Abstract
Interactions between the Anopheles mosquito vector and Plasmodium parasites shape how malaria is transmitted in endemic regions. The long association of these two organisms has led to evolutionary processes that minimize fitness costs of infection and benefit both players through shared nutrient resources, parasite immune suppression and mosquito tolerance to infection. In this review we explore recent data describing how Plasmodium falciparum, the deadliest malaria parasite, associates with one of its most important natural mosquito hosts, Anopheles gambiae, and we discuss the implications of these findings for parasite transmission and vector control strategies currently in development.
Keywords: vector-parasite interactions, fitness costs, Anopheles, Plasmodium
Revisiting questions of vector and parasite fitness in natural associations
Plasmodium parasites cause malaria, a tropical and sub-tropical disease that infected 229 million people and claimed the lives of 409,000 people in 2019 alone [1]. Human malaria parasites are transmitted by the infectious bite of Anopheles mosquitoes, with the most anthropophilic (see Glossary) vectors, including members of the Anopheles gambiae species complex, found in sub-Saharan Africa, where malaria burden is highest. As any organism, mosquitoes must allocate available resources to balance their energetic investment into multiple life processes, including development, flight, reproduction, immunity and survival. Should a Plasmodium infection occur, these allocations may be perturbed by costs associated with possible immune responses, tissue repair or nutrient theft by the parasites, causing trade-offs between fitness components such as decreased longevity or investment in reproduction.
We examine recent data on how natural combinations of Anopheles and Plasmodium have settled these allocations to minimize the fitness costs of infection, and compare them to non-natural associations (Figure 1, Key Figure). We consider how interactions between mosquitoes and parasites may have evolved, shaping current parasite development and transmission potential, and how they affect malaria control strategies currently in development.
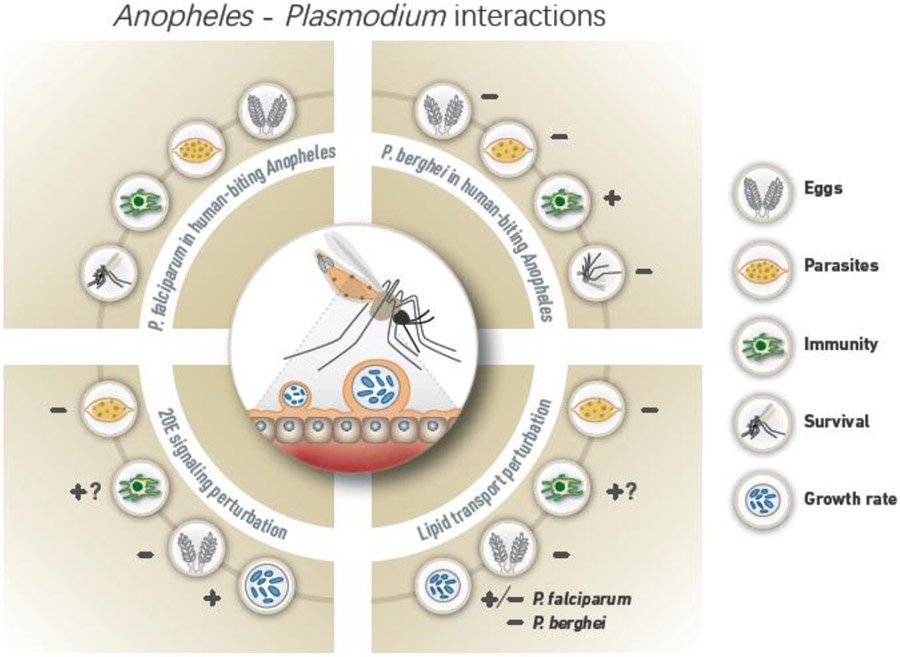
Figure 1, Key Figure. Summary of Plasmodium interactions with human-biting Anopheles.
The four quadrants represent four different conditions with outcomes on several mosquito and parasite factors relative to a natural association, P. falciparum infecting human-biting Anopheles (top left), indicated. Infection with P. falciparum does not reduce egg numbers or mosquito survival and parasites suppress TEP1-mediated immune responses. In contrast, when P. berghei infects human-biting Anopheles (non-natural association – top right), immune responses are increased and both egg and parasite numbers and mosquito survival are decreased. Manipulations to 20E signaling (bottom left) decrease egg numbers and P. falciparum parasites, although fewer in number through TEP1-independent immunity or otherwise, can exploit remaining resources to grow faster. When lipid transport is perturbed (bottom right), eggs and parasites are both decreased in number, and differences emerge between P. falciparum and P. berghei: while lack of lipophorin slows growth of P. berghei and renders parasites vulnerable to TEP1-mediated killing, the role of immunity is less clear for P. falciparum, which does not rely on lipids for growth.
Interactions between Plasmodium parasites and Anopheles mosquitoes have ancient origins
Anophelines only began transmitting Plasmodium falciparum, the deadliest human malaria parasite, relatively recently (10,000–100,000 years) [2-4]. The most closely related parasite species, P. praefalciparum, is one of three found in western lowland gorillas and is more genetically diverse than P. falciparum, suggesting a single transfer event was responsible for the creation of the deadliest disease in human history [3]. Despite this relatively short interaction, Plasmodium parasites have provided a strong genetic pressure in humans, causing the selection of multiple red blood cell variants that confer malaria protection in heterozygotes but have adverse effects in homozygotes (reviewed in [5]).
Plasmodium has undoubtedly also acted as a selective force on the genomes of its definitive host, the Anopheles mosquito, but many millions of years of evolution obscure the mosquito factors that are or have been under parasite pressure. This genus of mosquitoes, which contains more than 70 Plasmodium-competent vectors, broke off from the Culicinae lineage (containing Aedes and Culex mosquito species, which transmit avian and reptilian but not human malaria) about 217 million years ago [6]. Anopheles subsequently separated into three subgenera—Cellia, Anopheles and Nyssorhynchus—approximately 100 million years ago [7], concurrent with the split between the South American and African continents [8] and now occupy diverse geographic and ecological niches [9, 10]. With these ancient origins to the genus, anophelines have been transmitting the ancestral forms of P. falciparum and related parasites to our mammalian ancestors for 13–64 million years [11, 12].
Following a host transition from gorillas to humans, P. falciparum spread beyond Africa with human migration, adapting to local anopheline species along the way (reviewed in [13]), but it did not cross into the Americas until the 16th century, when it was introduced with the trans-Atlantic slave trade [14]. Therefore, while Old World vectors (species in the Cellia and Anopheles subgenera) have been transmitting P. falciparum for thousands of years, New World anophelines (in the Nyssorhynchus subgenus) have interacted with this parasite for a considerably shorter time.
Development of P. falciparum and its ancestors in the Anopheles vector has surely affected multiple facets of mosquito and parasite biology. On the mosquito side, adaptation must have shaped how fecundity and fertility as well as survival are impacted by infection. Conversely, parasite numbers, developmental rates and transmission efficiency must be the product of long-lasting interactions with the Anopheles female. Many of these parameters differ in natural associations (defined herein as human Plasmodia infecting human-biting Anopheles mosquitoes, including in a laboratory setting) versus non-natural (rodent Plasmodia infecting human-biting mosquitoes) so that their comparison provides useful insights into the evolutionary processes that may have influenced the mosquito-parasite partnership over time.
Physiological and temporal link between Plasmodium development and Anopheles oogenesis
The initial stages of P. falciparum development occur during a time period that is crucial for mosquito reproduction. Anopheles females may acquire Plasmodium parasites from an infected individual while feeding on blood to obtain nutrients for egg development. After bloodmeal ingestion, parasite gametocytes (the sexual stages) transform into gametes which fuse to produce a diploid zygote that differentiates into a motile ookinete within ~24 hours (h). Ookinetes then cross the midgut epithelial cells, entering at the apical surface membrane and egressing from the basal side, where they contact the basal lamina and round up to form oocysts by ~48 h (reviewed in [15]). Here, oocysts grow over the next 6–12 days and undergo amplification of the parasite genome, forming thousands of sporozoites [16, 17]. Once mature, the oocysts rupture, releasing into the mosquito hemolymph sporozoites that then invade the salivary glands [18, 19] from where they can be transmitted to a human host at the next bite the female takes. The time taken for parasite development from ingestion to potential transmission is known as the extrinsic incubation period (EIP), and it varies between parasite species and in different mosquito species. The EIP is a critical parameter of malaria transmission dynamics: mosquitoes harboring faster developing parasites are more likely to survive long enough to become infectious and spread malaria [20]. Identical events occur in the rodent malarias Plasmodium chabaudi and Plasmodium berghei, but the timing of these events is extended in P. berghei across 21 days, as this species must infect mosquitoes at 19–21 °C.
During the 48 h after blood ingestion, the Anopheles females will initiate and complete a gonotrophic cycle, consisting of egg development (oogenesis) and, if mated, egg laying (only mated females lay eggs). This cycle is largely orchestrated by the function of the steroid hormone 20-hydroxyecdysone (20E) via signaling cascades regulated by its nuclear receptor heterodimer ecdysone receptor (EcR)/ultraspiracle (USP) [21-23]. Additional blood meals taken by the mated female will trigger further batches of egg production, while also affecting the nutritional milieu surrounding the developing oocysts during their incubation period.
Due to this temporal overlap between parasite development and oogenesis cycles, researchers have long postulated that parasite infection may subtract resources or induce immune responses that could negatively affect fecundity and fertility or more broadly impair survival. Additionally, parasite invasion of the midgut and the ensuing tissue damage and immune response could all cause fitness costs to the mosquito host. Anopheles vectors may have therefore benefited from evolving mechanisms to regulate infection, by limiting either Plasmodium numbers (a strategy known as resistance) or costs associated with infection (referred to as tolerance). Conversely, Plasmodium, rather than enhancing its virulence, may have evolved mechanisms to preserve its viability while reducing the costs inflicted on the mosquito by either lowering its virulence or avoiding triggering immune responses. Such evolutionary developments are expected when costs of infection to mosquito fitness begin to negatively impact parasite transmission (reduced mosquito survival/quality) and have been theoretically proposed [24, 25].
Numerous studies using rodent model species have detected a fitness cost of infection to mosquito fecundity [26-29], fertility [27, 28] and survival [30], most likely due to the considerable investment by the infected female in immune responses (Box 1) . When Anopheles stephensi or An. gambiae were infected with Plasmodium yoelli nigeriensis, there was a reduction in fecundity of 21–41% [26-28], but with no significant correlation between egg numbers and oocyst loads [26, 28]. The reduction in fecundity was due to apoptosis of follicular epithelium cells, promoting resorption of ovarian follicles in infected females [31, 32]. The density independence between egg and oocyst numbers suggests an all-or-nothing cost to activation of the mosquito immune system preventing significant egg investment [33, 34]. An. stephensi also showed reduced survival when infected with a number of rodent malarias [26, 35-37]. Compared to uninfected controls, mortality rates were significantly higher when mosquitoes were infected with P. berghei or with P. yoelii nigeriensis, while infections with P. chabaudi reduced median survival (with parasite strain- and experiment-dependent effects).
Box 1. Rodent malaria parasites stimulate mosquito immune responses.
Infections involving non-natural combinations of rodent model malaria species with human-biting mosquitoes have been frequently used to decipher the molecular mechanisms of Anopheles immunity, in part because of the difficulties in establishing reliable infections with P. falciparum. Rodent malaria infections induce several potent immune factors that can greatly decrease parasite invasion of the midgut epithelium and survival. Immune functions are largely mediated by the fat body-produced complement-like factor thioester-containing protein 1 (TEP1), which when stabilized by two proteins of the leucine-rich repeat family, LRIM1 and APL1C, effectively kills P. berghei ookinetes that have crossed the midgut epithelium and come in contact with mosquito hemolymph, by lysis or encapsulation by melanization [89-92]. Lysis occurs because reactive oxygen and nitrogen species (ROS and RNS) in infected midgut cells, produced by JNK-mediated expression of nitric oxide synthase and heme peroxidase enzymes, mark parasites for killing by TEP1 [93-95]. In addition, cell-based immunity is stimulated through the proliferation of hemocytes, the circulating immune cells of the hemolymph, which express melanization enzymes, produce further ROS, phagocytose dead parasites, and play a role in unknown mechanisms of late-phase immunity targeting the oocyst [96, 97]. Recent studies have revealed a conserved role in mosquitoes for a class of C20 polyunsaturated fatty acids known as eicosanoids in activating immune pathways [98, 99] and increasing immune cell differentiation into circulating granulocytes [100, 101], priming an innate immune memory.
On the other hand, a meta-analysis on 11 published studies found that in infections involving P. falciparum and their natural mosquito vectors An. gambiae, Anopheles funestus, and An. stephensi, no costs to survival were detected [38]. Similarly, in recent studies An. gambiae and An. stephensi females infected with P. falciparum developed the same number of eggs than their uninfected sisters [39]. On the contrary, in An. gambiae an unexpected positive correlation existed between paired egg and oocyst numbers from individual infected mosquitoes, with females that invest more in reproduction supporting higher infection intensities [40]. These observations were not dependent on mosquito size [40], arguing against a greater blood meal volume or nutrient acquisition ability in larger females as the driver of this positive correlation [41]). These findings were confirmed using field colonies of Anopheles coluzzii and natural P. falciparum isolates, where again females that have a higher likelihood of egg development harbor increasing oocyst loads [39]. The egg-parasite correlation appears to be regulated at the level of the ookinete-to-oocyst transition (by yet unknown mechanisms), and interestingly, is lost when mosquito reproductive physiology is disrupted by impairing steroid hormone signaling as we will discuss later [40]. Combined, these observations suggest that evolution between naturally interacting organisms has acted to lessen infection virulence or fitness costs associated with infection, resulting in less/non-competitive strategies.
Evolution of mechanisms of immune suppression in P. falciparum
While rodent malarias induce strong immune responses that likely cause the fitness costs to survival and reproduction observed in infected females (Box 1), P. falciparum parasites have instead evolved mechanisms to silence the immune responses of nitration and the complement-like Thioester protein 1 (TEP1) system. The first mechanism to be identified is based on the parasite surface protein Pfs47 [42, 43], which interacts with the midgut transmembrane protein P47Rec during ookinete invasion [44]. This interaction is proposed to act as a ‘key’ in a ‘lock’ to inactivate TEP1-mediated immune responses within invaded cells, avoiding parasite killing. Effects of TEP1 silencing on P. falciparum survival are highly variable and depend on the mosquito-parasite combinations used [45-48]. This is largely because Pfs47 has many different haplotypes with strong geographical associations [49], such that haplotypes from a given region can suppress immune responses in Anopheles vectors from the same region, but not in species from other parts of the world [47]. How P47Rec is able to achieve this immune suppression is unclear, as its endogenous function is unknown and there are cases where Pfs47 does not seem to be wholly sufficient to silence immunity [44], suggesting the existence of additional parasite factors that are required for survival.
One such factor may be the ookinete (and sporozoite) surface protein PIMMS43 (Plasmodium Infection of the Mosquito Midgut Screen 43), which in An. coluzzii infections with P. berghei is required to avoid TEP1-mediated ookinete killing [50]. Analysis of the ortholog in natural P. falciparum isolates shows that PIMMS43 also has considerable geographic structure in its haplotypes even within Africa, leading to the hypothesis that regional differences in TEP1 alleles and the abilities of vectors to kill parasites may have driven parasite evolution of PIMMS43 to lessen immune activation [50]. Indeed, fluctuations in TEP1 allele frequencies among Anopheles species have been suggested to be driving Plasmodium transmission dynamics over multiple years [51]. By avoiding an immune response, parasites may not only increase their numbers, but also lessen costs to the vector that would be caused by a strong immune response. Together, these observations suggest that Plasmodium has adapted to its local natural vectors in order to ensure maximal survival and transmission.
Mosquito reproductive factors affecting parasite survival
Parasite numbers in the Anopheles midgut are also linked to 20E-regulated hormonal signaling pathways that are critical for mosquito reproductive biology, as briefly mentioned above. Upon blood feeding, 20E synthesis is initiated by the ovaries and is completed by the fat body, where 20E binds to its nuclear receptor heterodimer EcR/USP to control transcription of nutrient transporters [21-23]. Reducing 20E levels by ovary ablation or enzymatic inactivation, or impairing its function by silencing the EcR receptor result in a consistent reduction in parasite numbers, as well as an expected drop in egg production [40]. Additionally, EcR silencing removes the positive correlation between egg and parasite numbers. Our hypothesis is that 20E signaling may control a trade-off between reproduction and immunity or other somatic processes that can negatively affect parasite survival. While the precise killing mechanism is unknown, parasites in these 20E-disrupted backgrounds die during the ookinete-to-oocyst transition (around the time when 20E levels would peak) via TEP1-independent mechanisms, suggesting alternative processes such as disrupted midgut cell homeostasis or other immune pathways may be involved [40]. Other explanations involving competition for resources causing death by starvation, may be less likely as circulating lipids in the hemolymph are sufficient to produce (albeit a reduced number of) eggs, and resources accumulate in the very tissue where the parasite is found, the midgut. Whatever the mechanism, parasite survival is intimately connected to mosquito reproductive biology via the function of the 20E ecdysteroid.
Interestingly, reductions in survival of P. falciparum NF54, a common laboratory strain of African origin, are also observed in An. gambiae after knockdown of the major lipid transporter Lipophorin (Lp) [17, 24, 40, 52-54]. Lp transports lipids to the ovaries during egg development and its transcription is suppressed by 20E signaling [40]. Work in P. berghei shows Lp can cause neutral lipid accumulation in and around parasites and may obscure them from the immune system, antagonizing TEP1-mediated killing [24, 54]. It would be interesting to know if Lp mediates lipid accumulation also around P. falciparum and if in Lp-silenced mosquitoes parasites become recognized and killed by TEP1 (implied in [24]), circumventing the fact that African parasites should not trigger TEP1-dependent immune pathways in African mosquitoes. However, such a protective role for Lp does not restore parasite numbers in EcR-silenced females, where Lp levels increase from 24 h pIBM, since P. falciparum ookinetes have already crossed the midgut epithelium by this time, and parasite killing is TEP1-independent [40].
While the mechanisms need further investigation, 20E signaling post blood feeding shows a pivotal role in regulating parasite intensity of infection. Additionally, signaling via this same hormone after mating also regulates, albeit differently, how Anopheles females are affected by P. falciparum infection, as discussed below.
The evolution of mosquito tolerance to P. falciparum infections
A cost of P. falciparum infection has been observed in An. gambiae females depleted for a reproductive protein named Mating-Induced Stimulator of Oogenesis (MISO). MISO is induced in the female atrium by the sexual transfer of the same steroid hormone 20E that regulates oogenesis, this time however synthesized by the male in their reproductive glands. Upon sexual transfer, which occurs as part of a gelatinous mating plug containing other seminal secretions, male steroids first induce MISO and then interact with this protein to transduce hormonal signals into increased egg development [55]. In field infections using P. falciparum isolates circulating in Burkina Faso, MISO depletion caused decreased fecundity as the number of gametocytes in the blood increased, without affecting parasite numbers [39]. When levels of gametocytes were high in infectious blood meals, MISO-silenced females were far less likely than control females fed the same blood to develop any eggs [39]. Therefore, MISO supports the reproductive fitness of P. falciparum-infected Anopheles females not by limiting parasite numbers but rather by reducing fitness costs associated with infection, an evolutionary strategy known as tolerance. There are several explanations for these observations, invoking potential roles for steroid-MISO signaling in protecting the ovary from parasite-induced oxidative damage, or promoting the timely accumulation of resources towards the ovary and away from parasites. It is worth noting that the molecular mechanisms underlying tolerance are generally neither well understood nor universal, and can involve intrinsic genetic factors in the host, or extrinsic factors from the host’s environment [56, 57]. This is the first tolerance factor to P. falciparum identified in the Anopheles genome.
Intriguingly, sexual transfer of male 20E as part of a mating plug transfer has evolved specifically in anopheline mosquitoes of the Cellia subgenus, while this hormone is detected at negligible levels in species in the Anopheles subgenus and is absent in males from Nyssorhynchus species [58]. Previous work had indicated that the evolution of such mating system has induced reciprocal adaptations in females, causing the divergence of the 20E-interacting partner MISO [58]. As Cellia species comprise all major malaria vectors on the African continent, these findings suggest the hypothesis that tolerance mechanisms to P. falciparum may have been promoted by male-female sexual interactions driven by the evolution of male steroid hormone transfer during mating [39].
Through these two 20E-signaling dependent interactions, tolerance and a reproductive investment link with Plasmodium survival, it may appear that P. falciparum may be preferentially transmitted by females with high reproductive fitness. However, reproductive biology affects parasite development in other unexpected ways that strongly impact their chances of transmission.
Mosquito reproductive factors influencing oocyst growth rates
During the oocyst stage, parasites grow considerably in size under the basal lamina of the mosquito midgut and undergo multiple rounds of DNA replication. After several days, extensive invaginations of the plasma membrane surround each nucleus and coordinately subdivide the oocyst into individual sporozoites [16, 59]. Once this process is completed, many thousands of sporozoites are released from each oocyst into the mosquito hemolymph from where they invade the salivary glands, ready for transmission.
The factors and mechanisms behind this extensive oocyst growth are becoming better characterized, and mosquito nutrients appear to play a major role. Once again, the comparison between P. berghei and P. falciparum reveals interesting differences. Studies in P. berghei have shown that Lp and presumably Lp-transported lipids are required for oocyst growth and for the production and infectivity of sporozoites [24, 54], although there seems to be some variability as other studies do not report strong growth defects after Lp silencing [52, 60]. FITC-labelled lipoparticles have been visualized within oocysts of the bird malaria parasite Plasmodium gallinaceum [61] and neutral lipids appeared to be present in and accumulate around P. berghei oocysts [24]. Host phospholipids and neutral lipids transported by Lp may be required to generate the phospholipid membranes required for oocyst growth and sporozoite individualization [62]. Differences between oocysts in accessing Lp-transported lipids may contribute to the considerable variation in metabolic activity observed even in wild type sporozoites [24].
The role of lipids in facilitating P. falciparum growth is less clear. Here Lp silencing has very limited effects on oocyst growth [17, 40] or parasite protein expression (measured by pre-tRNATyr levels) [60], although decreased development has been noted in some studies [24, 53]. While strain-dependent or other experimental differences may explain this discrepancy, these results also suggest that P. falciparum may principally rely on other nutrients (amino acids or carbohydrates) to make its own phospholipids for synthesizing membranes. Notably, P. falciparum activates so-called FASII metabolism for de novo lipid synthesis during other life stages [63], and FASII pathway mutants cannot complete sporulation in the mosquito, among other defects [64]. In contrast, P. berghei FASII mutants develop normally during the mosquito stages [65], highlighting a difference between these species in their ability to scavenge host lipids.
Interestingly, disrupting egg development (via a number of different manipulations to 20E signaling post blood feeding) increases P. falciparum oocyst growth rates significantly, likely as resources not utilized for oogenesis become available to the parasite [40]. As a consequence, sporozoites reach the salivary glands in a shorter period of time and become infectious sooner. Although Lp does not appear to affect oocyst growth in normal conditions, when oogenesis is reduced accelerated growth can be substantially reversed by silencing Lp, suggesting that Lp-transported lipids are utilized when they become available [40]. P. falciparum is therefore capable of, but not reliant on, host lipid scavenging during the oocyst stage, plastically altering its growth rate in response to its physiological environment. This scavenging process does not impose a reduction in eggs on the Anopheles female, as it exploits only those resources remaining after egg development is completed [39, 40], although egg fertility and larval survival remain to be assessed. The oogenetic cycle associated with infection overlaps with ookinete-to-oocyst differentiation, rather than a significant oocyst growth phase, and it is possible the parasite is better adapted to utilize resources for growth from additional blood meals (below) rather than those destined for egg development. Conversely, high investment in oogenesis does appear to impact the speed of parasite development by limiting the remaining resources available. Indeed, we observe a negative correlation between numbers of eggs a female produces and the mean oocyst size in her midgut, a proxy for parasite growth rates [40]. From these observations a scenario emerges whereby P. falciparum parasites avoid a competitive interaction with egg development by entering their growth phase only as the gonotrophic cycle associated with infection is completed, although further work is required to assess exactly when parasites can begin to accelerate their development.
P. falciparum therefore perfectly tailor their development to the female reproductive investment, ensuring their transmission in most physiological circumstances.
Additional blood meals accelerate oocyst growth rates
Anopheles naturally have multiple cycles of blood feeding and oogenesis in their lifetime. At the second or subsequent gonotrophic cycles, the non-competitive relationship with P. falciparum may change in nature or become more complex. What is known is that the waves of available resources provided by an additional blood meal (provided between 3–11 d post infectious blood meal (pIBM)) considerably boost parasite growth rates [17, 60, 66, 67]. P. falciparum oocyst size in An. gambiae was much larger, and sporozoites were already detectable in the salivary glands at 7 d pIBM, a time when most oocysts are still developing in females receiving a single blood meal [17]. Both Plasmodium DNA content and protein expression increased at 9 d pIBM if a second blood meal was given at 3 d pIBM [60], molecularly confirming accelerated development.
This boost in oocyst development could come at a cost to the second gonotrophic cycle, although the accelerated growth is once again not due to Lp-transported lipids [17, 60]. P. falciparum oocysts developing in females depleted for Lp still increased in size and accelerated their development after a second blood meal as much as controls. Nevertheless, there is evidence supporting a greater impact of P. falciparum infection at subsequent cycles: infected and uninfected females produced similar numbers of eggs at the first blood meal, but when fed again on rabbits, eggs were reduced only in the infected group [68]. A second infectious blood meal may also introduce new parasites, and primed innate immune responses which limit the development of incoming parasites [69] could also expend resources intended for egg development.
Interestingly, a second proteinaceous meal (2% BSA + 2mM ATP in phosphate-buffered saline) was sufficient to accelerate P. falciparum development to some degree, indicating amino acid availability may be more important than lipids for this parasite [67]. Further support for a potential role for amino acids, especially branched chain amino acids (BCAA, isoleucine, leucine, valine), comes from manipulations to amino acid metabolism: prolonging BCAA breakdown in the mosquito after an infectious blood meal negatively affects sporozoite levels 14 days later [70]. This finding coincides with a requirement for isoleucine in growth of asexual stage parasites, where human hemoglobin lacks isoleucine entirely and therefore parasites must scavenge this amino acid from the blood [71].
Additionally, the content of amino acids or other nutrients in the bloodmeal, which varies in profile between mammalian hosts, can affect how parasites develop. In side-by-side experiments, the anthropophilic An. gambiae and the zoophilic Anopheles arabiensis were infected with P. falciparum and fed a second uninfectious meal of either human or bovine blood. At 16 d pIBM, a higher prevalence of sporozoites in the salivary glands was observed when An. gambiae fed on human rather than bovine blood, and when An. arabiensis fed on bovine rather than human blood [72]. This suggests that parasites can benefit from a certain composition and/or processing of metabolites by the mosquito to increase development speed in a manner consistent with their vectors’ host feeding preferences.
Interplay between mosquito reproduction and parasite transmission
Increased parasite development rates have consequences for the EIP and malaria transmission, with sporozoites appearing sooner in the salivary glands after either a second bloodmeal or impairment to oogenesis [17, 40]. Parasites are reliant on the mosquito survival exceeding the EIP for successful transmission to a human host and would therefore benefit from shortening their developmental time, especially considering the short, 2–3 week lifespan of a female mosquito in the field [73]. The EIP has been shown to depend on environmental temperature (higher temperatures shorten the EIP up to a point) but also on mosquito resources, with larval and adult nutrition playing a role [74, 75]. Following a blood meal, the appearance of sporozoites was delayed in egg-bearing females fed a restricted diet during larval stages compared to those fed a control diet. This finding suggests that lower adult reserves remaining after oogenesis limit parasite development rates. In contrast, our experimental females, manipulated to invest less in reproduction, have higher available reserves which accelerate parasite development compared to controls [40]. The decision of a female to invest in egg development may have substantial consequences for parasite development in malaria endemic settings, as many mosquitoes exhibit pre-gravid behavior and do not produce eggs following an initial blood meal due to poor larval nutrition [76, 77] and might therefore harbor faster developing parasites than their gravid sisters.
As described earlier for P. falciparum in An. gambiae, we expect that other combinations of Anopheles and Plasmodium will also show accelerated parasite development after a second blood meal. In support of this hypothesis, this effect can be seen in multiple vector-pathogen combinations [78-81] and may be a generalized mechanism for pathogens to increase their chances of transmission. This also has implications for malaria transmission potential, with a greater proportion of younger mosquitoes, known to be more resistant to insecticide killing [82, 83], contributing to the force of infection. Importantly, salivary gland sporozoites derived from faster developing oocysts are as infectious as those dissected from the salivary glands of control mosquitoes: impairing 20E signaling in mosquitoes caused sporozoite accumulation sooner but did not affect the numbers of exo-erythrocytic forms present in primary liver cells 3 days after infection [40]. How mosquito nutrients affect sporozoite invasion of liver cells is largely unknown, with only one report showing that in Lp-silenced An. gambiae, P. berghei salivary glands sporozoites are severely compromised in their infectiousness [24]. In these mosquitoes, oocysts show abnormal intracellular vacuoles and electron-dense membranes, and although low numbers of sporozoites can be recovered that appear normal in structure and gene expression, they are limited in their infectivity to mammalian hepatocytes, perhaps due to impaired formation of mitochondrial membranes [24]. Given the lack of strong effects of Lp silencing on P. falciparum oocyst growth, however, it is unlikely that the same abnormal phenotype will be observed in this human parasite. Recent profiling of transcriptomes from immature and infectious sporozoites [84-87] may reveal which pathways might be important for infectivity and could be targeted to prevent malaria transmission in transmission blocking strategies or by using recently developed methods to target parasite development directly within the mosquito [88].
Concluding Remarks
Plasmodium and Anopheles have endured a long but dynamic relationship whereby fitness costs of infection to survival and reproduction have been lessened over time. While many of the molecular players dictating this interplay have been discovered by studying non-natural associations, their relative importance must be considered in the context of natural infection, which may be quite different due to many millions of years of evolution of these two organisms. Many outstanding questions remain regarding if and how mosquito reproductive processes interact with immune pathways and affect Plasmodium survival and growth, and how and which nutrients support parasite infectivity (see Outstanding Questions). The strength of the vector-parasite association means manipulations to mosquitoes may reasonably be expected to also impact parasite development in ways that may not always be easily predictable. Specifically, targeting egg development may increase the likelihood of malaria transmission due to an unexpected effect of accelerating parasite development. Interactions of Plasmodium parasites with the physiological environment of their Anopheles hosts must therefore be carefully evaluated when methods aimed at controlling mosquito reproduction are implemented in the field, as these strategies may have unintended consequences for parasite transmission and malaria control.
Outstanding Questions.
How does reproductive signaling in the ovary feedback on the fat body and midgut?
Does reproduction interact with immunity to affect Plasmodium survival and growth? Which immune pathways are affected?
What are the molecular mechanisms of Plasmodium tolerance and how do 20E and MISO fit into the tolerance network?
Which nutrients support parasite growth and infectivity and how do parasites acquire these nutrients?
Can we target parasite development for malaria control?
Can we manipulate mosquito reproduction safely?
Highlights.
Parasites can exploit mosquito resources for their own development.
Reproductive fitness costs are minimized in natural combinations of Plasmodium and Anopheles.
A mating-induced tolerance mechanism has evolved in An. gambiae to limit reproductive fitness costs of high intensity Plasmodium infections.
Nutrient resources within the mosquito can affect parasite development, sporozoite function and infectivity.
Manipulations to mosquito physiology have consequences for malaria transmission and proposed vector control strategies.
Acknowledgements
FC is funded on research related to the topic discussed here by a Faculty Research Scholar Award by the Howard Hughes Medical Institute (HHMI) and the Bill & Melinda Gates Foundation (BMGF) (Grant ID: OPP1158190), and by the National Institutes of Health (NIH) (R01 AI124165 and R01 AI148646). The findings and conclusions within this publication are those of the authors and do not necessarily reflect positions or policies of the HHMI, the BMGF, or the NIH.
Glossary
- Anthropophilic
preferring to bite and feed on humans
- Apoptosis
an orchestrated program of cell death in which proteases are activated to destroy the cell
- Atrium
organ of the female lower reproductive tract into which the mating plug is received and through which eggs are laid
- Basal lamina
a thin external extracellular matrix layer containing fibrous proteins that acts to support tissues and organs
- Definitive host
the host in which sexual reproduction of the parasite takes place
- Ecdysteroid
a steroid hormone found in arthropods with roles in molting, development or reproduction
- Epithelium
a tissue layer containing polarized cells that is exposed to an exterior environment such as the lining of the gut
- Exo-erythrocytic form
the liver stage of the parasite which produces liver merozoites to establish infection in red blood cells (erythrocytes)
- Fecundity
the reproductive potential of a female, her physiological capability to produce eggs or offspring
- Fertility
the number of live offspring produced by a female
- Follicle
an egg-producing unit of the ovary, consisting of the oocyte (the future egg) and nurse cells which provision the developing egg, all surrounded by a single layer of epithelial cells
- Gonotrophic cycle
the cycle of blood feeding, egg production and laying, which is repeated to give multiple egg batches
- Hemolymph
the fluid that surrounds the organs of the mosquito in an open circulatory system.
- Isolate
a strain of parasites that is isolated from a patient blood sample
- Neutral lipid
a lipid such as cholesterol or triglyceride with no charged groups
- Primary liver cells
cultured liver cells (hepatocytes) isolated from human donors
- Resorption
the process of unloading of nutrients from ovarian follicles when egg development is aborted
- Species complex
a group of closely related species that are difficult to distinguish, particularly by morphology, often occurring in the same or overlapping regions, requiring DNA sequencing to be able to assign individuals to one species or another
- Sporulation
the production of sporozoites
- Thioester protein 1 (TEP1)
is a component of the mosquito immune system analogous to complement in mammalian blood that coats invading pathogens, triggering their lysis or destruction by immune cells
- Zoophilic
preferring to bite and feed on animals
Footnotes
Declaration of Interests
The authors declare no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO, World Malaria Report, World Health Organization, Geneva, 2020. [Google Scholar]
- 2.Joy DA et al. (2003) Early origin and recent expansion of Plasmodium falciparum. Science 300 (5617), 318–21. [DOI] [PubMed] [Google Scholar]
- 3.Liu W et al. (2010) Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature 467 (7314), 420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundararaman SA et al. (2016) Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria. Nat Commun 7, 11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatkowski DP (2005) How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet 77 (2), 171–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reidenbach KR et al. (2009) Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol Biol 9, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neafsey DE et al. (2015) Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science 347 (6217), 1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valencio DA and Vilas JF (1969) Age of the Separation of South America and Africa. Nature 223, 1353–1354. [Google Scholar]
- 9.Kiszewski A et al. (2004) A global index representing the stability of malaria transmission. Am J Trop Med Hyg 70 (5), 486–98. [PubMed] [Google Scholar]
- 10.Sinka ME et al. (2012) A global map of dominant malaria vectors. Parasit Vectors 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricklefs RE and Outlaw DC (2010) A molecular clock for malaria parasites. Science 329 (5988), 226–9. [DOI] [PubMed] [Google Scholar]
- 12.Silva JC et al. (2015) A new method for estimating species age supports the coexistence of malaria parasites and their Mammalian hosts. Mol Biol Evol 32 (5), 1354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina-Cruz A et al. (2016) Mosquito Vectors and the Globalization of Plasmodium falciparum Malaria. Annu Rev Genet 50, 447–465. [DOI] [PubMed] [Google Scholar]
- 14.Yalcindag E et al. (2012) Multiple independent introductions of Plasmodium falciparum in South America. Proc Natl Acad Sci U S A 109 (2), 511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baton LA and Ranford-Cartwright LC (2005) Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol 21 (12), 573–80. [DOI] [PubMed] [Google Scholar]
- 16.Sinden RE and Strong K (1978) An ultrastructural study of the sporogonic development of Plasmodium falciparum in Anopheles gambiae. Trans R Soc Trop Med Hyg 72 (5), 477–91. [DOI] [PubMed] [Google Scholar]
- 17.Shaw WR et al. (2020) Multiple blood feeding in mosquitoes shortens the Plasmodium falciparum incubation period and increases malaria transmission potential. PLoS Pathog 16 (12), e1009131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sultan AA et al. (1997) TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90 (3), 511–22. [DOI] [PubMed] [Google Scholar]
- 19.Kappe S et al. (1999) Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J Cell Biol 147 (5), 937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macdonald G (1956) Epidemiological basis of malaria control. Bull World Health Organ 15 (3-5), 613–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Hagedorn HH et al. (1975) The ovary as a source of alpha-ecdysone in an adult mosquito. Proc Natl Acad Sci U S A 72 (8), 3255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao TP et al. (1993) Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature 366 (6454), 476–9. [DOI] [PubMed] [Google Scholar]
- 23.Attardo GM et al. (2005) Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol 35 (7), 661–75. [DOI] [PubMed] [Google Scholar]
- 24.Costa G et al. (2018) Non-competitive resource exploitation within mosquito shapes within-host malaria infectivity and virulence. Nat Commun 9 (1), 3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koella JC and Boete C (2003) A model for the coevolution of immunity and immune evasion in vector-borne diseases with implications for the epidemiology of malaria. Am Nat 161 (5), 698–707. [DOI] [PubMed] [Google Scholar]
- 26.Hogg JC and Hurd H (1995) Plasmodium yoelii nigeriensis: the effect of high and low intensity of infection upon the egg production and bloodmeal size of Anopheles stephensi during three gonotrophic cycles. Parasitology 111(Pt 5) (5), 555–62. [DOI] [PubMed] [Google Scholar]
- 27.Jahan N and Hurd H (1997) The effects of infection with Plasmodium yoelii nigeriensis on the reproductive fitness of Anopheles stephensi. Ann Trop Med Parasitol 91 (4), 365–9. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed AM et al. (1999) The effects of infection withPlasmodium yoelii nigeriensison the reproductive fitness of the mosquitoAnopheles gambiae. Invertebrate Reproduction & Development 36 (1-3), 217–222. [Google Scholar]
- 29.Marrelli MT et al. (2007) Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci U S A 104 (13), 5580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson HM et al. (2003) Mosquito mortality and the evolution of malaria virulence. Evolution 57 (12), 2792–804. [DOI] [PubMed] [Google Scholar]
- 31.Hopwood JA et al. (2001) Malaria-induced apoptosis in mosquito ovaries: a mechanism to control vector egg production. J Exp Biol 204 (Pt 16), 2773–80. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed AM and Hurd H (2006) Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect 8 (2), 308–15. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed AM et al. (2001) Effects of malaria infection on vitellogenesis in Anopheles gambiae during two gonotrophic cycles. Insect Mol Biol 10 (4), 347–56. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed AM et al. (2002) The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. OIKOS 97 (3), 371–377. [Google Scholar]
- 35.Gad AM et al. (1979) Pathology of Anopheles stephensi after infection with Plasmodium berghei berghei. I. Mortality rate. Z Parasitenkd 60 (3), 249–61. [DOI] [PubMed] [Google Scholar]
- 36.Hogg JC and Hurd H (1995) Malaria-induced reduction of fecundity during the first gonotrophic cycle of Anopheles stephensi mosquitoes. Med Vet Entomol 9 (2), 176–80. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson HM and Read AF (2002) Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc Biol Sci 269 (1497), 1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson HM and Read AF (2002) Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol 18 (6), 256–61. [DOI] [PubMed] [Google Scholar]
- 39.Marcenac P et al. (2020) A mating-induced reproductive gene promotes Anopheles tolerance to Plasmodium falciparum infection. PLoS Pathog 16 (12), e1008908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werling K et al. (2019) Steroid Hormone Function Controls Non-competitive Plasmodium Development in Anopheles. Cell 177 (2), 315–325 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reznick D et al. (2000) Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol Evol 15 (10), 421–425. [DOI] [PubMed] [Google Scholar]
- 42.Molina-Cruz A et al. (2013) The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340 (6135), 984–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramphul UN et al. (2015) Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci U S A 112 (5), 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina-Cruz A et al. (2020) Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc Natl Acad Sci U S A 117 (5), 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molina-Cruz A et al. (2012) Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes. Proc Natl Acad Sci U S A 109 (28), E1957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eldering M et al. (2016) Variation in susceptibility of African Plasmodium falciparum malaria parasites to TEP1 mediated killing in Anopheles gambiae mosquitoes. Sci Rep 6, 20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molina-Cruz A et al. (2015) Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc Natl Acad Sci U S A 112 (49), 15178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nsango SE et al. (2012) Genetic clonality of Plasmodium falciparum affects the outcome of infection in Anopheles gambiae. Int J Parasitol 42 (6), 589–95. [DOI] [PubMed] [Google Scholar]
- 49.Manske M et al. (2012) Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487 (7407), 375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ukegbu CV et al. (2020) PIMMS43 is required for malaria parasite immune evasion and sporogonic development in the mosquito vector. Proc Natl Acad Sci U S A 117 (13), 7363–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gildenhard M et al. (2019) Mosquito microevolution drives Plasmodium falciparum dynamics. Nat Microbiol 4 (6), 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlachou D et al. (2005) Functional genomic analysis of midgut epithelial responses in Anopheles during Plasmodium invasion. Curr Biol 15 (13), 1185–95. [DOI] [PubMed] [Google Scholar]
- 53.Mendes AM et al. (2008) Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog 4 (5), e1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rono MK et al. (2010) The major yolk protein vitellogenin interferes with the anti-plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol 8 (7), e1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldini F et al. (2013) The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol 11 (10), e1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goic B et al. (2016) Virus-derived DNA drives mosquito vector tolerance to arboviral infection. Nat Commun 7, 12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sternberg ED et al. (2012) Food plant derived disease tolerance and resistance in a natural butterfly-plant-parasite interactions. Evolution 66 (11), 3367–76. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell SN et al. (2015) Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347 (6225), 985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer M and Frischknecht F (2021) Fluorescent tagging of Plasmodium circumsporozoite protein allows imaging of sporozoite formation but blocks egress from oocysts. Cell Microbiol 23 (5), e13321. [DOI] [PubMed] [Google Scholar]
- 60.Habtewold T et al. (2020) Plasmodium oocysts respond with dormancy to crowding and nutritional stress. bioRxiv, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atella GC et al. (2009) The major insect lipoprotein is a lipid source to mosquito stages of malaria parasite. Acta Trop 109 (2), 159–62. [DOI] [PubMed] [Google Scholar]
- 62.Atella GC et al. (2006) Anopheles gambiae lipophorin: characterization and role in lipid transport to developing oocyte. Insect Biochem Mol Biol 36 (5), 375–86. [DOI] [PubMed] [Google Scholar]
- 63.Mi-Ichi F et al. (2006) Intraerythrocytic Plasmodium falciparum utilize a broad range of serum-derived fatty acids with limited modification for their growth. Parasitology 133 (Pt 4), 399–410. [DOI] [PubMed] [Google Scholar]
- 64.van Schaijk BC et al. (2014) Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot Cell 13 (5), 550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaughan AM et al. (2009) Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 11 (3), 506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ponnudurai T et al. (1989) Sporozoite load of mosquitoes infected with Plasmodium falciparum. Trans R Soc Trop Med Hyg 83 (1), 67–70. [DOI] [PubMed] [Google Scholar]
- 67.Kwon H et al. (2021) Additional Feeding Reveals Differences in Immune Recognition and Growth of Plasmodium Parasites in the Mosquito Host. mSphere 6 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alout H et al. (2017) Consequences of insecticide resistance on malaria transmission. PLoS Pathog 13 (9), e1006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodrigues J et al. (2010) Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329 (5997), 1353–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lampe L et al. (2019) Metabolic balancing by miR-276 shapes the mosquito reproductive cycle and Plasmodium falciparum development. Nat Commun 10 (1), 5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J et al. (2006) Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci U S A 103 (23), 8840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emami SN et al. (2017) The transmission potential of malaria-infected mosquitoes (An.gambiae-Keele, An.arabiensis-Ifakara) is altered by the vertebrate blood type they consume during parasite development. Sci Rep 7, 40520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gillies MT and Wilkes TJ (1965) A study of the age-composition of populations of Anopheles gambiae Giles and A. funestus Giles in North-Eastern Tanzania. Bull Entomol Res 56 (2), 237–62. [DOI] [PubMed] [Google Scholar]
- 74.Shapiro LL et al. (2016) Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc Biol Sci 283 (1834). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shapiro LLM et al. (2017) Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol 15 (10), e2003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takken W et al. (1998) Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol 35 (5), 639–45. [DOI] [PubMed] [Google Scholar]
- 77.Scott TW and Takken W (2012) Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol 28 (3), 114–21. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong PM et al. (2020) Successive blood meals enhance virus dissemination within mosquitoes and increase transmission potential. Nat Microbiol 5 (2), 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serafim TD et al. (2018) Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity. Nat Microbiol 3 (5), 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Travi BL and Orihel TC (1987) Development of Brugia malayi and Dirofilaria immitis in Aedes aegypti: effect of the host's nutrition. Trop Med Parasitol 38 (1), 19–22. [PubMed] [Google Scholar]
- 81.Chandler LJ et al. (1996) Analysis of La Crosse virus S-segment RNA and its positive-sense transcripts in persistently infected mosquito tissues. J Virol 70 (12), 8972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rowland M and Hemingway J (1987) Changes in malathion resistance with age in Anopheles stephensi from Pakistan. Pesticide Biochemistry and Physiology 28 (2), 239–247. [Google Scholar]
- 83.Lines JD and Nassor NS (1991) DDT resistance in Anopheles gambiae declines with mosquito age. Med Vet Entomol 5 (3), 261–5. [DOI] [PubMed] [Google Scholar]
- 84.Lindner SE et al. (2019) Transcriptomics and proteomics reveal two waves of translational repression during the maturation of malaria parasite sporozoites. Nat Commun 10 (1), 4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Real E et al. (2020) A single-cell atlas of Plasmodium falciparum transmission through the mosquito. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruberto AA et al. (2021) Single-cell RNA sequencing reveals developmental heterogeneity among Plasmodium berghei sporozoites. Sci Rep 11 (1), 4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bogale HN et al. (2021) Transcriptional heterogeneity and tightly regulated changes in gene expression during Plasmodium berghei sporozoite development. Proc Natl Acad Sci U S A 118 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paton DG et al. (2019) Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 567 (7747), 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blandin S et al. (2004) Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116 (5), 661–70. [DOI] [PubMed] [Google Scholar]
- 90.Povelones M et al. (2009) Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324 (5924), 258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fraiture M et al. (2009) Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5 (3), 273–84. [DOI] [PubMed] [Google Scholar]
- 92.Povelones M et al. (2011) Structure-function analysis of the Anopheles gambiae LRIM1/APL1C complex and its interaction with complement C3-like protein TEP1. PLoS Pathog 7 (4), e1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oliveira Gde A et al. (2012) Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science 335 (6070), 856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garver LS et al. (2013) The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog 9 (9), e1003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molina-Cruz A et al. (2008) Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem 283 (6), 3217–23. [DOI] [PubMed] [Google Scholar]
- 96.Smith RC et al. (2015) Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc Natl Acad Sci U S A 112 (26), E3412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith RC and Barillas-Mury C (2016) Plasmodium Oocysts: Overlooked Targets of Mosquito Immunity. Trends Parasitol 32 (12), 979–990. [DOI] [PubMed] [Google Scholar]
- 98.Kwon H and Smith RC (2019) Inhibitors of Eicosanoid Biosynthesis Reveal that Multiple Lipid Signaling Pathways Influence Malaria Parasite Survival in Anopheles gambiae. Insects 10 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barletta ABF et al. (2020) Prostaglandins regulate humoral immune responses in Aedes aegypti. PLoS Negl Trop Dis 14 (10), e0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramirez JL et al. (2015) A mosquito lipoxin/lipocalin complex mediates innate immune priming in Anopheles gambiae. Nat Commun 6, 7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barletta ABF et al. (2019) Mosquito Midgut Prostaglandin Release Establishes Systemic Immune Priming. iScience 19, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]