Abstract
Objective
Ceftriaxone (CTRX) is a widely used antibiotic because of its long plasma half-life and good tissue transmission. Many of the reported studies on CTRX-associated pseudolithiasis were performed in children. Although some studies have been published in adults, there are no studies limited to elderly people. The present study investigated CTRX-associated pseudolithiasis and explored its risk factors in the elderly.
Methods
We retrospectively reviewed 133 elderly patients (≥65 years old) treated with CTRX. Pseudolithiasis was defined as stones or sludge newly appearing in the gallbladder, as detected by computed tomography after the administration of CTRX. We evaluated the risk factors for pseudolithiasis using multivariate regression and inverse probability of treatment weighting analyses.
Results
Among the 133 patients, 24 (18%) developed CTRX-associated pseudolithiasis. In a multivariate analysis, the CTRX dose [odds ratio (OR) 4.54, 95% confidence interval (CI) 1.36-15.07, p=0.012] and CTRX treatment duration (OR 2.80, 95% CI 1.06-8.04, p=0.043) were significantly associated with pseudolithiasis formation. The cut-off value of the total CTRX dose associated with pseudolithiasis formation was 19 g. A propensity analysis determined that the frequency of pseudolithiasis was increased in patients treated with >19 g total CTRX compared with those who received ≤19 g in total (OR 4.06, 95% CI 1.45-11.32, p=0.008).
Conclusion
The incidence rate of CTRX-induced pseudolithiasis is high in elderly people, and the CTRX dose and CTRX treatment duration are significant risk factors for pseudolithiasis. A total dose of >19 g increases the likelihood of pseudolithiasis formation in elderly people treated with CTRX.
Keywords: ceftriaxone, pseudolithiasis, elderly people, cut-off value, IPTW
Introduction
Ceftriaxone (CTRX) is a third-generation cephalosporin that has good tissue transmission. It is used for the treatment of many infectious diseases, such as respiratory infection, urinary tract infection and meningitis. In addition, because this antibiotic has a long plasma half-life (8 hours), it can be administered once a day and is often used in outpatient settings.
In 1986, Schaad et al. first reported a case of gallstone-like substance that precipitated in the gallbladder after CTRX administration (1). Because this gallstone-like substance has the property of spontaneously disappearing relatively early, it was named “pseudolithiasis” (2). Since then, there have been reports on pseudolithiasis associated with CTRX, mainly in children. Pseudolithiasis often spontaneously disappears after the discontinuation of CTRX (3,4). However, complications such as cholecystitis, cholangitis, and pancreatitis can occur, and biliary drainage (5) and surgical resection (6) are required when discontinuation of CTRX does not improve the symptoms.
Japan has an aging society, and the number of elderly inpatients is increasing. Many elderly patients with various infectious diseases are treated with CTRX because of its effectiveness. Studies on CTRX-associated pseudolithiasis have been well reported in children thus far; however, there have been no studies limited to elderly people. The incidence rate is expected to be high in elderly people because they often have conditions such as dehydration, a bedridden status, and renal dysfunction, which are reported risk factors for pseudolithiasis formation (7,8).
The purpose of the present study was to investigate CTRX-associated pseudolithiasis and to identify its risk factors in elderly people.
Materials and Methods
Definition of pseudolithiasis
A transient CTRX-induced gallstone detected with ultrasonography was first reported by Schaad et al. in 1986, and this reversible gallstone (i.e., has the property of spontaneously disappearing) was named “pseudolithiasis” (1,2). In the present study, pseudolithiasis was defined as stones or sludge newly appearing in the gallbladder, as detected with computed tomography (CT) performed within nine weeks after CTRX administration. The nine-week period was set based on a previous report (9) that pseudolithiasis was still observed even nine weeks after CTRX administration. We confirmed the absence of gallstones before CTRX administration by reviewing CT scans performed at the time of hospitalization. CT before CTRX administration was performed to diagnose the disease necessitating the hospitalization, and CT after CTRX administration was performed as a follow-up for the disease necessitating the hospitalization or to search for the cause of clinical symptoms, such as a fever, during the course.
Abdominal CT was performed using a Revolution EVO (GE Healthcare, Waukesha, USA). Images of the entire abdomen were obtained at a 5-mm thickness (axial image; width 300 HU; level 25 HU). All of the patients were examined by plain CT. The presence of high-density material in the gallbladder was considered the criterion for positive gallbladder stones and sludge. Positive CT findings were diagnosed by radiologists.
Study subjects and measurements
We retrospectively reviewed elderly patients (≥65 years old) treated with CTRX at Higashisumiyoshi Morimoto Hospital between April 2019 and March 2020. First, patients without CT scans before CTRX administration and within nine weeks after CTRX administration were excluded in order to ensure the same background characteristics for comparative cases. Second, patients with a history of cholelithiasis or who had undergone cholecystectomy before CTRX treatment were also excluded. Fig. 1 shows a flowchart of the selection of participants.
Figure 1.
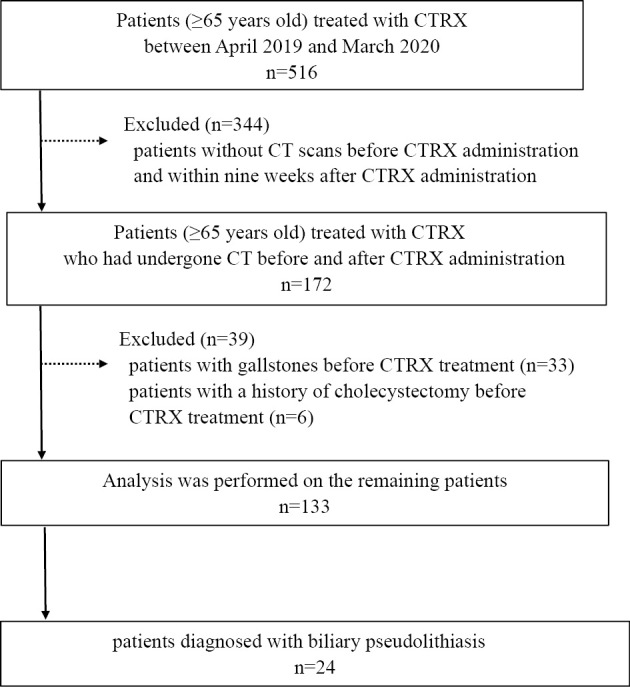
Flowchart of participant selection. CT: computed tomography, CTRX: ceftriaxone
We examined the age, sex, body mass index (BMI), CTRX dose, CTRX treatment duration, fasting status, bedridden status, blood test results [creatinine (Cr), estimated glomerular filtration rate (eGFR)], and the day of the CT examination after CTRX administration (CT day) to identify the risk factors for pseudolithiasis. Blood samples were collected before CTRX therapy.
Statistical analyses
The chi-square test and Fisher's exact test were used to compare categorical variables between patients with and without pseudolithiasis. Categorical variables are summarized as frequencies with percentages. The Mann-Whitney U test or two-sample t-test was used to compare continuous variables. Continuous variables are summarized as medians with interquartile ranges. The risk factors for the development of pseudolithiasis were evaluated using a logistic regression analysis, and data are reported as the estimated odds ratios (ORs) and 95% confidence intervals (CIs). Based on several previous reports that the CTRX dose, treatment duration, and renal dysfunction were significant risk factors for pseudolithiasis formation (2,8,10,11), a multivariate analysis was performed using these three risk factors. To determine the cut-off values of the dose, treatment duration, and total CTRX dose associated with pseudolithiasis formation, we constructed receiver operating characteristic (ROC) curves and determined their area under the curve (AUC). In addition, the inverse probability of treatment weighting (IPTW) method based on propensity scoring was used. We adjusted for confounding factors without reducing the sample size by using the estimated propensity scores to assign weights to the data (12,13). Analyses involving IPTW linear regressions for pseudolithiasis formation were performed. The variables included in the IPTW analysis were the age, sex, BMI, fasting status, bedridden status, eGFR, and CT day. A p value of ≤0.05 was considered statistically significant. All statistical analyses were performed using the R software program (version 3.5; R Core Team, Vienna, Austria).
Ethical considerations
This study was performed in accordance with the Declaration of Helsinki, and the ethics committee of our hospital approved this study.
Results
Clinical patient characteristics and comparisons
A total of 516 patients (≥65 years old) were treated with CTRX during the study period. After excluding 344 patients without CT scans before CTRX administration and within nine weeks after CTRX administration, 33 patients with gallstones, and 6 patients with a history of cholecystectomy before CTRX treatment, the remaining 133 patients were enrolled in this study (Fig. 1).
The baseline patient characteristics are summarized in Table 1. Among the 133 patients, 24 (18%) developed CTRX-associated pseudolithiasis. The comparison of the groups with and without pseudolithiasis demonstrated no significant differences with respect to the age, sex, BMI, fasting status, bedridden status, Cr level, eGFR, or CT day. The development of pseudolithiasis showed a significant relationship with the CTRX dose (p=0.005) and CTRX treatment duration (p=0.002).
Table 1.
Baseline Patient Characteristics.
| Background | Pseudolithiasis group | Non-pseudolithiasis group | p value |
|---|---|---|---|
| Number of patients (%) | 24 (18%) | 109 (82%) | |
| Age (years) (IQR) | 82 (77-88) | 84 (78-89) | 0.429 |
| Sex (male/female) | 12/12 | 55/54 | 0.968 |
| BMI (IQR) | 19 (17-22) | 20 (17-22) | 0.745 |
| CTRX dose (g/day), n (%) | |||
| 1 g/day | 0 (0%) | 12 (11%) | 0.005* |
| 2 g/day | 17 (71%) | 89 (82%) | |
| 4 g/day | 7 (29%) | 8 (7%) | |
| CTRX treatment duration (days) (IQR) | 10 (7-12) | 7 (4-10) | 0.002* |
| Fasting, n (%) | |||
| No | 20 (83%) | 89 (82%) | 1 |
| Yes | 4 (17%) | 20 (18%) | |
| Bedridden, n (%) | |||
| No | 12 (50%) | 50 (46%) | 0.714 |
| Yes | 12 (50%) | 59 (54%) | |
| Cr (mg/dL) (IQR) | 1.1 (0.8-1.5) | 0.9 (0.7-1.3) | 0.245 |
| eGFR (mL/min/1.73m2) (IQR) | 44 (28-59) | 51 (35-71) | 0.204 |
| CT day (IQR) | 14 (9.8-19) | 9 (6-22) | 0.121 |
BMI: body mass index, Cr: creatinine, CTRX: ceftriaxone, eGFR: estimated glomerular filtration rate, IQR: interquartile range, CT day: the day of CT examination after CTRX administration
Characteristics of all cases of pseudolithiasis at our hospital
The characteristics of all cases of pseudolithiasis at our hospital are shown in Table 2. The median patient age was 82 (range 67-95) years old. Of the 24 patients with pseudolithiasis, 12 were men, and 12 were women. The median dose of CTRX was 2 g/day (range 2-4 g/day). The median duration of CTRX treatment was 10 days (range 5-34 days). 3 patients (13%) had abdominal pain, and 3 patients (13%) had abnormal bilirubin levels. The median time until the detection of pseudolithiasis was 13 (range 3-47) days.
Table 2.
Incidence of Biliary Pseudolithiasis.
| No. | Age (years)/ Sex |
Clinical Diagnosis |
CTRX dose (g/day) |
CTRX treatment duration (days) |
Abdominal pain |
Abnormal bilirubin level |
Time to detect pseudolithiasis (days) |
Pseudolithiasis location |
Pseudolithiasis pattern |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 82/M | Rectal cancer | 2 | 16 | - | - | 8 | GB | Sludge |
| 2 | 79/M | Bacterial pneumonia | 4 | 8 | - | - | 9 | GB | Sludge |
| 3 | 88/F | Lung cancer | 2 | 7 | - | - | 14 | GB | Stone |
| 4 | 91/F | Bacterial pneumonia | 2 | 8 | - | - | 30 | GB | Sludge |
| 5 | 87/F | Bacterial pneumonia | 2 | 11 | - | - | 13 | GB | Sludge |
| 6 | 67/F | Pleuritis | 4 | 7 | - | - | 4 | GB | Sludge |
| 7 | 72/M | Pyelonephritis | 2 | 11 | - | - | 3 | GB | Sludge |
| 8 | 88/M | Bacterial pneumonia | 2 | 10 | - | - | 11 | GB | Sludge |
| 9 | 93/M | Purulent spondylitis | 2 | 6 | - | - | 47 | GB | Sludge |
| 10 | 95/M | Bacterial pneumonia | 4 | 14 | - | - | 13 | GB | Sludge |
| 11 | 89/F | Colon cancer | 2 | 12 | - | - | 7 | GB | Sludge |
| 12 | 70/M | Lung cancer | 2 | 7 | - | + | 13 | GB | Sludge |
| 13 | 79/M | Bronchitis | 2 | 8 | + | - | 15 | GB | Sludge |
| 14 | 84/F | Empyema | 2 | 6 | - | - | 19 | GB | Sludge |
| 15 | 85/F | Bacterial pneumonia | 2 | 5 | - | - | 18 | GB | Sludge |
| 16 | 85/F | Bacterial pneumonia | 2 | 9 | - | - | 10 | GB | Sludge |
| 17 | 71/M | Lung cancer | 2 | 14 | - | - | 12 | GB | Sludge |
| 18 | 82/M | Empyema | 4 | 9 | - | - | 25 | GB | Stone |
| 19 | 77/F | Ileus | 2 | 13 | - | - | 18 | GB | Sludge |
| 20 | 91/F | Bacterial pneumonia | 4 | 11 | - | - | 8 | GB | Sludge |
| 21 | 77/M | Brain abscess | 4 | 34 | - | - | 7 | GB | Sludge |
| 22 | 82/F | Pyelonephritis | 2 | 10 | + | + | 19 | GB | Sludge |
| 23 | 82/F | Bacterial pneumonia | 4 | 24 | - | - | 32 | GB | Sludge |
| 24 | 77/M | Bacterial pneumonia | 2 | 7 | + | + | 7 | GB, CBD | Sludge |
GB: gallbladder, CBD: common bile duct
All patients showed pseudolithiasis in the gallbladder, and 1 (4%) showed pseudolithiasis in both the gallbladder and common bile duct. With regard to the pseudolithiasis pattern, most patients showed sludge, and 2 (8%) showed stones. One patient underwent endoscopic retrograde cholangiopancreatography (ERCP) (patient no. 24). The patient presented with abdominal pain and abnormal bilirubin levels due to cholangitis caused by pseudolithiasis. Abdominal CT revealed pseudolithiasis in the common bile duct. CTRX-associated pseudolithiasis was proven by the detection of a pattern similar to CTRX in the component analysis of the stones extracted by ERCP.
Risk factors for CTRX-associated pseudolithiasis
We examined the risk factors for CTRX-associated pseudolithiasis using a logistic regression analysis (Table 3). On a multivariate analysis, the CTRX dose (OR 4.54, 95% CI 1.36-15.07, p=0.012) and treatment duration (OR 2.80, 95% CI 1.06-8.04, p=0.043) were significantly associated with pseudolithiasis.
Table 3.
Uni- and Multivariate Regression Analyses for Risk Factors of Pseudolithiasis with CTRX Treatment.
| n | Case (%) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |||
| Age | 133 | 24 (18) | 0.98 (0.92-1.04) | 0.426 | ||
| Gender | ||||||
| Male | 67 | 12 (18) | 1 | |||
| Female | 66 | 12 (18) | 1.02 (0.42-2.49) | 0.968 | ||
| BMI | ||||||
| ≤25 kg/m2 | 109 | 19 (17) | 1 | |||
| >25 kg/m2 | 11 | 2 (18) | 1.05 (0.15-4.51) | 0.950 | ||
| CTRX dose | ||||||
| ≤2 g/day | 118 | 17 (14) | 1 | 1 | ||
| >2 g/day | 15 | 7 (47) | 5.20 (1.63-16.42) | 0.004* | 4.54 (1.36-15.07) | 0.012* |
| CTRX treatment duration | ||||||
| ≤7 days | 69 | 7 (10) | 1 | 1 | ||
| >7 days | 64 | 17 (27) | 3.20 (1.27-8.87) | 0.017* | 2.80 (1.06-8.04) | 0.043* |
| CT day | ||||||
| 0≤ day <3 week | 99 | 19 (19) | 1 | |||
| 3≤ day <6 week | 24 | 4 (17) | 0.60 (0.13-1.97) | 0.444 | ||
| 6≤ day <9 week | 10 | 1 (10) | 0.44 (0.02-2.57) | 0.454 | ||
| Fasting | ||||||
| No | 109 | 20 (18) | 1 | |||
| Yes | 24 | 4 (17) | 0.89 (0.24-2.67) | 0.846 | ||
| Bedridden | ||||||
| No | 62 | 12 (19) | 1 | |||
| Yes | 71 | 12 (17) | 0.85 (0.35-2.07) | 0.714 | ||
| Cr | ||||||
| ≤1.5 mg/dL | 107 | 18 (17) | 1 | |||
| >1.5 mg/dL | 26 | 6 (23) | 1.48 (0.49-4.06) | 0.459 | ||
| eGFR | ||||||
| ≥60mL/min/1.73m2 | 46 | 6 (13) | 1 | 1 | ||
| <60mL/min/1.73m2 | 84 | 18 (21) | 1.82 (0.70-5.36) | 0.243 | 2.17 (0.77-7.02) | 0.162 |
CTRX: ceftriaxone, OR: odds ratio, CI: confidence interval, BMI: body mass index, Cr: creatinine, eGFR: estimated glomerular filtration rate, CT day: the day of CT examination after CTRX administration
Influence of total CTRX dose on pseudolithiasis formation
For the prediction of pseudolithiasis formation, the cut-off values of the total dose, treatment duration, and dose of CTRX administration were determined using an ROC curve analysis (Fig. 2). The total CTRX dose had the highest predictive values, with an AUC of 0.746. In contrast, the treatment duration and dose had lower predictive values, with AUCs of 0.706 and 0.648, respectively. The optimal cut-off value of the total CTRX dose associated with pseudolithiasis formation was 19 g.
Figure 2.

Receiver operating characteristic (ROC) curve comparing the total dose, duration, and dose for the prediction of pseudolithiasis formation in elderly people treated with CTRX. AUC: area under the curve, CI: confidence interval, CTRX: ceftriaxone, ROC: receiver operating characteristic
Using this cut-off value, we determined that the frequency of pseudolithiasis formation was higher in patients treated with a total CTRX dose of >19 g (31%, 15/48) than in those who received ≤19 g in total (11%, 9/85) (p=0.003). A logistic regression analysis determined that pseudolithiasis formation was increased in patients treated with a total CTRX dose of >19 g (OR 3.84, 95% CI 1.55-9.99, p=0.004) (Table 4). The effect of the total CTRX dose on pseudolithiasis formation persisted after several adjustments (OR 4.75, 95% CI 1.66-15.01, p=0.005) (Table 4).
Table 4.
Multivariate and IPTW Logistic Odds Ratio of Pseudolithiasis Formation Associated with Total CTRX Dose.
| Odds ratio (95% CI) | p value | |
|---|---|---|
| Unadjusted | 3.84 (1.55-9.99) | 0.004 |
| Adjusted for age, sex, BMI, fasting, Bedridden, eGFR, CT day |
4.75 (1.66-15.01) | 0.005 |
| IPTW | 4.06 (1.45-11.32) | 0.008 |
IPTW: inverse probability of treatment weighting, CTRX: ceftriaxone, CI: confidence interval, BMI: body mass index, eGFR: estimated glomerular filtration rate, CT day: the day of CT examination after CTRX administration
In addition, after adjusting the model for differences related to background factors using the IPTW method, we determined that the likelihood of pseudolithiasis formation was increased in cases with a total CTRX dose of >19 g (OR 4.06, 95% CI 1.45-11.32, p=0.008) (Table 4).
Discussion
In the present study, the incidence rate of pseudolithiasis induced by CTRX was high in elderly people, and the CTRX dose and CTRX treatment duration were significant risk factors for pseudolithiasis. In addition, the optimal cut-off value of total CTRX dose associated with pseudolithiasis formation was 19 g. After adjusting for other confounding factors using the IPTW method based on propensity scores, a total dose of >19 g was found to increase the likelihood of pseudolithiasis formation in elderly people treated with CTRX.
CTRX, a third-generation cephalosporin, is widely used in the treatment of various infectious diseases because of its long half-life (8 hours) and good tissue transmission. Approximately 85-95% of CTRX in blood is bound to albumin, with approximately 60% of the unchanged drug excreted in the urine and approximately 40% excreted in the bile. The concentration of CTRX in bile reaches 20-150 times that in blood (3,10). In addition, CTRX has a high affinity for calcium ions and easily forms calcium stones (14). A previous in vitro study reported that a dose of ≥2 g/day causes precipitation of calcium-CTRX complexes (10). Furthermore, CTRX reportedly inhibits bile acid excretion and increases the calcium ion concentration in bile (14). In addition, an animal experiment showed that CTRX reduces gallbladder contractility (15).
CTRX-associated pseudolithiasis is usually asymptomatic and reversible after the discontinuation of CTRX (16). Therefore, failure to diagnose pseudolithiasis may lead to unnecessary treatment. However, complications such as cholecystitis, cholangitis, and pancreatitis can occur, which may require biliary drainage (5) and surgical resection (6). When discontinuation of CTRX does not improve the symptoms, treatment according to the condition is needed. Clinicians should consider pseudolithiasis when abdominal symptoms appear or when elevated biliary system enzymes are detected and should provide prompt and appropriate treatment. In the present study, we found 1 case of cholangitis requiring biliary drainage via ERCP (4%, 1/24). Pseudolithiasis mainly showed a high-density sludge pattern on CT (17). Most of the pseudolithiasis cases found in this study showed a sludge pattern, and the stone extracted by ERCP also showed a sludge-like appearance. Although less common than pseudolithiasis, the formation of nephrolithiasis is also a notable complication during treatment with CTRX (18). The incidence of nephrolithiasis, including small asymptomatic stones, is reported to be 0.6-8.3% (19).
Many studies on CTRX-associated pseudolithiasis have been reported in children. Some have been performed in adults, but no study has been limited to elderly people. The incidence rate of pseudolithiasis is expected to be high in elderly people because they often have conditions that are reported risk factors for pseudolithiasis formation, such as dehydration, a bedridden status, and renal dysfunction (7,8). In particular, reduced gallbladder contractility is believed to be involved in the development of gallstones (10,20). Elderly people are more likely to have decreased dietary intake and to be bedridden, which reduce the contractility of the gallbladder and consequently increase the risk of pseudolithiasis formation. In fact, in the present study limited to elderly people, a high incidence rate of pseudolithiasis (18%) was found.
Table 5 summarizes the studies on pseudolithiasis in adults thus far, as searched in PubMed, including the present study in elderly people. The reported incidence rates (3-25%) revealed that, as in children, pseudolithiasis is not uncommon in adults. With respect to risk factors, in the present study, the CTRX dose and CTRX treatment duration were significant. An older age (18), female sex (8,22), and renal dysfunction (8) were reported as other significant risk factors in previous studies; however, no significant association with pseudolithiasis was observed for these factors in the present study. Because previous studies have reported various significant risk factors, evidence concerning risk factors for pseudolithiasis remains insufficient. Using an ROC curve analysis, we determined that the optimal cut-off value of the total CTRX dose associated with pseudolithiasis formation was 19 g. After determining the cut-off value, we demonstrated that a total CTRX dose of >19 g was associated with increased pseudolithiasis formation. In addition, we evaluated the incidence of pseudolithiasis formation with respect to the cut-off value using the IPTW method with propensity scoring (12,13). In this study, selection bias may still be present because the relationship between pseudolithiasis formation and the total CTRX dose may have been affected by confounding factors, including the age, sex, eGFR, and CT day. The IPTW method, which is based on propensity scores and can be applied without reducing the sample size, was used to assess the sensitivity of the results and to evaluate the causal effects statistically, free from confounding effects. After adjusting for background factors using the IPTW method, we demonstrated that the likelihood of pseudolithiasis formation increased in association with a total CTRX dose of >19 g. This is the first study to use the IPTW method to evaluate the relationship between pseudolithiasis formation and the total CTRX dose.
Table 5.
Seven Studies of Pseudolithiasis with CTRX Treatment in Adults and This Study in Elderly People.
| Study | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | 121 | 222 | 311 | 418 | 58 | 623 | 717 | This study |
| Study method | Pro | Pro | Pro | Pro | Retro | Retro | Retro | Retro |
| Pseudolithiasis (%) | 5 | 21 | 25 | 7 | 3 | 6 | 8 | 18 |
| Number of patients | 40 | 28 | 20 | 84 | 478 | 211 | 208 | 133 |
| Mean age (years) | 51 | 40 | 56 | 55 | 65 | 69 | NR | 83 |
| Mean CTRX dose (g/day) | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 |
| Range of days to | 10-14 | 14 | 4-17 | NR | 2-28 | 9-41 | 4-32 | 3-47 |
| Pseudolithiasis formation | ||||||||
| Significant risk factor | NR | Female sex |
CTRX dose CTRX treatment duration |
Age | Renal dysfunction Female sex |
Hemodialysis CTRX dose |
NR | CTRX dose CTRX treatment duration |
CTRX: ceftriaxone, Pro: prospective, Retro: retrospective, NR: not reported
As the number of elderly patients with infectious diseases is expected to continue to increase, CTRX will likely be more frequently used because of its long half-life and good tissue transmission. As elderly people tend to have few complaints and many complications, such as dehydration, pseudolithiasis formation tends to develop unnoticed until it becomes severe. Therefore, clinicians should be fully aware of the potential for pseudolithiasis formation in elderly people treated with CTRX.
Several limitations associated with the present study warrant mention. First, this was a retrospective study with a relatively small number of patients, and all patients were recruited from a single institution. Second, the time point of the post-treatment CT examination varied. In this study, post-treatment CT was defined as CT performed within nine weeks after CTRX administration. Pseudolithiasis might have formed and have already spontaneously disappeared by the time post-treatment CT was performed. Therefore, it is possible that many pseudolithiasis cases were overlooked in this study. In addition, the time to detect pseudolithiasis means the length of time from CTRX administration to CT day; however, the exact number of days of pseudolithiasis formation within that period could not be determined. We used the IPTW method to reduce the confounding effect from differences in the CT timing, as we evaluated the incidence of pseudolithiasis formation based on the cut-off value. Because post-treatment CT was mainly performed as a follow-up examination for the disease that necessitated the hospitalization regardless of pseudolithiasis, there are limitations in regard to the CT timing. Third, the CT findings were not diagnosed by the same radiologist, so there may have been some bias in the diagnosis. Further prospective studies with a large sample size will be required to resolve these issues.
In conclusion, the findings of this study suggest that the incidence rate of pseudolithiasis induced by CTRX is high in elderly people and that the CTRX dose and CTRX treatment duration are significant risk factors for pseudolithiasis formation. Furthermore, the risk of pseudolithiasis formation is increased in cases with a total CTRX dose >19 g.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Tsutomu Maruhama, Hideo Kurosawa from Higashisumiyoshi Morimoto Hospital for their assistance with the data collection.
References
- 1. Schaad UB, Tschäppeler H, Lentze MJ. Transient formation of precipitations in the gallbladder associated with ceftriaxone therapy. Pediatr Infect Dis 5: 708-710, 1986. [DOI] [PubMed] [Google Scholar]
- 2. Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet 332: 1411-1413, 1988. [DOI] [PubMed] [Google Scholar]
- 3. Brogard JM, Blickle JF, Jehl F, Arnaud JP, Paris-Bockel D, Monteil H. High biliary elimination of ceftriaxone in man. Int J Clin Pharmacol Ther Toxicol 26: 167-172, 1988. [PubMed] [Google Scholar]
- 4. Riccabona M, Kerbl R, Schwinger W, Spork D, Millner M, Grubbauer HM. Ceftriaxone-induced cholelithiasis--a harmless side-effect? Klin Padiatr 205: 421-423, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Lebovics E, Halata MS, Rosario JA, Lantin J, Schwarz SM, Rosenthal WS. Endoscopic management of ceftriaxone pseudolithiasis involving the common bile duct and gallbladder. Gastrointest Endosc 40: 246-248, 1994. [DOI] [PubMed] [Google Scholar]
- 6. Famularo G, Polchi S, De Simone C. Acute cholecystitis and pancreatitis in a patient with biliary sludge associated with the use of ceftriaxone: a rare but potentially severe complication. Ann Ital Med Int 14: 202-220, 1999. [PubMed] [Google Scholar]
- 7. Murata S, Aomatsu T, Yoden A, Tamai H. Fasting and bed rest, even for a relatively short period, are risk factors for ceftriaxone associated pseudolithiasis. Pediatr Int 57: 942-946, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Imafuku A, Sawa N, Sekine A, et al. Risk factors of ceftriaxone-associated biliary pseudolithiasis in adults: influence of renal dysfunction. Clin Exp Nephrol 22: 613-619, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Becker CD, Fischer RA. Acute cholecystitis caused by ceftriaxone stones in an adult. Case Rep Med 2009: 1-2, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiffman ML, Keith FB, Moore EW. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology 99: 1772-1778, 1990. [DOI] [PubMed] [Google Scholar]
- 11. Pigrau C, Pahissa A, Gropper S, Sureda D, Martinez Vazquez JM. Ceftriaxone-associated biliary pseudolithiasis in adults. Lancet 2: 165, 1989. [DOI] [PubMed] [Google Scholar]
- 12. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 32: 2837-2849, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med 23: 2937-2960, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Park HZ, Lee SP, Schy AL. Ceftriaxone associated gallbladder sludge. Identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology 100: 1665-1670, 1991. [DOI] [PubMed] [Google Scholar]
- 15. Arpacik M, Ceran C, Kaya T, Karadas B, Sarac B, Koyluoğlu G. Effects of ceftriaxone sodium on in vitro gallbladder contractility in guinea pigs. J Surg Res 122: 157-161, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Palanduz A, Yalçin I, Tonguç E, et al. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound 28: 166-168, 2000. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida R, Yoshizako T, Katsube T, Kitagaki H. Computed tomography findings of ceftriaxone-associated biliary pseudocholelithiasis in adults. Jpn J Radiol 37: 826-831, 2019. [DOI] [PubMed] [Google Scholar]
- 18. Azarkar G, Birjand MM, Ehsanbakhsh A, Bijari B, Abedini MR, Ziaee M. Ceftriaxone-associated nephrolithiasis and gallstone in adults. Drug Healthc Patient Saf 10: 103-108, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Youssef DM, Sherief LM, Sherbiny HS, et al. Prospective study of nephrolithiasis occurrence in children receiving ceftriaxone. Nephrology (Carton) 21: 432-437, 2016. [DOI] [PubMed] [Google Scholar]
- 20. Bolondi L, Gaiani S, Testa S, Labò G. Gall bladder sludge formation during prolonged fasting after gastrointestinal tract surgery. Gut 26: 734-738, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cometta A, Gallot-Lavallee-Villars S, Iten A, et al. Incidence of gallbladder lithiasis after ceftriaxone treatment. J Antimicrob Chemother 25: 689-695, 1990. [DOI] [PubMed] [Google Scholar]
- 22. Heim-Duthoy KL, Caperton EM, Pollock R, Matzke GR, Enthoven D, Peterson PK. Apparent biliary pseudolithiasis during ceftriaxone therapy. Antimicrob Agents Chemother 34: 1146-1149, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ubukata M, Ohsawa I, Suzuki H, et al. Hemodialysis as a risk factor for ceftriaxone-associated pseudolithiasis in adults. Ther Apher Dial 24: 393-399, 2019. [DOI] [PubMed] [Google Scholar]


