Abstract
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is a rare progressive neurodegenerative disease caused by either homozygous or compound heterozygous mutations in the SACS gene. The original ARSACS cases found in Quebec showed very homogenous phenotypes characterized by cerebellar ataxia, spasticity, and polyneuropathy. However, many cases with atypical phenotypes have been found in other regions and ethnic groups. We herein present a Japanese patient with atypical ARSACS who showed cerebellar ataxia and polyneuropathy, but no spasticity. She carried novel compound heterozygous mutations (p.Lys4326Glu and p.Leu1412Lysfs*16) in the SACS gene. The brain MRI findings were useful for making a diagnosis of ARSACS.
Keywords: ARSACS, SACS, spasticity, cerebellar ataxia, MRI
Introduction
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is a rare neurodegenerative disease originally described in the French-Canadian population derived from the regions of Charlevoix and Saguenay-Lac-Saint-Jean of Quebec (1). In the originally reported cases, clinical features were remarkably homogenous and included progressive gait disturbance appearing during the ‘toddler’ stage, cerebellar ataxia, spasticity, peripheral neuropathy, distal muscle wasting, foot deformity, and retinal striation (hypermyelination of retinal nerve fibers) on fundoscopy (1). In 2000, the causative gene for ARSACS, named SACS, was identified (2). In the Quebec cases, two major SACS gene mutations, c.8844delT (p.Ile2949Phefs*4) and c.7504 C>T (p.Arg2502*), were identified in 92,6% and 3.7% of the total pathogenic alleles, respectively (2,3). The gene encodes a large, 4,579 amino acid protein named sacsin, which is highly expressed in neurons, particularly within brain motor systems, including cerebellar Purkinje cells (4). Sacsin is a multi-domain protein containing a ubiquitin-like domain at the N-terminus and a functional DnaJ domain very close to the C-terminus. The DnaJ domain is the defining component of the DnaJ/heat shock protein (Hsp) 40 class chaperones. Therefore, sacsin is thought to have co-chaperone activity and it also integrates the ubiquitin-proteasome system and Hsp70 chaperone-mediated protein folding (4).
At first, ARSACS was considered to be confined to the Quebec area as a result of the founder effect. However, after discovery of the SACS gene, over 200 pathogenic mutations have been described in patients with ARSACS outside of Quebec. It is now known that ARSACS patients are distributed worldwide and they are not limited to a particular ethnic group. In addition, greater phenotypic variability of ARSACS has been reported in the non-Quebec cases (5,6).
We herein present a patient with ARSACS harboring novel compound heterozygous mutations in the SACS gene. This patient exhibited neither spasticity nor hypermyelination of the retinal nerve fibers. The phenotype of this patient was atypical for the classical ARSACS observed in Quebec, and it induced a delay in the clinical diagnosis. However, the brain MRI findings were characteristic of ARSACS.
Case Report
A 44-year-old Japanese woman with childhood-onset slowly progressive gait disturbance and cerebellar ataxia was referred to our neurology department. She had demonstrated no obvious symptoms in infancy. The patient began to walk at the age of 14 months without any clumsiness. Although the exact age of onset was unclear, she was less good at exercising than other children at elementary school. As a junior high school student, she could walk for over an hour but could not ride a bicycle without training wheels. The patient received good grades at both high school and junior college, thus indicating normal intelligence. At 22 years of age, she visited a hospital because of progressive gait disturbance and was diagnosed with spinocerebellar ataxia (SCA). At 29 years of age, she needed a cane to walk. Thereafter, the patient became wheelchair-bound from the age of 32 years, when an examination revealed increased patellar tendon reflexes and positive Babinski signs.
At the referring hospital, SCA1, SCA2, SCA3, SCA6, SCA7, SCA8, SCA10, SCA17, and SCA31 were ruled out based on the results of genetic testing. Thus, the patient was clinically diagnosed with SCA of unknown etiology. Her parents were not consanguineous. There was no significant family history of any neurologic disorders.
Neurological examinations conducted at our hospital revealed saccadic eye movement, nystagmus, scanning speech, cerebellar ataxia of the upper and lower extremities. Distal dominant muscle atrophy and weakness of lower extremities was evident. Her muscle tone was not spastic, but rather hypotonic in all extremities. The deep tendon reflexes were either diminished or absent in the lower extremities. Babinski signs were negative. Her bladder and rectal function were normal. Mild pes cavus was confirmed; however, intrinsic hand muscle wasting was not observed. Her cognitive function was normal.
Hypermyelination of the retinal nerve fibers was not observed by fundoscopy.
In nerve conduction studies, no sensory nerve action potentials were evoked in the ulnar, median, and sural nerves. Compound muscle action potential (CMAP) was not evoked in the tibial nerve. In the ulnar and median nerves, the amplitudes of CMAP were moderately reduced and the motor nerve conduction velocities were slightly decreased. A detailed biochemical evaluation for ataxia and polyneuropathy, including serum albumin and vitamin E, was normal.
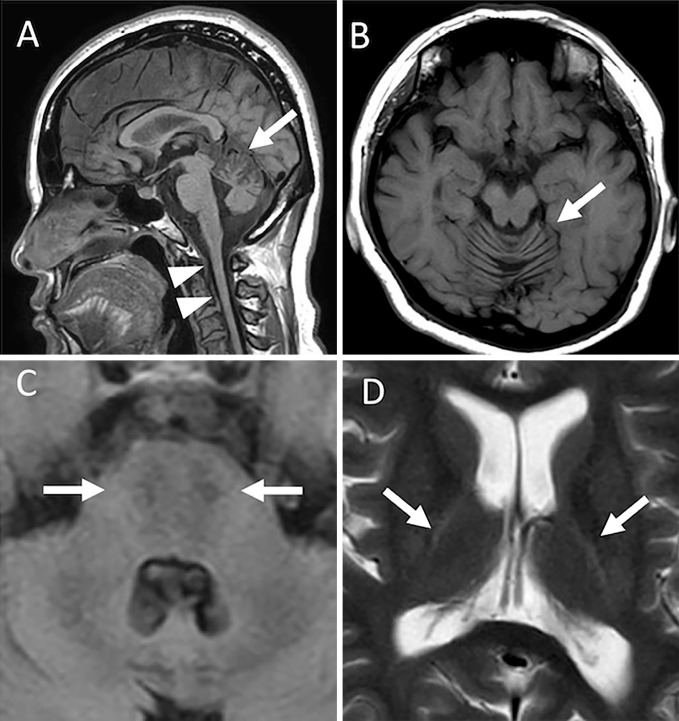
Brain and cervical T1-weighted images (T1W1) of magnetic resonance imaging (MRI) revealed atrophy of the cerebellum, especially in the upper vermis, and the cervical spinal cord (Fig. 1A, B). Axial fluid-attenuated inversion recovery (FLAIR) images showed linear hypointensities in the pons (Fig. 1C). Axial T2-weighted images (T2WI) displayed linear hyperintensities around the thalami (Fig. 1D).
Figure 1.
Magnetic resonance image findings of the brain and cervical spinal cord. A: Midsagittal T1-weighted image showing atrophy of the cerebellar superior vermis (arrow) and upper cervical cord (arrow head). B: An axial T1-weighted image showing atrophy of the vermis of the cerebellum. C: An axial fluid-attenuated inversion recovery image showing footprint shaped hypointensities in the pons (arrow). D: An axial T2-weighted image showing linear hyperintensities around the thalami (arrow).
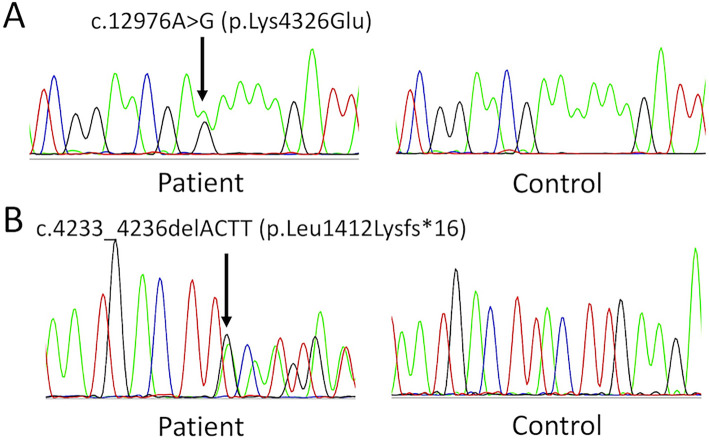
After obtaining the patient's written informed consent, we analyzed the exons of the genes related to spinocerebellar degeneration by next-generation sequencing (NGS) (Illumina, TruSight One). The gene analysis protocol was approved by the institutional ethics committee of the National Hospital Organization, Niigata National Hospital. The results revealed this patient to have a compound heterozygote for a missense mutation (NM_014363.5:c.12976A>G, p.Lys4326Glu) and a frameshift mutation (NM_014363.5:c.4233-4236delACTT, p.Leu1412Lysfs*16) in the SACS gene (Fig. 2). To evaluate the pathogenicity of the missense mutation p.Lys4326Glu in silico, we used two computational prediction tools, PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) and SIFT (http://sift.jcvi.org). These algorithms predicted a pathogenic effect of the mutation (PolyPhen-2 score 0.997, SIFT score 0.017). Based on these characteristic MRI findings and the results of genetic testing, we diagnosed the patient to have ARSACS.
Figure 2.
Results of the SACS gene mutation analysis. Electropherograms of a novel missense mutation p.Lys4326Glu, which is located within the DnaJ domain (A) and a very rare frameshift mutation p.Leu1412Lysfs*16, which was first found in a patient with autosomal recessive spastic ataxia of Charlevoix-Saguenay (B).
Discussion
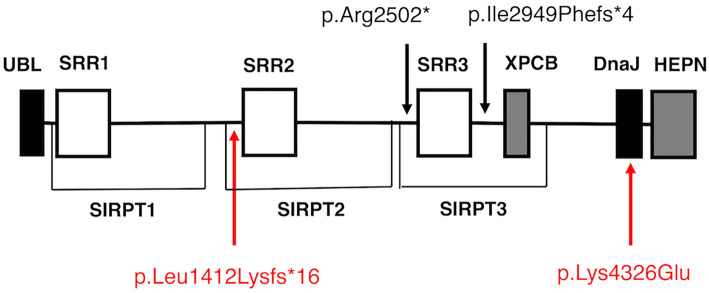
We herein report a Japanese woman with ARSACS harboring novel compound heterozygous mutations in the SACS gene. A multi-gene panel analysis using NGS confirmed the diagnosis. To our knowledge, p.Lys4326Glu is a novel missense mutation that is located within helix II of the DnaJ domain. Since the lysin at position 4326 is highly conserved across species, it is likely that the substitution of lysin to glutamic acid at this position has a significant effect on the function of sacsin. The other mutation, p.Leu1412Lysfs*16, is a very rare variant listed in the Genome Medical Alliance-Japan Whole Genome Aggregation, an open variant panel of the Japanese population (TogoVar ID, tgv110704992, allele frequency is 1/15,192). This frameshift mutation is located just upstream of the sacsin repeating region 2 (SRR2) and it is presumed to disrupt sacsin synthesis at that position (Fig. 3).
Figure 3.
Domain organization of sacsin and the location of the selected SACS gene mutations. Sacsin contains a ubiquitin-like (UBL) domain at the N-terminus and a DnaJ domain immediately followed by a higher eukaryotes and prokaryotes nucleotide-binding (HEPN) domain at the C-terminus. Three repeated sequences, termed sacsin repeating regions (SRR), and a xeroderma pigmentosum group C binding (XPCB) domain exist between the UBL domain and the DnaJ domain. Besides SRR, sacsin internal repeat (SIRPT) supradomain, a larger homologous repeating architecture including SRR, was also proposed. Two SACS gene mutations identified in this case, p.Leu1412Lysfs*16 and p.Lys4326Glu, are located just before the SRR2 and within the DnaJ domain, respectively. The two major founder mutations identified in over 95% of the Quebec cases, p.Ile2949Phefs*4 and p.Arg2502*, exist across SRR3.
The phenotype of this patient was not typical of the classical phenotype of ARSACS described in Quebec cases, but it was consistent with the milder atypical forms of ARSACS often seen in non-Quebec cases. It is noteworthy that this patient did not show spasticity in the lower limbs on neurological examinations. Spasticity is an element of the clinical triad (cerebellar ataxia, spasticity, and peripheral neuropathy) of classical ARSACS. In classical ARSACS, spasticity progresses with the course of the disease and it is normally observed in older patients. However, several cases without spasticity have been reported in Japan and other countries (5-8). We investigated the genotypes of these reported cases but found no commonality between them (Table). In this case, the masking effect caused by both severe axonal sensori-motor neuropathy with mild demyelinating features and advanced distal dominant muscular atrophy in lower extremities might be a possible explanation for the absence of spasticity (7-9). However, the absence of spasticity cannot be simply explained by the masking effect because a case lacking both spasticity and neuropathy has been reported (6).
Table.
Genotypes of the ARSACS Patients without Spasticity.
| Case | Genotype | Country (Origin) | AAO (years) | AAE (years) | Peripheral neuropathy |
Reference |
|---|---|---|---|---|---|---|
| 1 | p.Phe304Ser/p.Phe304Ser | Japan | <10 | 30 | + | 7 |
| 2 | p.Phe304Ser/p.Phe304Ser | Japan | early childhood | 32 | + | 7 |
| 3 | p.Ser4007Phe/p.Ser4007Phe | Japan | <2 | 54 | + | 8 |
| 4 | p.Cys72Cysfs*4/{p.Arg3636Gln; p.Pro3652Thr} | Belgium | >25 | 52 | + | 5 |
| 5 | p.Arg1575Pro/p.Ser2032del | Belgium (Serbia) | 3 | 23 | + | 5 |
| 6 | p.Ile3848del/p.Ile3848del | Turkey | 3 | 13 | + | 6 |
| 7 | p.Leu796Tyrfs*13/p.Leu3995dup | German | 2 | 25 | + | 6 |
| 8 | p.Arg2426Pro/p.Tyr2975Phefs*29 | German | 2 | 6 | + | 6 |
| 9 | p.Thr4581Ile/p.Thr458Ile (VUS) | German | 30 | 44 | + | 6 |
| 10 | p.Leu1412Lys*16/p.Lys4326Glu | Japan | <7 | 44 | + | This case |
Case 1 and case 2 are siblings. AAO: age at onset, AAE: age at examination, VUS: variant of unknown significance
In the present case, the atypical clinical features caused a delay in the diagnosis of ARSACS. However, brain MRI showed highly characteristic findings for ARSACS, such as atrophy of the cerebellar vermis, linear T2WI hypointensity on either side of the midline in the pons, and peri-thalamic linear T2WI hyperintensity (10,11). These brain MRI findings reinforced the results of the genetic diagnosis.
Conclusion
An increasing number of non-Quebec ARSACS cases have expanded the clinical and genetic spectrum of this disease. As a result, it is now known that the absence of spasticity cannot rule out such a diagnosis. Our findings demonstrated the diagnostic usefulness of a multi-gene analysis by NGS together with brain MRI in an ARSACS patient with atypical clinical features.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank the patient and her family, who provided the data for this report.
References
- 1. Bouchard JP, Barbeau A, Bouchard R, Bouchard RW. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can J Neurol Sci 5: 61-69, 1978. [PubMed] [Google Scholar]
- 2. Engert JC, Bérubé P, Mercier J, et al. ARSACS, a spastic ataxia common in northeastern Québec, is caused by mutations in a new gene encoding an 11.5-kb ORF. Nat Genet 24: 120-125, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Thiffault I, Dicaire MJ, Tetreault M, et al. Diversity of ARSACS mutations in French-Canadians. Can J Neurol Sci 40: 61-66, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Parfitt DA, Michael GJ, Vermeulen EG, et al. The ataxia protein sacsin is a functional co-chaperone that protects against polyglutamine-expanded ataxin-1. Hum Mol Genet 18: 1556-1565, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baets J, Deconinck T, Smets K, et al. Mutations in SACS cause atypical and late-onset forms of ARSACS. Neurology 75: 1181-1188, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Synofzik M, Soehn AS, Gburek-Augustat J, et al. Autosomal recessive spastic ataxia of Charlevoix Saguenay (ARSACS): expanding the genetic, clinical and imaging spectrum. Orphanet J Rare Dis 8: 41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimazaki H, Takiyama Y, Sakoe K, Ando Y, Nakano I. A phenotype without spasticity in sacsin-related ataxia. Neurology 64: 2129-2131, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Miyatake S, Miyake N, Doi H, et al. A novel SACS mutation in an atypical case with autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). Intern Med 51: 2221-2226, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Takiyama Y. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Neuropathology 26: 368-375, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Prodi E, Grisoli M, Panzeri M, et al. Supratentorial and pontine MRI abnormalities characterize recessive spastic ataxia of Charlevoix-Saguenay. A comprehensive study of an Italian series. Eur J Neurol 20: 138-146, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Shimazaki H, Takiyama Y, Honda J, et al. Middle cerebellar peduncles and pontine T2 hypointensities in ARSACS. J Neuroimaging 23: 82-85, 2013. [DOI] [PubMed] [Google Scholar]