiotherapy for inoperable stage III non‐squamous non‐small‐cell lung cancer. Clin Lung Cancer. 2019;20:e256–64. [DOI] [PubMed] [Google Scholar]
- 25. Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non‐small‐cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–6. [DOI] [PubMed] [Google Scholar]
- 26. Akamatsu H, Kaira K, Murakami H, Serizawa M, Koh Y, Ono A, et al. The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who received concurrent chemoradiotherapy. Am J Clin Oncol. 2014;37:144–7. [DOI] [PubMed] [Google Scholar]
- 27. Verma V, Simone CB 2nd, Werner‐Wasik M. Acute and late toxicities of concurrent chemoradiotherapy for locally‐advanced non‐small cell lung cancer. Cancers (Basel). 2017;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR‐mutated advanced non‐small‐cell lung cancer. J Clin Oncol. 2018;36:3290–97. 10.1200/JCO.2018.78.3118 [DOI] [PubMed] [Google Scholar]
- 29. Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M‐positive advanced non‐small‐cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36:2702–9. [DOI] [PubMed] [Google Scholar]
- 30. Hsia TC, Liang JA, Li CC, Chien CR. Comparative effectiveness of concurrent chemoradiotherapy versus EGFR‐tyrosine kinase inhibitors for the treatment of clinical stage IIIb lung adenocarcinoma patients with mutant EGFR. Thorac Cancer. 2018;9:1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jazieh A, Onal H, Tan D, Soo R, Prabhash K, Kumar A, et al. OA05.03 real‐world global data on targeting epidermal growth factor receptor in stage III non‐small cell lung cancer: the results of the KINDLE study. J Thoracic Oncol. 2021;16:S110–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Diagram of survival analysis.
TABLE S1 The performance status, distant metastasis rate, and subsequent osimertinib use among group 1 and group 2 patients with disease progression at first‐line therapy
TABLE S2. Cox proportional hazard regression analysis, including the presence of EGFR mutations, of PFS, PFS2, and OS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKIs.
TABLE S3. Cox proportional hazard regression analysis, including the cancer stage, of PFS, PFS2, and OS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKIs.
TABLE S4. Cox proportional hazard regression analysis, including the use of EGFR‐TKIs, of PFS, PFS2, and OS among patients with unresectable adenocarcinoma receiving CCRT or upfront EGFR‐TKIs.


