Abstract
Purpose
Osteoporosis has detrimental consequences for frail older adults. The effects on those with both osteoporosis and cognitive impairment are compounded due to increased risk of falls and changes in mobility, both of which can lead to fracture. However, there are limited data on treatment benefits for osteoporotic individuals with cognitive impairment.
Methods
This post-hoc, secondary analysis of data from a randomized, double-blind, placebo-controlled clinical trial of single dose zoledronic acid included 179 women age ≥ 65 years residing in assisted living facilities or nursing homes, 43 of whom had mild to severe cognitive impairment. We assessed bone mineral density (BMD) of the total hip, femoral neck, and lumbar spine by dual-energy x-ray absorptiometry and serum bone turnover markers (C-terminal crosslinking telopeptide of type I collagen and procollagen type I N propeptide) at 6 and 12 months.
Results
In participants with cognitive impairment, those who received zoledronic acid had 4.3% greater BMD at the total hip (p=.005) and 5.3% greater BMD at the femoral neck (p<001) after 12 months compared to those in the placebo group. Bone turnover markers demonstrated significant decreases at 6 months in those with cognitive impairment who received active treatment compared to the placebo group. Improvements in bone health measures with zoledronic acid were similar to those seen in participants without cognitive impairment.
Conclusion
Zoledronic acid improves bone health in frail older women with cognitive impairment similar to those without impairment. Further studies are warranted to assess the benefit for fracture reduction in this undertreated population.
Keywords: Zoledronic acid, Cognitive impairment, Bone density, Osteoporosis
Mini Abstract
Fracture prevention in cognitively impaired individuals is lacking. This work highlights the benefits of zoledronic acid on bone health in cognitively impaired older adults. Demonstrating benefits of therapy may increase treatment uptake and reduce fracture risk in this group.
INTRODUCTION
Osteoporosis is a common and costly disease with the greatest impact on frail older adults. There are over 1.5 million osteoporotic fractures in the US annually [1] and approximately one-third of women and 1 out of 6 men will have a fracture by age 90 [2]. Fractures have detrimental effects on mortality, morbidity, and independence. Mortality is 12-20% higher in individuals with hip fractures compared to those without [2]. Hip fractures are also associated with long-term care placement and an increased need for assistive devices to maintain mobility [2]. The financial burden is significant due to hospitalization and post-fracture costs for the roughly 500,000 patients hospitalized annually for fractures [1]
Patients with both osteoporosis and cognitive impairment are particularly at risk for fracture-related consequences. Studies have shown that Alzheimer’s disease, a common cause of cognitive impairment, can disrupt bone homeostasis and remodeling [3]. Individuals with Alzheimer’s disease are at a two-times greater risk of falls given the disease’s impact on coordination [4]. Falls can be devastating for an osteoporotic individual who is already at risk for fracture. We have previously demonstrated that a fall, low bone density and poor mobility contribute to hip fractures for geriatric patients [5]. Bonafede and colleagues [6] examined patient characteristics contributing to imminent risk for fracture in the ensuing 12 months and reported that a history of falls increased the odds of imminent fracture in the subsequent year over 6-fold and Alzheimer’s disease increased the odds by 35%.
There are limited data on the efficacy of osteoporosis treatments in patients with Alzheimer’s disease or cognitive impairment. This is consequential given the potential relationship between Alzheimer’s disease and bone health [3]. Low bone mineral density (BMD) and Alzheimer’s disease may be connected via multiple pathways including one pertaining to tauopathy effects on the dorsal raphe nucleus (DRN), which is pivotal in parasympathetic promotion of bone formation [3]. Diminished function in the DRN may lead to increased osteoclast activity via a sympathetic response that is without its parasympathetic regulation [3].
We may then consider appropriate interventions in these individuals, including bisphosphonates given the hypothesized effects on osteoclast activity in Alzheimer’s disease. However, the large pivotal trials for osteoporosis treatment routinely exclude these patients. Two previous trials with risedronate for hip fracture reduction in patients with Alzheimer’s disease were retracted from publication [7,8]. A recent secondary analysis of data from the Horizon Recurrent Fracture Trial reported that fracture reduction with zoledronic acid was maintained in patients with cognitive impairment presenting with a hip fracture [9]. However, mental status was assessed by the Short Portable Mental Status Questionnaire (SPMSQ) collected in hospital before hip repair. The authors acknowledge that patients may have had confusion or delirium prior to surgery when the questionnaire was performed. Because the average life expectancy from the diagnosis of cognitive impairment or Alzheimer’s disease is 8-10 years but can range up to 20 years [10]. it is imperative to know if osteoporosis treatment is safe and effective in this vulnerable cohort to maintain quality of life.
The goal of this secondary analysis was to examine frail older women with cognitive impairment, previously enrolled in a randomized, double-blind, placebo-controlled clinical trial with zoledronic acid for osteoporosis, to determine if those with cognitive impairment responded in a similar manner to those without cognitive impairment.
METHODS
Study Design and Intervention
We performed a post-hoc secondary analysis using data from the previously completed Zoledronic acid in frail Elders to STrengthen bone (ZEST) study [11]. Briefly, this study was a randomized, double-blinded, and placebo-controlled trial that assessed efficacy and safety following administration of a single 5 mg intravenous dose of zoledronic acid. Participants were frail women aged 65 years and older residing in long-term care facilities who met osteoporosis treatment guidelines based on BMD and/or history of fracture [11]. Individuals currently on osteoporosis therapy, those with limited life duration (less than two years), and those with impaired renal function (estimated GFR less than 30 ml/min) were excluded [11]. Outcome variables included BMD of the hip and spine and markers of bone turnover. We assessed baseline physical function with the Nursing Home Physical Performance Test [12] and self-reported function with Instrumental Activities of Daily Living [13] and Katz Activities of Daily Living [14] questionnaires. All participants provided informed consent or, in the case of cognitive impairment, provided assent with informed consent obtained from their representative [11].
Cognitive Assessment
Assessments for cognitive function were performed at baseline prior to randomization using the 10-item Short Portable Mental Status Questionnaire (SPMSQ) [15]. Participants were categorized as having no (0-2 errors), mild (3-4 errors), moderate (5-7 errors), or severe cognitive impairment (8 or more errors) based on the number of incorrect responses [15]. For our analysis, we considered a score of 3 or greater to indicate cognitive impairment.
Bone Mineral Density and Bone Turnover Markers
Bone health indicators were assessed at baseline, 6-month, and 12-month time points as previously described [11]. BMD was determined using dual energy x-ray absorptiometry (DXA; Discovery densitometer, Hologic Inc., Bedford, MA) at various skeletal sites including the total hip, femoral neck, and lumbar spine (posterior-anterior projection) with precision errors of 1.2% (hip), 1.5% (spine), and 1.9% (femoral neck) [16]. Serum markers of bone turnover included the bone resorption marker C telopeptide crosslinks type I collagen (CTX; Crosslaps, Osteometer Biotech, Herlev, Denmark) and the bone formation marker N-terminal propeptide type I procollagen (PINP; Orion Diagnostica, Espoo, Finland).
Statistical Analyses
We used appropriate descriptive statistics to summarize participant characteristics and baseline to 6- and 12-month changes. To draw main conclusions, we fitted a series of linear mixed models with baseline to follow-up (percent) change in each of the BMD and marker measures as the dependent variable; cognitive status (unimpaired/impaired), treatment group (zoledronic acid/placebo), follow-up time (6/12 months) and their interaction as factors of interest; baseline value of the measure as a covariate; and a participant random effect to account for multiple observations from each person. We used appropriately constructed contrasts to estimate zoledronic acid vs placebo treatment effect at 6 and 12 months separately within those impaired and unimpaired, and assess a potential differential treatment effect between impaired and unimpaired. SAS® version 9.4 (SAS Institute, Inc., Cary, North Caroline) was used for analysis.
RESULTS
Participants
Baseline characteristics of participants with (n=43) and without (n=136) cognitive impairment are detailed in Table 1. The average age was 85 years. Baseline body mass index and comorbidity burden were similar between those with and without cognitive impairment and for those receiving active treatment and placebo. BMD of the lumbar spine, total hip and femoral neck were similar between those with cognitive impairment and those without; as well as those who received active treatment and those who did not. Ten participants died and another 9 did not complete (Online Resource 2) due to moving (7), refusal (1) and loss to follow-up (1).
Table 1:
Participant Characteristics at Baseline [mean ± standard deviation or N (%)]
| Participant Characteristic | Cognitively Impaired (N=43) | Cognitively Unimpaired (N=136) | ||||
|---|---|---|---|---|---|---|
| Zoledronic Acid (N=21) | Placebo (N=22) | p-Value | Zoledronic Acid (N=67) | Placebo (N=69) | p-Value | |
| Age (years) | 85.9±6.1 | 84.7±5.7 | 0.517 | 85.1±5.1 | 85.7±4.7 | 0.484 |
| BMI (kg/m2) | 28.3±5.0 | 26.7±5.1 | 0.304 | 28.1±5.7 | 26.9±5.1 | 0.179 |
| ADL (0-14 points)a | 10.4±3.1 | 11.0±3.0 | 0.509 | 11.8±2.2 | 12.0±2.1 | 0.548 |
| IADL (0-14 points)a | 4.7±3.1 | 5.9±3.0 | 0.201 | 8.7±3.8 | 9.0±3.6 | 0.673 |
| Comorbidity Index (0-8 domains)b | 3.1±1.2 | 2.9±1.4 | 0.560 | 3.6±1.5 | 3.4±1.3 | 0.500 |
| NHPPT (0-24 points)a | 17.6±3.5 | 18.0±5.5 | 0.781 | 19.8±4.5 | 20.8±3.9 | 0.155 |
| Lumbar spine BMD (g/cm2) | 0.96±0.21 | 0.98±0.18 | 0.713 | 0.92±0.18 | 0.96±0.20 | 0.204 |
| Total hip BMD (g/cm2) | 0.70±0.17 | 0.68±0.12 | 0.724 | 0.67±0.13 | 0.70±0.13 | 0.224 |
| Femoral neck BMD (g/cm2) | 0.61±0.14 | 0.62±0.13 | 0.741 | 0.60±0.12 | 0.62±0.11 | 0.483 |
Lower score worse
Higher score worse
BMI = Body mass index, ADL= Activities of Daily Living, IADL= Instrumental Activities of Daily Living, NHPPT = Nursing Home Physical Performance Test, BMD= Bone mineral density
Changes in BMD and Bone Turnover Following Treatment
Among participants with cognitive impairment, those in the zoledronic acid group had an adjusted 1.9 percentage point greater improvement (p=.117) in spine BMD after 12 months than the placebo group (mean ± standard deviation 2.7 ± 4.4 vs 0.2 ± 2.6, respectively; see Figure 1A and Online Resource 1), although this difference was not statistically significant between the treatment groups. The treatment effect on spine BMD was similar to that observed in cognitively unimpaired participants (adjusted difference 1.8 percentage points with p=.009; 3.2 ± 3.8 vs 1.4 ± 4.5, respectively). After 12 months, participants with cognitive impairment had a 4.3 percentage point greater improvement (p<.001) than placebo in total hip BMD (3.7 ± 5.1 vs −0.3 ± 3.8, respectively; Figure 1B) as well as a 5.3 percentage point improvement (p=.005) than placebo in femoral neck BMD (3.7 ± 5.8 vs −1.3 ± 5.5, respectively; Figure 1C). Participants without cognitive impairment who received active treatment had a 2.8 percentage point greater increase (p<.001) in BMD than placebo at the total hip (2.6 ± 3.8 vs −0.6 ± 3.6, respectively) and a 3.0 percentage point greater increase (p=.004) than placebo in femoral neck BMD (2.0 ±6.1 vs −1.3 ± 5.2, respectively) compared to the placebo group. The difference in treatment effect (i.e., treatment × cognitive impairment interaction effect) was not significantly different between cognitively impaired and unimpaired participants at any skeletal site (Online Resource 1, last column).
Fig. 1. Bone Density and Bone Marker Changes by Cognitive Status and Treatment Group.
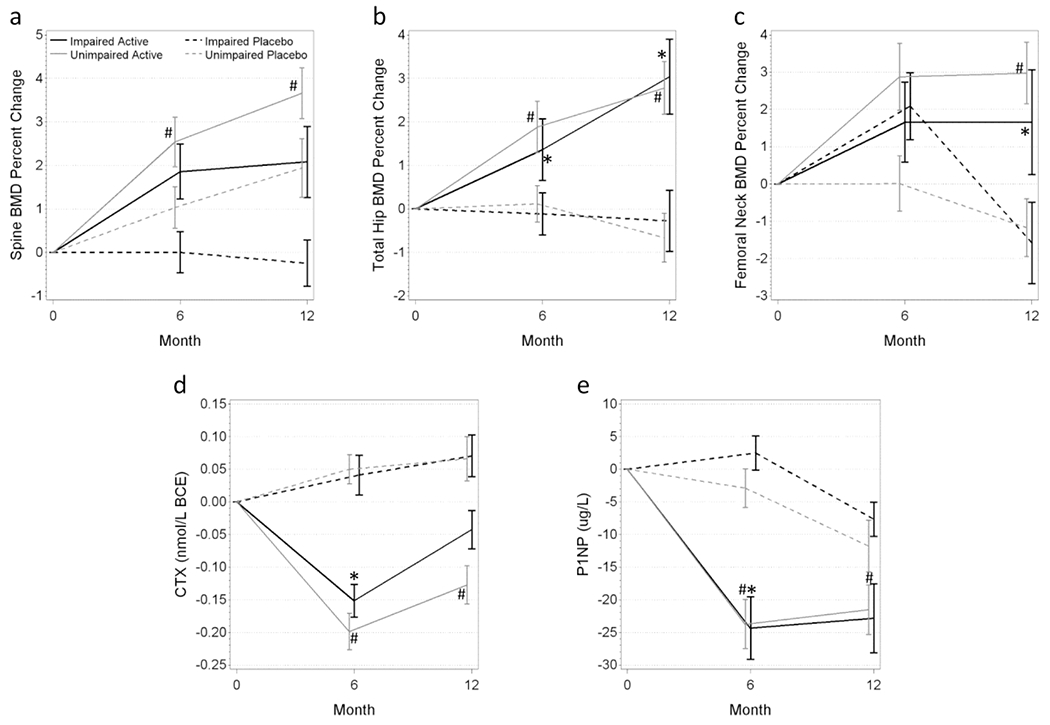
Participants who received one dose of zoledronic acid had improvements in bone density (A-C) and attenuation of bone turnover markers (D-E). Black lines indicate cognitively impaired participants in the active (solid line) or placebo group (dashed line). Gray lines indicate cognitively unimpaired participants in the active (solid line) or placebo group (dashed line). *p<.05, active vs placebo among the cognitively impaired, #p<.05, active vs placebo among the cognitively unimpaired.
BMD= Bone mineral density, CTX = C telopeptide crosslinks type I collagen, PINP = N-terminal propeptide type I procollagen
CTX, a marker of bone resorption, decreased 0.17 nmol/L BCE more at 6 months with zoledronic acid compared to placebo among cognitively impaired participants (p=.001; −0.10 ± 0.13 vs 0.04 ± 0.20, respectively; Figure 1D). Similar decreases were observed at 6 months for PINP, a marker of bone formation. PINP decreased 20.1 μg/L more in those who received zoledronic acid compared to those in the placebo group (p<.001; −24.0 ± 22.6 vs −0.1 ± 15.7, respectively; Figure 1E). Those without cognitive impairment had significantly greater decreases compared to the placebo group at both 6 and 12 months (both p<0.01; −24.2±25.6 vs −1.1±18.9 and −22.7 ± 28.1 vs −10.0 ± 25.5, respectively; Figure 1D–E and Online Resource 1). There was no significant difference in treatment effect on bone marker turnover between participants with and without cognitive impairment (i.e., treatment × cognitive impairment interaction effect; Online Resource 1 last column).
Safety
Adverse event rates were not significantly different, although expected symptoms (52.4% vs 36.4%) and falls (71.4% vs 45.5%) are descriptively greater among those with cognitive impairment (Online Resource 2).
DISCUSSION
This secondary analysis of patients with cognitive impairment from a large zoledronic acid trial demonstrated that hip BMD improved by 4.3-5.3 more percentage points with treatment compared to placebo. Bone turnover markers also demonstrated a treatment effect with concurrent decreases in markers 6 months after treatment. Participants with cognitive impairment had treatment responses to zoledronic acid similar to participants without cognitive impairment. Treatment appears to be safe, with the difference in fall rate largely attributed to greater baseline frailty in the active arm of the parent study [11].
Lyles et al [17] showed that BMD increased at sites including the total hip and femoral neck at 12 months following intravenous zoledronic acid treatment. This study included individuals 50 or older with recent hip fracture repair [17]. Our study shows similar BMD results in a population that was a decade older (average age approximately 85 versus 75) of frail women with cognitive impairment and without a recent hip fracture. The concurrence of our results supports the use of zoledronic acid to improve BMD in frail older adults with cognitive impairment. Treatment is especially important in this population as those with Alzheimer’s disease, compared to those without, have been shown to have lower BMD of the hip [18]. Skeletal fractures have a significant impact on the quality of life. Since the average life expectancy from the diagnosis of Alzheimer’s disease to death is 8-10 years but can range up to 20 years, preservation of quality of life is crucial for both patients and their caregivers [10]. Annual infusions of zoledronic acid address concerns regarding poor compliance with oral osteoporosis medications and reduces staff burden for daily or weekly administration of pills. Zoledronic acid is also a cost-effective treatment option, particularly in those who cannot tolerate an oral bisphosphonate [19].
Alzheimer’s disease has disruptive effects on bone regulation through a variety of mechanisms, including within the central nervous system as well as a direct effect on bone [3]. Improving bone turnover with treatment in cognitively impaired individuals should be considered given such disruptions. Liang et al [20] showed suppressive effects on bone turnover following annual infusions of 5 mg zoledronic acid, with a decrease in PINP and CTX levels at 6 months and 24 months compared to baseline. In an older cohort, we demonstrate a similar decrease from baseline in both CTX and PINP after zoledronic acid. There does not appear to be other studies that examine bone turnover marker levels in cognitively impaired individuals, signifying value of future studies for comparison.
Our study has several limitations. Our sample size for cognitively impaired individuals was small, and this was a secondary analysis. We focused on surrogate bone health variables that are associated with fracture risk rather than fractures themselves. We were also limited in our assessment of cognitive function. More comprehensive assessments may provide additional information about cognitive abilities and underlying pathologies. Our cognitive assessment was done at one point in time, and cognitive function could have changed over time with illness and medication alterations that are common in this population. Finally, our sample population included individuals who were able to stand and pivot with assistance and who had physical performance and activities of daily living scores similar to those without cognitive impairment (Table 1). Such sample characteristics may limit the generalizability of our findings to populations with more severe cognitive impairment who are primarily bedbound with limited ambulatory abilities.
Our study also has several strengths. There are limited data about osteoporosis therapy in frail older adults with cognitive impairment. Since these individuals can live up to a decade after diagnosis, it is important to maintain bone health as long as possible. Our study demonstrates that zoledronic acid infusions are a feasible medication to maintain bone health in an underserved, understudied vulnerable population. Our data provide the foundation for a larger fracture reduction trial in this population with cognitive impairment that are routinely excluded from clinical trials.
This secondary analysis sheds light upon an increasingly growing subpopulation of individuals with cognitive impairment and osteoporosis. With increasing longevity, it is important to recognize ways we can improve the quality of life for these individuals by maintaining bone health with the goal of preventing future fractures.
Supplementary Material
Funding
This work was supported by National Institutes of Health, National Institute on Aging grants R01 AG028086, T32 AG021885, and the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827).
Conflicts of interest:
Susan L. Greenspan reports research grant support from Amgen to the University of Pittsburgh. Bryce M. Churilla, Subashan Perera, Neil M. Resnick, and Mary P. Kotlarczyk declare they have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical Approval: All procedures performed involving human participants were in accordance with the ethical standards of the University of Pittsburgh Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the parent clinical trial as previously described [11].
REFERENCES
- 1.Office of the Surgeon General (2004) Bone Health and Osteoporosis: A Report of the Surgeon General. Office of the Surgeon General (US), Rockville (MD) [PubMed] [Google Scholar]
- 2.Kelsey JL, Hoffman S (1987) Risk factors for hip fracture. N Engl J Med 316 (7):404–406. doi: 10.1056/NEJM198702123160709 [DOI] [PubMed] [Google Scholar]
- 3.Frame G, Bretland KA, Dengler-Crish CM (2020) Mechanistic complexities of bone loss in Alzheimer’s disease: a review. Connect Tissue Res 61 (1):4–18. doi: 10.1080/03008207.2019.1624734 [DOI] [PubMed] [Google Scholar]
- 4.Wang HK, Hung CM, Lin SH, Tai YC, Lu K, Liliang PC, Lin CW, Lee YC, Fang PH, Chang LC, Li YC (2014) Increased risk of hip fractures in patients with dementia: a nationwide population-based study. BMC Neurol 14:175. doi: 10.1186/s12883-014-0175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenspan SL, Myers ER, Maitland LA, Resnick NM, Hayes WC (1994) Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA 271 (2):128–133 [PubMed] [Google Scholar]
- 6.Bonafede M, Shi N, Barron R, Li X, Crittenden DB, Chandler D (2016) Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos 11 (1):26. doi: 10.1007/s11657-016-0280-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato Y, Honda Y, Umeno K, Hayashida N, Iwamoto JUN, Takeda T, Matsumoto H (2010) Retraction : The prevention of hip fracture with menatetrenone and risedronate plus calcium supplementation in elderly patients with alzheimer disease: a randomized controlled trial. The Kurume Medical Journal 57 (4):117–124. doi: 10.2739/kurumemedj.57.117 [DOI] [PubMed] [Google Scholar]
- 8.Statement of Retraction (2017). Current Medical Research and Opinion 33 (6):1179–1179. doi: 10.1080/03007995.2017.1300004 [DOI] [PubMed] [Google Scholar]
- 9.Prieto-Alhambra D, Judge A, Arden NK, Cooper C, Lyles KW, Javaid MK (2014) Fracture prevention in patients with cognitive impairment presenting with a hip fracture: secondary analysis of data from the HORIZON Recurrent Fracture Trial. Osteoporos Int 25 (1):77–83. doi: 10.1007/s00198-013-2420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolk DABCDM (2018) Clinical Features and Diagnosis of Alzheimer Disease. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-alzheimer-disease. Accessed September 3 2020
- 11.Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM (2015) Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med 175 (6):913–921. doi: 10.1001/jamainternmed.2015.0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder EF, Miller JP, Ball LJ (2001) Development of a test of physical performance for the nursing home setting. Gerontologist 41 (5):671–679 [DOI] [PubMed] [Google Scholar]
- 13.Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9 (3):179–186 [PubMed] [Google Scholar]
- 14.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 185:914–919 [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer E (1975) A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 23 (10):433–441 [DOI] [PubMed] [Google Scholar]
- 16.Varney LF, Parker RA, Vincelette A, Greenspan SL (1999) Classification of osteoporosis and osteopenia in postmenopausal women is dependent on site-specific analysis. J Clin Densitom 2 (3):275–283. doi: 10.1385/jcd:2:3:275 [DOI] [PubMed] [Google Scholar]
- 17.Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S, Trial HRF (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357 (18):1799–1809. doi: 10.1056/NEJMoa074941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Shen L, Ji HF (2012) Alzheimer’s disease and risk of hip fracture: a meta-analysis study. ScientificWorldJournal 2012:872173. doi: 10.1100/2012/872173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins RB, Goeree R, Pullenayegum E, Adachi JD, Papaioannou A, Xie F, Thabane L (2011) The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 12:209. doi: 10.1186/1471-2474-12-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang BC, Shi ZY, Wang B, Wu P, Kong LC, Yao JL, Li CW, Shi XL (2017) Intravenous zoledronic acid 5 mg on bone turnover markers and bone mineral density in east china subjects with newly diagnosed osteoporosis: a 24-month clinical study. Orthop Surg 9 (1):103–109. doi: 10.1111/os.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


