Abstract
Left ventricular (LV) apical hypoplasia is a rare restrictive cardiomyopathy subtype with an unclear pathophysiology. LV apical hypoplasia typically presents with elongated right ventricle (RV) wrapping around a truncated and spherical LV with a deficient apex (the “banana-shape” of the RV). Here we report a case of a young boy with apparent LV apical hypoplasia that developed after birth; no “banana-shaped” RV was observed during the fetal period. Moreover, suprasystemic pulmonary hypertension (PH) developed even after a mitral valve replacement was performed for progressive mitral stenosis and regurgitation at 14 months of age. He underwent surgery for the Potts shunt, a shunt between the pulmonary artery and aorta, at 13 years to secure systemic output. His PH ameliorated and his heart failure remained stable for 3 years after the operation. This case indicates that the “banana-shaped” RV seen in this condition is not always congenital but that it can form and develop after birth. Furthermore, this case supports the usefulness of the Potts shunt as a therapeutic option in patients with severe PH due to LV apical hypoplasia.
<Learning objective: Left ventricular apical hypoplasia typically presents with elongated right ventricle wrapping around a truncated and spherical left ventricle with a deficient apex. However, this characteristic may not be always congenital and can also form and develop after birth. The Potts shunt, a shunt between the pulmonary artery and aorta, may be a therapeutic option in patients with severe pulmonary hypertension due to left ventricular apical hypoplasia.>
Keywords: Left ventricular apical hypoplasia, Restrictive cardiomyopathy, Potts shunt, Pulmonary hypertension, Mitral valve replacement
Introduction
Left ventricular (LV) apical hypoplasia is a subtype of restrictive cardiomyopathy that has been recognized as a disease since 2004 [1]. Even though most reported pediatric and adult cases are not severe [2], some are accompanied by severe pulmonary hypertension (PH) [3,4], and fatal cases have also been reported [3]. Although LV apical hypoplasia as a disease entity is not yet fully understood, it is believed to be congenital [1,5]. Here we present a case with apparent LV apical hypoplasia that was followed since the fetal stage. This case demonstrates not only that the characteristic morphology of LV apical hypoplasia may not always be congenital but also that it can form and progress after birth. We also reveal that the Potts shunt [6,7] may be a useful treatment option for patients with severe PH.
Case report
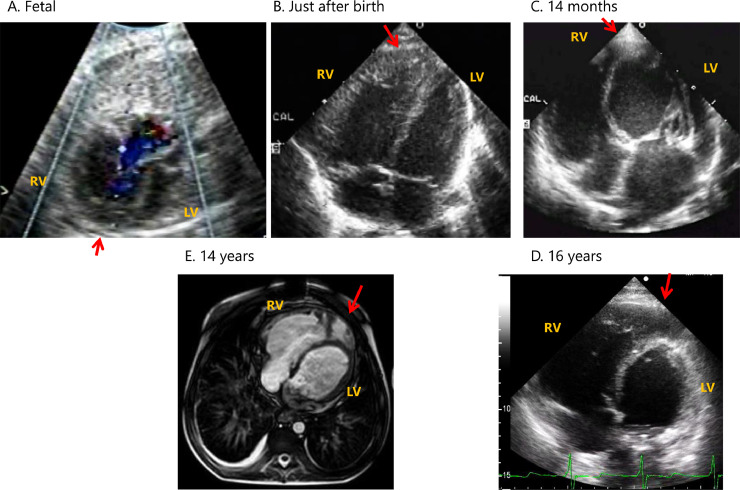
Our case was a 17-year-old male who had his clinical course reported up to 4 years of age [8]. Fetal echocardiography indicated a small left heart size during development (Fig. 1 and Video A). He was born at 37 weeks gestational age. Echocardiography after birth (Fig. 1 and Online Video B) ruled out hypoplastic left heart syndrome but did reveal premature closure of the foramen ovale. PH after birth was ameliorated, and his hemodynamic state was almost normal at 8 months old. However, the boy showed mitral valve thickening (Fig. 1 and Online Video C), severe mitral valve stenosis, and regurgitation at 14 months, and he became acutely symptomatic with suspected PH, as evidenced by a 25 mmHg pulmonary regurgitation pressure gradient. He subsequently underwent mitral valve replacement surgery. Operative findings during mitral valve replacement indicated a hammock mitral valve with all of the chordae fused together [8]. Postoperatively, his PH continued to progress to a suprasystemic level despite a well-functioning prosthetic mitral valve. Cardiac catheterization at 2 years of age revealed suprasystemic PH with elevated pulmonary vascular resistance (20.3 U*m2) and left ventricular end-diastolic pressure (15 mmHg) (Table 1).
Fig. 1.
Formation and completion of the “banana-shape.”
(A) During fetal development, there was only mild hypoplasia of the left-sided heart and no “banana-shape” of the right ventricle (RV).
(B) After birth, the size of the left side heart was normalized, but there was still no “banana-shape” of the RV.
(C) At 14 months of age, the extent of the left ventricle (LV) apex was shortened relative to the RV. The RV is surrounded by the left ventricular apex, revealing the typical “banana-shape” of the RV.
(D) At 16 years age, the typical “banana-shape” of the RV progressed.
(E) Magnetic resonance imaging at 14 years showed objective finding of the “banana-shape” of the RV and small LV.
(Red arrows indicate the position of the apex of the RV).
Table 1.
Hemodynamics by cardiac catheterization.
| Age | State | PAP (mmHg) | PCWP (mmHg) | LV EDP (mmHg) | RAP (mmHg) | AoP (mmHg) | QpI(L/min/m2) | RpI(U*m2) | Rp/Rs | SaO2(%) | PaO2(mmHg) | Qp/Qs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | BL | 80/44(61) | 15 | 15 | 15 | 78/47(60) | 2.3 | 20.3 | 1.02 | 89 | ||
| O2 10 L/min | 79/43(59) | 12 | 83/54(69) | 2.7 | 17.7 | 100 | 352 | |||||
| 8 | BL | 137/80(99) | 16 | 18 | 17 | 89/52(66) | 3.3 | 25.0 | 1.69 | 96 | 70 | |
| O2 10 L/min | 130/74(94) | 23 | 24 | 16 | 108/62(80) | 4.0 | 17.7 | 1.11 | 100 | 332 | ||
| O2 + PGI2 4 ng/kg/min | 121/65(87) | 32 | 32 | 15 | 110/60(81) | 4.3 | 12.9 | 0.83 | 100 | 191 | ||
| 14 | BL | 119/49(75) | 20 | 20 | 12 | 86/57(71) | 5.0 | 11.0 | 1.24 | 98 | 98 | 0.77 |
| FiO2 0.8 | 137/59(85) | 23 | 24 | 15 | 99/67(83) | 5.4 | 11.4 | 1.10 | 100 | 375 | 0.85 | |
| FiO2 0.8 + NO20 ppm | 131/57(82) | 35 | 31 | 14 | 101/63(81) | 7.6 | 6.2 | 0.80 | 100 | 379 | 0.90 | |
| FiO2 0.8 + PGI2 10 ng/kg/min | 115/43(69) | 29 | 29 | 14 | 92/52(68) | 7.6 | 5.3 | 0.61 | 100 | 455 | 1.21 | |
| FiO2 0.8, post BD | 116/51(75) | 21 | 21 | 13 | 93/59(72) | 6.4 | 8.4 | 0.79 | 100 | 401 | 1.16 |
RV, right ventricle; LV, left ventricle; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; EDP, end-diastolic pressure; RAP, right atrial pressure; AoP, aortic pressure; QpI, pulmonary output index; RpI, pulmonary arterial resistance; Rs, systemic arterial resistance; SaO2, arterial oxygen saturation; PaO2, partial pressure of arterial oxygen; Qs, systemic output; BL, baseline; PGI2, prostaglandin I2; NO, nitrogen oxide; BD, balloon dilation.
Despite continuing treatment with home oxygen, pulmonary vasodilator, and other anti-heart failure therapies, he started to show hemoptysis from the age of 7 years. We performed a second cardiac catheterization at this stage that revealed worsened suprasystemic PH compared to the initial catheterization. Intravenous prostacyclin was not indicated because of his highly elevated left ventricular end-diastolic pressure despite a reduction in pulmonary resistance. We were unable to identify the etiology of this high left ventricular end-diastolic pressure.
Because of the difficult situation regarding cardiopulmonary transplantation in Japan, neither the boy nor his parents wished for him to undergo transplantation. Instead, they selected a surgical intervention using a Potts shunt (a shunt between the descending aorta and the left pulmonary artery [6,7]). At 13 years old, the Potts shunt procedure was performed using a 12-mm expanded polytetrafluoroethylene (ePTFE) graft with 5 mm of banding at the center, and re-mitral valve replacement (On-X, 23 mm, CryoLife, Kennesaw, GA, USA) was performed to relieve his worsened circulation and dyspnea. Cardiac catheterization performed 1 year after the Potts shunt at 14 years (Table 1) showed ameliorated circulation and decreased pulmonary resistance (to 11.0 U*m2) by increased pulmonary flow and reduced mean pulmonary arterial pressure. His pulmonary arterial pressure was 119/49 (75) mmHg, and his systemic arterial pressure was 86/57 (71) mmHg. Balloon dilation of the Potts shunt was subsequently performed to secure the patency of the shunt. Currently, more than 3 years have passed since the last surgery, and he is now 17 years old. His suprasystemic PH has improved such that it is now almost balanced with his systemic circulation, as evidenced by low-velocity bilateral flow across the Potts shunt and tricuspid regurgitation pressure gradient. There were differences in percutaneous oxygen saturation between the upper and lower extremities (95–98% and 80–92%, respectively) under oxygen administration through the nasal canula. His quality of life is maintained as he can walk short distances, eat well, and enjoy his life. As a result of the highly elevated LV end-diastolic pressure, a round-shaped LV lacking its apex, and LV apex surrounded by enlarged right ventricle (RV), we recently came to the realization that his heart condition mimicked that of LV apical hypoplasia (Fig. 1C–E and Online Videos C, D). Retrospective echocardiographic reviews of this patient revealed an LV apex surrounded by enlarged RV; the typical “banana-shape [2],” was not observed in the fetal period but formed and developed after birth (Fig. 1A–D and Online Videos A–D).
Discussion
The LV morphology and physiology of this pediatric case with severe PH mimic those of LV apical hypoplasia [1,3,4] despite some differences. If we assume that this patient had LV apical hypoplasia, this case provides four novel points to consider for the disease: 1) This case was complicated by hammock mitral valves; 2) suprasystemic PH was ameliorated by a Potts shunt; 3) the characteristic “banana-shape” morphology seen in LV apical hypoplasia is not always congenital but can appear and develop after birth; and 4) the characteristic properties of LV and markedly elevated pulmonary vascular resistance may be responsible for the uneven increase in the sizes of the RV and LV and the formation of the “banana-shape.”
To the best of our knowledge, there have been no reported cases of LV apical hypoplasia complicated by a hammock mitral valve. In the current case, the PH progressed despite the fact that mitral valve replacement was performed at 15 months of age. The patient's PH was a combination of pulmonary arterial hypertension and PH due to left heart disease (Groups 1 and 2 of the updated classification, respectively). Previous reports have indicated that most patients with LV apical hypoplasia have mild disease, and some are even asymptomatic. In contrast, our search found three reported cases outside Japan [3,4] and three cases in Japan [9] that had severe PH. In Japan, two of the three cases with LV apical hypoplasia underwent cardiopulmonary transplantation. The patient in our case and his parents did not consider cardiopulmonary transplantation as an option; instead, he received a Potts shunt and mitral valve re-replacement. His PH status changed from “suprasystemic” to “almost balanced” after the Potts shunt, and his general status was maintained until the age of 17 years. Previous reports of Potts shunt use in PH patients have demonstrated its effectiveness, as evidenced by prolongation of 6-min walks, discontinuation of intravenous epoprostenol, reduced pulmonary vasodilator use, improvement in the New York Heart Association grade [10], and reduced mortality [7]. We believe that the Potts shunt contributed to the improvement seen in the PH in this case and that this clinical course supports the fact that a Potts shunt may be an important therapeutic option for treating patients with severe and suprasystemic PH due to LV apical hypoplasia as well as due to other etiologies [7].
LV apical hypoplasia has previously been reported to have four clinical features: 1) A truncated and spherical LV with abnormal diastolic and systolic function, 2) invagination of fatty material into the myocardium of the defective LV apex, 3) origin of a complex papillary network in the antero-apical LV, and 4) an elongated RV wrapping around the deficient LV apex [1] (“banana-shape”). Although the current case lacks some of these features [points 2) and 3), in particular], it does present with the other features, which are more likely to be specific for LV apical hypoplasia. Therefore, in the future, it should be clarified whether the presence of all four features is truly mandatory for the diagnosis of LV apical hypoplasia. In addition, we were able to obtain echocardiography information from the fetal stage, which showed us that the characteristic “banana-shape” is not always congenital but can appear and develop after birth in patients with LV apical hypoplasia (Fig. 1A–D and Online Videos A–D). Generally, the RV enlarges in response to pressure overload in patients with severe PH. Such changes may contribute to form the “banana-shape” in some patients with severe PH and LV apical hypoplasia, as in this case. The lack of histological examination of the myocardium or lung is a limitation of this report. More cases with long-term follow-up from the fetal stage are needed to elucidate the exact nature and subgrouping of this disease.
Declarations of Competing Interest
None.
Acknowledgments
We thank all the doctors and medical staff who were involved in the treatment of this patient in Shizuoka Children's Hospital, Toho University Omori Medical Center, and Saitama Medical University. We also thank Drs Shigetoyo Kogaki and Hidekazu Ishida for their comments on LV apical hypoplasia.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2021.03.006.
Appendix. Supplementary materials
(A) During fetal development, there was only mild hypoplasia of the left-sided heart and no “banana-shape” of the right ventricle (RV).
(B) After birth, the size of the left side heart was normalized, but there was still no “banana-shape” of the RV.
(C) At 14 months, the extent of the left ventricle (LV) apex was shortened relative to the RV. The RV is surrounded by the left ventricular apex, revealing the typical “banana-shape” of the RV.
(D) At 16 years, the typical “banana-shape” of the RV progressed.
(Red arrows indicate the position of the apex of the RV).
References
- 1.Fernandez-Valls M., Srichai M.B., Stillman A.E., White R.D. Isolated left ventricular apical hypoplasia: a new congenital anomaly described with cardiac tomography. Heart. 2004;90:552–555. doi: 10.1136/hrt.2003.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sousa A., Pinho T., Almeida P., Madureira A., Macedo F., Maciel M.J. Left ventricular apical hypoplasia: an unusual diagnosis. Rev Port Cardiol. 2013;32:265–267. doi: 10.1016/j.repc.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Irving C.A., Chaudhari M.P. Fatal presentation of congenital isolated left ventricular apical hypoplasia. Eur J Cardiothorac Surg. 2009;35:368–369. doi: 10.1016/j.ejcts.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Meng H., Li J.R., Sun X. Left ventricular apical hypoplasia: a case series and review of the literature. Acta Cardiol. 2013;68:339–342. doi: 10.1080/ac.68.3.2983433. [DOI] [PubMed] [Google Scholar]
- 5.Marin C., Sanchez M.L., Maroto E., Ossaba S., Ruiz Y., Zabala J.I. MR imaging of isolated left ventricular apical hypoplasia. Pediatr Radiol. 2007;37:703–705. doi: 10.1007/s00247-007-0459-4. [DOI] [PubMed] [Google Scholar]
- 6.Blanc J., Vouhe P., Bonnet D. Potts shunt in patients with pulmonary hypertension. N Engl J Med. 2004;350:623. doi: 10.1056/NEJM200402053500623. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster T.S., Shahanavaz S., Balzer D.T., Sweet S.C., Grady R.M., Eghtesady P. Midterm outcomes of the Potts shunt for pediatric pulmonary hypertension, with comparison to lung transplant. J Thorac Cardiovasc Surg. 2021;161:1139–1148. doi: 10.1016/j.jtcvs.2020.10.163. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto Y., Tamai A., Kawasaki H., Taketazu M., Senzaki H. Late clinical manifestations of mitral valve disease and severe pulmonary hypertension in a patient diagnosed with premature closure of foramen ovale during fetal life. World J Pediatr. 2011;7:182–184. doi: 10.1007/s12519-011-0276-6. [DOI] [PubMed] [Google Scholar]
- 9.Narita J., Kogaki S., Uchikawa T., Okada Y., Fukushima N., Ozono K., Inamura N., Kayatani F., Ishikawa Y., Ishikawa S. The three cases of RCM with left ventricular apex dysplasia. J Jpn Pediatr Soc. 2011;115:490. (in Japanese) [Google Scholar]
- 10.Baruteau A.E., Belli E., Boudjemline Y., Laux D., Levy M., Simonneau G., Carotti A., Humbert M., Bonnet D. Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: updated data from the first 24 patients. Eur J Cardiothorac Surg. 2015;47:e105–e110. doi: 10.1093/ejcts/ezu445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) During fetal development, there was only mild hypoplasia of the left-sided heart and no “banana-shape” of the right ventricle (RV).
(B) After birth, the size of the left side heart was normalized, but there was still no “banana-shape” of the RV.
(C) At 14 months, the extent of the left ventricle (LV) apex was shortened relative to the RV. The RV is surrounded by the left ventricular apex, revealing the typical “banana-shape” of the RV.
(D) At 16 years, the typical “banana-shape” of the RV progressed.
(Red arrows indicate the position of the apex of the RV).