Abstract
Glycans are expressed on the surface of nearly all host and bacterial cells. Not surprisingly, glycan-mediated molecular interactions play a vital role in bacterial pathogenesis and host responses against pathogens. Glycan-mediated host–pathogen interactions can benefit the pathogen, host, or both. Here, we discuss (i) bacterial glycans that play a critical role in bacterial colonization and/or immune evasion, (ii) host glycans that are utilized by bacteria for pathogenesis, and (iii) bacterial and host glycans involved in immune responses against pathogens. We further discuss (iv) opportunities and challenges for transforming these research findings into more effective antibacterial strategies, and (v) technological advances in glycoscience that have helped to accelerate progress in research. These studies collectively offer valuable insights into new perspectives on antibacterial strategies that may effectively tackle the drug-resistant pathogens that are rapidly spreading globally.
Overview
Glycans are commonly found on the surface of host and bacterial cells. These glycans are highly diverse, yet commonalities between host and bacterial glycans exist [1]. Not surprisingly, glycointeractions are at the center of bacterial pathogenesis and host responses against invading pathogens [2] (Figure 1). Core concepts relevant to host–pathogen interactions are discussed in this review, with examples reported in the recent literature. We start the review by discussing concepts concerning bacterial glycans used for colonization and/or immune evasion. For instance, some bacterial pathogens express cell-surface glycans that are vital for successful colonization on/in host cells; some bacterial pathogens express molecular mimicry of host glycans on their cell surfaces, enabling them to disguise themselves from host immune surveillance; and some bacterial pathogens cover their cell surface with layers of glycans to hamper host recognition of common pathogen-associated molecular patterns (PAMPs) – such as lipoteichoic acid (LTA), lipopolysaccharides (LPS), and flagellin [1]. We continue by discussing concepts of bacterial utilization of host glycans for pathogenesis. Many bacterial pathogens and toxins are equipped with lectin-like features that recognize specific host glycans expressed on a set of host cells for their colonization and virulence [2]. Conversely, in the following section, we discuss concepts concerning host immune cells utilizing bacterial glycans as molecular patterns to trigger bactericidal immune responses [3] and utilizing host glycans for immune responses against pathogens whose outcomes can be altered by input from microbiota. As a result, glycointeractions between pathogens and the host can benefit bacteria, host, or both. The review continues by discussing recent examples of antibacterial strategies designed on the basis of the glycobiology of host–pathogen interactions, and finally, we deal with the technological advances in glycoscience that have helped to accelerate the progress in research.
Figure 1. Glycointeractions in bacterial pathogenesis.
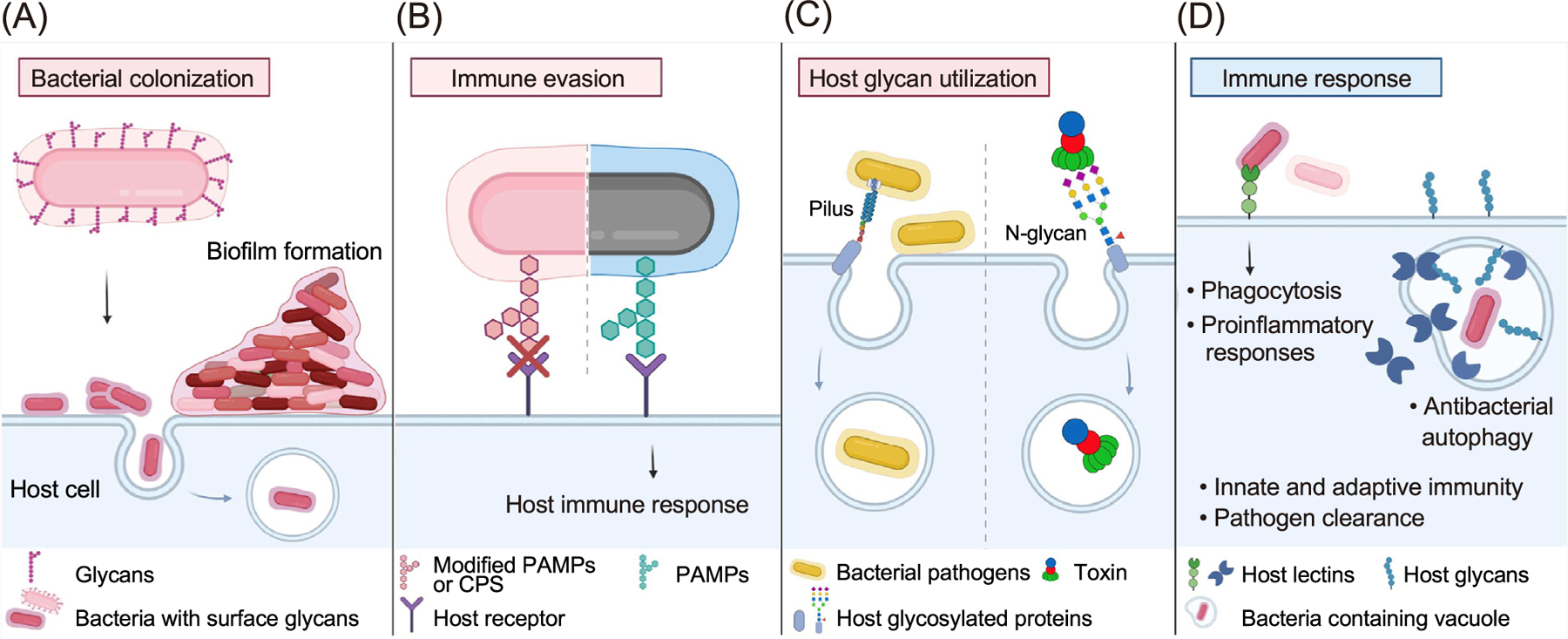
(A) Bacterial surface glycans play a vital role in adherence and colonization on host cells, which in some cases results in bacterial invasion of host cells. Bacterial glycans are also crucial for biofilm formation. (B) Some bacterial pathogens express molecular mimicry of host glycans on their cell surface (bacteria with pink hexagons) that can disguise themselves from host immune surveillance. Some bacteria cover their cell surface with layers of glycans [capsular polysaccharide (CPS), light pink and/or pink hexagons] to hamper host recognition of common pathogen-associated molecular patterns (PAMPs). In comparison, the recognition of PAMPs (green hexagons) and downstream immune responses is depicted in the right half of the graphic. (C) Many bacterial pathogens and toxins are equipped with lectin-like features that recognize specific host glycans (depicted as a gray rod in the left panel or gray rod with codes for N-glycans in the right) expressed on a set of host cells for their colonization and/or virulence. (D) Conversely, host immune cells can utilize bacterial glycans as molecular patterns to trigger bactericidal immune responses (depicted in the left) and host glycans (blue), resulting in various immune responses and/or pathogen clearance. Multiple pathogens have developed evasion strategies. This review discusses the mechanisms involved.
Bacterial glycans in bacterial colonization and/or immune evasion
Most bacterial glycoconjugates are located on cell surfaces and membranes. The membranes of Gram-positive bacteria consist of the inner membrane (IM) and cell wall comprised of peptidoglycan (PG), capsular polysaccharide (CPS), wall teichoic acid (WTA), and LTA (Figure 2). The roles of these bacterial glycans in host cell colonization or immune evasion are considerably well characterized. For instance, the functions of CPS in colonization, invasion of host cells, and immune evasion are perhaps best demonstrated in group A streptococci (GAS), such as S. pyogenes [4]. GAS glycans contain a polyrhamnose core where N-acetylglucosamine (GlcNAc) side chains are added by glycosyltransferase GacI, contributing to colonization and immune evasion [5], while ~25% of GlcNAc of GAS glycans can be further modified by glycerol phosphate via GacH, contributing to bacterial survival and pathogenesis [6]. Similarly, O-acetylation and deacetylation of glycans in the cell wall of Streptococcus pneumoniae contribute to enhancing resistance to lysozyme-mediated killing [7]. Glycosylated adhesins (e.g., cnm) on the surface of many Streptococcus spp. are vital for the binding to host cells and successful colonization [8,9]. Another example is Staphylococcus aureus WTA. The glycosylation of S. aureus WTA by TarS inhibits the binding of alternative penicillin-binding protein (PBP2a), contributing to resistance to β-lactam antibiotics, while methicillin-resistant S. aureus (MRSA) is known to utilize TarP-modified WTA to evade host defenses [10].
Figure 2. Common bacterial glycoconjugates located on bacterial cell surfaces and membranes.
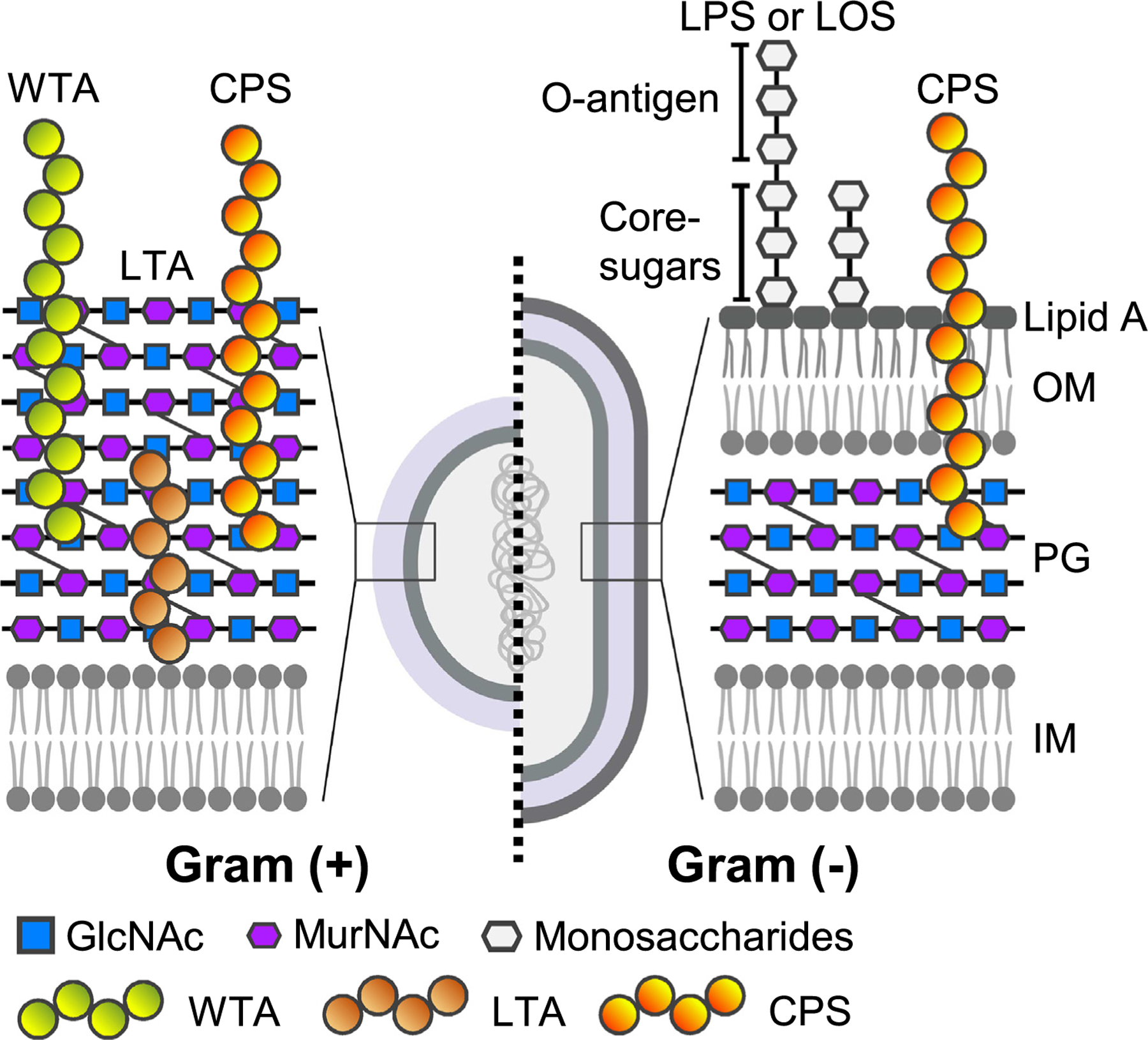
As depicted on the left, the membranes of Gram-positive bacteria consist of the inner membrane (IM) and cell wall comprised of peptidoglycan (PG, a polymer of GlcNAc and MurNAc), capsular polysaccharide (CPS), wall teichoic acid (WTA), and lipoteichoic acid (LTA). As depicted on the right, the membranes of Gram-negative bacteria consist of the PG layer sandwiched between the IM and outer membrane (OM) with the space between the two membranes referred to as the periplasm. The outer surfaces of Gram-negative bacteria are coated by glycans that are part of the lipopolysaccharide (LPS) O-antigen, lipo-oligosaccharide (LOS) core-sugars, and/or the CPS.
The membrane structure of Gram-negative bacteria consists of the PG layer sandwiched between the IM and outer membrane (OM) with the space between the two membranes referred to as the periplasm. The outer surface of Gram-negative bacteria is coated by glycans that are part of the LPS O-antigen, core-sugars, and/or the CPS (Figure 2). The PG is a highly ordered glycan repeat synthesized by MurE and MurF. In the context of host–pathogen interactions, the PG can activate the immune system, and thus the modification of PG to noncanonical variants prevents this activation [11,12]. Likewise, noncanonical variants of LPS, such as extended LPS O-antigens and the lack of O-antigens, are expressed by multiple bacteria for immune evasion and/or successful colonization. For instance, Salmonella enterica serovar Paratyphi A produces very long O-antigen chains that resemble the length and function of the Vi CPS of S. Typhi, both of which hamper immune surveillance [13]. S. Typhi and S. Paratyphi cause the life-threatening systemic diseases typhoid fever and paratyphoid fever, respectively. A very long O-antigen is also observed in Pseudomonas aeruginosa that is controlled by the Wzz system [14], contributing to immune evasion [15]. Conversely, multiple bacteria produce lipo-oligosaccharides (LOS) in place of LPS, including species of Campylobacter and Neisseria. LOS is analogous to LPS for Lipid A and core oligosaccharides but lacks O-antigens (Figure 2). Some of these bacteria, such as Acinetobacter baumannii, have further evolved to carry either LOS modified with phosphatidylethanolamine and galactosamine (GalN) or can tolerate the total loss of LOS/LPS, contributing to resistance to antimicrobial peptides and/or antibiotics targeting Lipid A, such as a ‘last-resort’ antibiotic colistin [16,17].
In A. baumannii, several O-oligosaccharyltransferases (O-OSTs) glycosylate other surface molecules [18], which are also associated with antibiotic resistance and/or immune evasion. These include general O-OSTs, like PglL, that is responsible for glycosylating serine and threonine residues of multiple proteins, PglC, known for attaching GalNAc to the lipid carrier [18], and substrate-specific O-OSTs such as TfpO known for glycosylating C-terminal serine residues of type IV pilin [19]. Lastly, multiple Gram-negative bacteria have evolved to express CPS for pathogen survival and/or immune evasion. Notable examples are the CPSs of S. Typhi [20], A. baumannii, and Neisseria meningitidis [21,22], protecting the pathogens from complement-mediated bactericidal activities and preventing the recognition of PAMPs on bacterial cells. These bacteria can modify the CPSs by O-acetylation that is known to enhance the stability of these capsular glycans, contributing to the prolonged protection from bactericidal activities and the inhibition of triggering effective immune responses against the pathogens.
Host glycans utilized by bacteria for pathogenesis
Physical barriers to the entry of pathogens, including the gastrointestinal (GI) tract, urogenital tract, and respiratory tract, are heavily glycosylated, which is vital for the host barrier function. Paradoxically, those host glycans benefit multiple pathogens for colonization, tropism, and/or a source of nutrients during infection (Figure 3). Relating to colonization, in the GI tract, S. Typhimurium Std fimbriae [23] and Bacillus subtilis YesU [24] use fucosylated glycans abundant in the gut to adhere and colonize. Vibrio cholerae adhesins, such as V. cholerae cytolysin (VCC) and RbmC, bind complex-type N-linked glycans expressed on intestinal epithelial cells to support colonization [25], while enterotoxigenic Escherichia coli (ETEC) adhesin EtpA binds blood group A glycans to adhere and deliver AB5 toxins, heat-labile, and heat-stable toxins [26]. Helicobacter pylori infection alters glycan expression in the gastric tissues, which elevates an autophagy response in infected cells, supporting H. pylori replication [27].
Figure 3. Utilization of host glycans by bacterial pathogens.
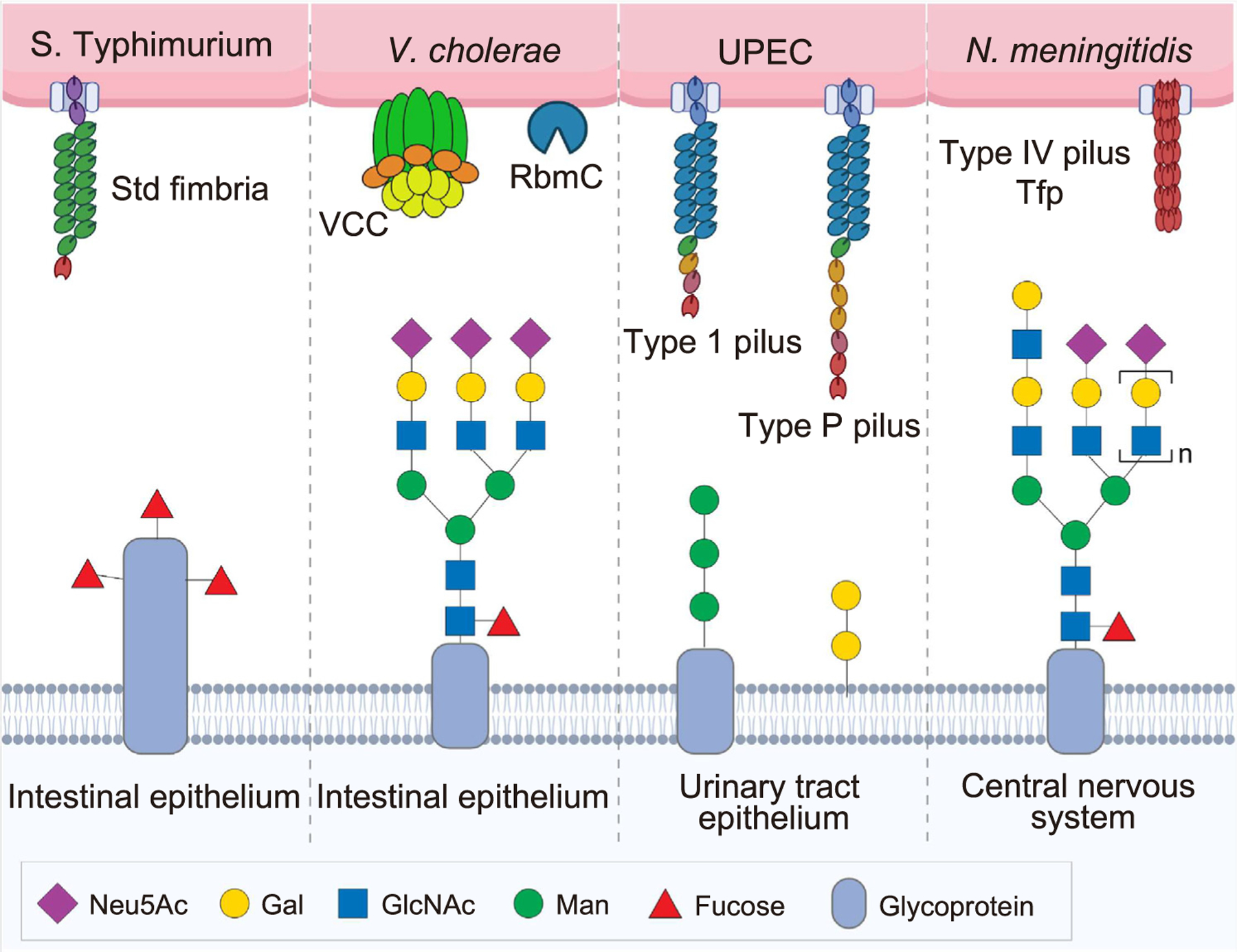
Physical barriers to the entry of pathogens (the light blue shaded area in this graphic), including the gastrointestinal (GI) tract, urogenital tract, and respiratory tract, are heavily glycosylated, which is vital for the host barrier function. Paradoxically, those host glycans benefit multiple pathogens for colonization, tropism, and/or as a source of nutrients during infection. Bacterial names and virulence factors involved are indicated in the top portion of this graphic. Some notable examples are highlighted in this figure and are discussed under the heading ‘Host glycans utilized by bacteria for pathogenesis’. Abbreviations: S. Typhimurium, Salmonella enterica serovar Typhimurium; V. cholerae, Vibrio cholerae; UPEC, uropathogenic Escherichia coli; N. meningitidis, Neisseria meningitidis; VCC, Vibrio cholerae cytolysin; RbmC, the biofilm matrix protein.
Glycan-mediated tropisms of pathogens are exemplified in the urogenital tract [28]. Predominant glycans differently expressed in cells and tissues are associated with tropism of pathogens affecting these tissues. For example, uropathogenic E. coli (UPEC) adhesins type 1 and type P fimbriae bind mannose-containing glycans and galactose (Gal)α1–4Gal moieties of glycolipids predominantly expressed on the lower and upper urinary tract epithelium, respectively [29], corresponding to their primary target sites. Similarly, N. gonorrhoeae binds to mannosyl glycans expressed on cervical and urethral epithelial cells, while Chlamydia trachomatis recognizes N-glycosylated galectin-1 (Gal1) expressed on the genital tract, promoting bacterial attachment and entry in their primary infection sites [30,31]. Furthermore, the recognition of triantennary sialylated N-glycans containing poly-N-acetyllactosamine by N. meningitidis type IV pili Tfp is an example of glycan-mediated pathogen tropism to the central nervous system (CNS) [32].
In the respiratory tract, among several invasive groups of streptococci named groups A, B, C, and G, the GAS bind glycans terminating in sialic acid (N-acetylneuraminic acid, Neu5Ac) and GalNAc to colonize in the oral cavity and respiratory tract [33,34]. Coinfection of group B streptococci (GBS) with Influenza A virus (IAV), that utilizes α2,3-linked sialic acids/Neu5Acs on epithelial cells in the respiratory tract, results in more severe infectious outcomes [35]. Intriguingly, as the infection progresses, some streptococci produce bacterial glycoside hydrolases (GHs) to degrade host glycans to utilize as nutrients to benefit their growth during infection [36,37]. Similarly, P. aeruginosa, also infecting the respiratory tract, can exploit monosaccharides digested from host mucins as sources of nutrients for successful colonization [38].
Bacterial toxins
Given that almost all AB toxins use host glycans as cellular receptors [39], bacterial AB toxins are prime examples that help us to understand multiple important concepts of glycan-mediated host–pathogen interactions, including the roles in tropism to a specific set of target host cells, host adaptation, and toxin trafficking pathways (Figure 4). For instance, Salmonella typhoid toxin, an A2B5 toxin consisting of two enzymatic subunits CdtB and PltA linked to a homopentamer of glycan receptor binding subunits PltB5 [40], exhibits tropism to specific target host cells. The in vivo tropism of typhoid toxin is achieved through high-affinity multivalent binding to glycan receptor moieties [41]. Consistently, multiantennary N-linked glycans, terminated in Neu5Acα2–6 or α2–3-linked to the underlying Gal and GlcNAc disaccharides, serve as cellular receptors for typhoid toxin, contributing to in vivo tropism to a range of host cells in the GI tract, systemic sites, and biliary tract that match with the dynamic infectious cycle of the bacteria [40–42]. 9-O-acetylation of the terminal sialic acid Neu5Ac further increases the binding affinity of typhoid toxin to glycan receptor moieties, also contributing to in vivo tropism of typhoid toxin [41,43]. Intriguingly, in certain conditions, typhoid toxin CdtB and PltA subunits are known to be able to assemble an alternate A2B5 toxin with alternate glycan receptor-binding subunits PltC5 [44]. Glycans recognized by PltC subunits remain to be identified, but we predict that the glycan target matches with the infectious cycle of this pathogen.
Figure 4. Utilization of host glycans by bacterial AB toxins.
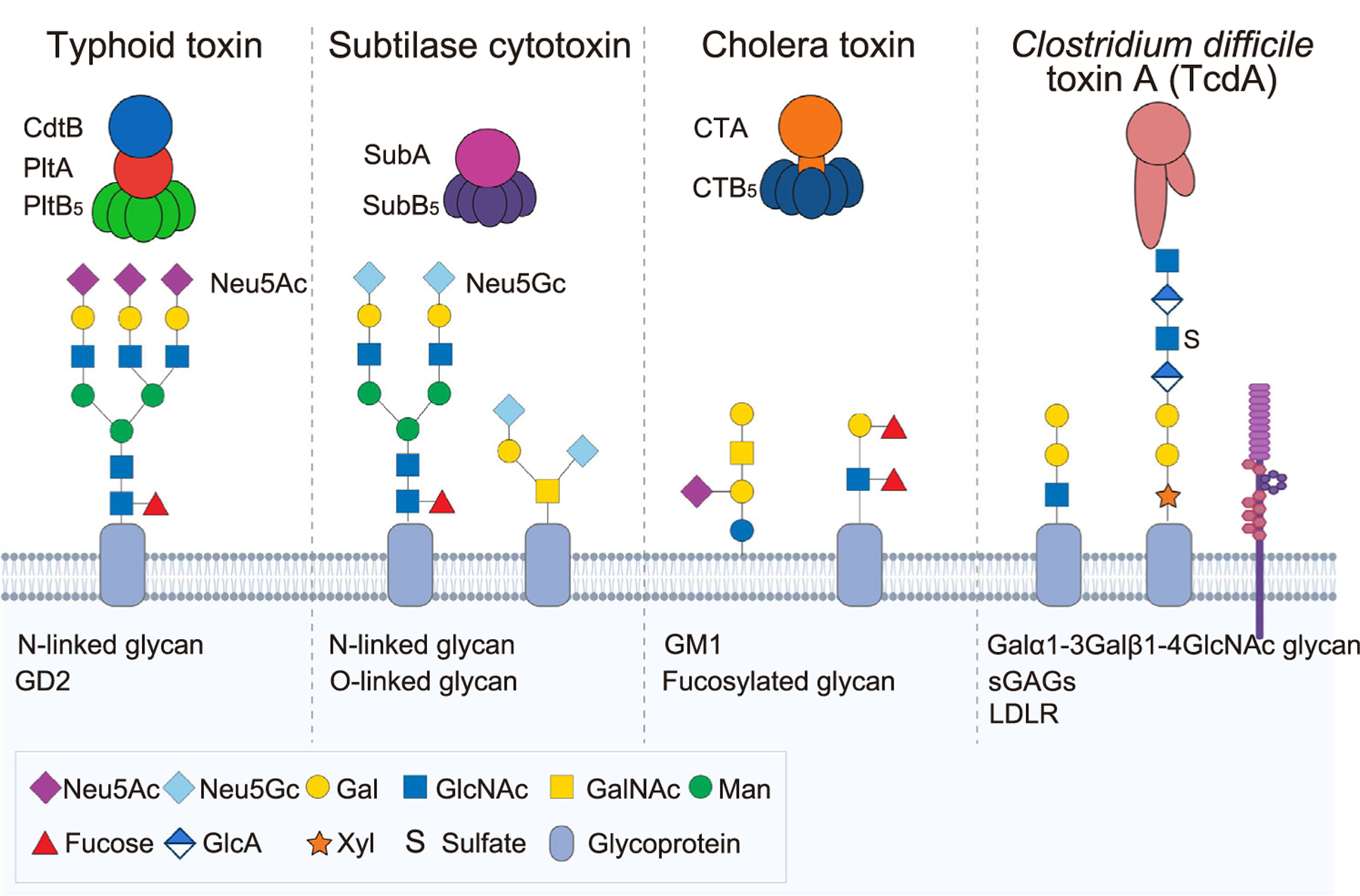
Almost all bacterial AB toxins use host glycans as cellular receptors. Toxin names and their glycan receptor moieties identified are indicated in this graphic. The light blue shaded area depicts target host cells. Some notable examples are highlighted in this figure and are discussed under the heading ‘Bacterial toxins’. Abbreviations: LDLR, low-density lipoprotein receptor; sGAGs, sulfated glycosaminoglycans.
Salmonella A2B5 toxins also help us to understand the role of glycan-mediated host–pathogen interaction in cell, tissue, and host adaptations. Typhoid toxin orthologs with some amino acid sequence variations on subunits, including their glycan-receptor binding subunits, are encoded in the genomes of some nontyphoidal Salmonella (NTS) serovars. One such toxin characterized at the molecular level is Javiana toxin expressed by S. Javiana [42]. The glycan receptor-binding subunit of Javiana toxin JaPltB contains three amino acid sequence variations that are sufficient for changing the tropism of this toxin to intestinal epithelial cells. This change is considered a niche-specific cell/tissue adaptation because the GI tract is the primary infection site of NTS serovars that, in general, do not cause systemic infection in immunocompetent humans [42].
About host adaptation, typhoid toxin recognizes sialosides terminated in Neu5Ac but not to ‘nonhuman-type’ N-glycolylneuraminic acid (Neu5Gc) that is identical to Neu5Ac in structure except for the hydroxyl group added at the C11 position of Neu5Ac [45]. Neu5Gc is often called ‘nonhuman-type’ because, unlike various animal species, humans cannot synthesize Neu5Gc due to the lack of the functional cytidine monophospho-Neu5Ac hydroxylase (CMAH) that converts Neu5Ac to Neu5Gc. Thus, humans can make Neu5Ac only. The preference of typhoid toxin to ‘human-type’ Neu5Ac correlates to the human-specific feature of S. Typhi [45]. This aspect of typhoid toxin is often contrasted to subtilase cytotoxin (SubAB) from the enteric pathogen Shiga-toxigenic Escherichia coli (STEC) to highlight host adaptation of bacterial toxins. SubAB is an AB5 toxin that binds glycans terminating in Neu5Gc and exhibits a strong binding preference toward Neu5Gc over similar glycans but terminating in Neu5Ac [46]. As noted previously, human intestinal epithelial cells cannot synthesize Neu5Gc, but a small amount of Neu5Gc can be incorporated in human cells from diets such as red meat [46], thus serving as target host cells for this toxin.
Typhoid toxin, and possibly SubAb, can control their trafficking mechanisms through toxin–glycan interactions. A characteristic two-stage trafficking mechanism is involved in typhoid toxin, consistent with the observation that typhoid toxin is produced only by S. Typhi which has invaded host cells. S. Typhi situated in the extracellular environment does not produce typhoid toxin. Environmental cues found in the Salmonella-containing vacuole (SCV) are vital for inducing the expression of typhoid toxin subunits [47,48]. The first stage of typhoid toxin trafficking is toxin export from infected host cells to the extracellular environment. The second stage of toxin trafficking is toxin-receptor binding and endocytosis [47,49]. PltB binding to its specific glycan receptor moieties expressed on the SCV membrane in infected host cells regulates the first stage toxin of export [49]. The interaction of the exported toxin with multiantennary N-linked glycans on target host cells is essential for the second stage of toxin endocytosis [40,41]. SubAB toxin is known to use complex-type N-glycans and core 1 O-glycans for entry and subsequent cellular toxicity [50]; it is intriguing to hypothesize that the glycan-binding preferences of SubB5 to these glycans change during the retrograde toxin trafficking to facilitate the process.
Several concepts described previously are also shared among other bacterial AB toxins. Glycan receptor binding profiles of cholera toxin, an AB5 toxin secreted by the enteric pathogen V. cholerae, have been revisited recently. In addition to GM1 gangliosides, fucosylated glycans linked to glycoproteins are proposed as a preferred glycan receptor for subsequent endocytosis processes in intestinal epithelial cells [51]. The binding pockets for fucosylated glycoprotein glycans are located on the lateral side of the B subunit pentamer, apart from the binding pockets for GM1 situated on the bottom side of the pentamer [52], suggesting multiple combinations that can result in multivalent high-affinity bindings between the toxin and glycans. The similar multivalent interaction mechanisms to intestinal epithelial cells through multiple binding pockets available in the B subunit pentamer have been shown using two Salmonella A2B5 toxins, typhoid toxin and Javiana toxin [41,42]. Furthermore, the roles of toxin–glycan interactions in toxin tropism are highlighted in the following recent discoveries. Bacterial pore-forming toxins, such as Tc toxin complexes and cholesterol-dependent-cytolysins (CDCs), have been characterized for their uses of glycans as cellular receptors and toxin tropism [53,54]. Clostridium difficile toxin A (TcdA) is a single-chain AB-type toxin contributing to the disruption of the colonic epithelium during C. difficile infection, causing fluid secretion, inflammation, and cell death [55]. TcdA contains two glycan receptor-binding domains which use Galα1–3Galβ1–4GlcNAc glycans, sulfated glycosaminoglycans (sGAGs), and low-density lipoprotein receptors (LDLRs) as its cellular receptor expressed on colonic epithelial cells [56,57].
Bacterial and host glycans for immune responses against pathogens
Bacterial glycans
Host immune cells recognize bacterial glycans and induce bactericidal immune reactions. C-type, Siglecs, and galectins are three major classes of lectins involved in this process, preferentially binding to mannose-, sialic acid-, and N-acetyllactosamine-rich glycoconjugates, respectively [58]. C-type lectins recognize many pathogens and induce proinflammatory responses, although not surprisingly, many pathogens have evasion mechanisms [58]. The mannose-binding lectin (MBL), a kind of C-type lectin, recognizes a variety of pathogen oligosaccharides, including those expressed on Borrelia burgdorferi, and induces bactericidal responses [59]. Another type of C-type lectin, the macrophage galactose-binding lectin (MGL), recognizes GalNAc on S. aureus and induces cytokine production, which may affect downstream adaptive immune responses and pathogen clearance [60]. Siglecs-mediated interactions to GBS, N. meningitidis, and N. gonorrhoeae, can induce host inflammatory responses [58]. Galectins bind to glycans of several important pathogens, including the opportunistic P. aeruginosa, and mediate the up-take by phagocytic cells [61].
Host glycans
Host glycans are crucial for innate and adaptive immune responses against bacteria. Regarding the roles in innate immune responses, lectins can control pathogens after the invasion of host cells by modulating cellular autophagy processes by recognizing specific types of host glycan. Intracellular galectins recognize host glycans expressed on vacuoles harboring intracellular pathogens, resulting in antibacterial autophagy [62]. For instance, the cytosolic galectin-3 recognizes damage-exposed host glycans, which induces autophagic responses against damaged lysosomes, offering protection against Mycobacterium tuberculosis [63].
An additional example highlighting the role of host glycan in innate immunity is the mucus that is produced by many pathogen entry sites in the body; it guards against pathogens while serving as a niche for microbiota and a protective layer to keep critical organs sterile [64]. For instance, mucin glycans can directly inhibit the expression of virulence genes of pathogens, including P. aeruginosa [65]. Furthermore, the microbiota can modulate host immune responses to infection by regulating cytokine expression [66,67], alleviating neurotoxicity [68], or influencing mucosal immunity [69]. One mechanism is by using host- and/or diet-derived glycans; gut microbiota, such as Bacteroides, uses enzymes encoded in the polysaccharide utilization loci (PULs) to utilize available glycans [70,71].
Host glycans also play a critical role in the development of immune cells and adaptive immune responses. For instance, the role of N-glycan branching in thymocyte development has been established [3]. N-glycan branching of the T cell receptor and CD4/CD8 coreceptors modulates the affinity for the positive selection of T cells. Their role in B cell development was also reportedly characterized in studies in which a lack of branched N-glycans resulted in the impaired maturation of B cells [72]. N-glycan branching played a critical role in B cell receptor and coreceptor expression modulation, essential in promoting B cell selection. Glycosaminoglycans (GAGs), such as heparin, chondroitin, and dermatan sulfate, bind to chemokine receptors, such as CXCL1 and CXCL5, to play a diverse role in the immune system [73]. Furthermore, critical functions regarding the N-glycosylation of immunoglobulins in antibody stability and placental IgG transfer efficiency have been demonstrated [74,75].
Antibacterial strategies
Host glycan mimicry (Table 1)
Table 1.
Examples of antibacterial strategies targeting glycan-mediated host–pathogen interactions, which are discussed in this review
| Names | Targets | Challenges |
|---|---|---|
| Glycan mimicry | ||
| Aryl galactoside and GalNAc | FmlH adhesin of the F9 pilus used by UPEC [76,77] | Requires structure-based optimizations of high-affinity ligands to antagonize bacterial lectins [76]. |
| Zanamivir sialic acid mimic | Periodontal bacterial sialidases [78] | |
| STARFISH Gb3 mimic | Homopentameric receptor-binding B subunits of Shiga-like toxin I | Must overcome the low intrinsic affinity of carbohydrate-protein interactions through the use of an oligovalent ligand [79]. |
| L-fucose analogs | Homopentameric receptor-binding B subunits of cholera toxin | Requires optimization of the analog candidates through the structure-activity relationship analysis of the CTB-fucose interaction [80]. |
| Antibodies | ||
| Anti-CPS IgG MAbs | CPS of carbapenem-resistant K. pneumoniae | Only elicit passive immunity against the bacterial target [81]. |
| Anti-KDO MAb | KDO added to a terminal N-acetyllactosamine structure of LOS on NTHi | NTHi expresses KDO only under certain conditions in vivo, and bactericidal effects were observed only in a limited number of strains [82]. |
| Anti-PltB MAbs | Glycan receptor-binding pockets on the receptor-binding subunits [83,84] | A-subunit-mediated asymmetry-associated interference of antibody binding to the lateral side-located epitopes on the receptor-binding subunits [84]. |
| Vaccines | ||
| Pneumococcal vaccines | CPSs of specific S. pneumoniae strains | Serotype-specific protection has prompted changes in serotype prevalence, requiring multivalent vaccines incorporating synthetic glycoconjugates to cover more serotypes [85]. |
| Vi CPS vaccine | Vi polysaccharide of S. Typhi | Emergence of S. Typhi strains lacking Vi polysaccharide [87]. |
| Hypervirulent K. pneumoniae bioconjugate bivalent vaccine candidate | CPSs of the K1 and K2 K. pneumoniae serotypes | Uses a relatively new, in vivo method of glycan-carrier protein conjugation [88,89]. |
| C. difficile synthetic glycoconjugate vaccine candidates | C. difficile surface glycans PS-I, PS-II, and PS-III | Requires synthetic glycan conjugation to a carrier protein [90]. |
| Gonococcal peptide mimotope vaccine candidate | Glycan epitope on the LOS of N. gonorrhoeae | Requires the development of a stable and homogeneous peptide mimotope derivative of the glycan epitope [91]. |
High-affinity synthetic glycans resembling host glycans recognized by bacteria or toxins have been demonstrated as effective antibacterial strategies. Various mannoside compounds effectively compete over endogenous host glycans recognized by the type 1 fimbriae of UPEC and N. gonorrhoeae [30,76,77]. A similar strategy has been developed against the adhesin FmlH in F9 fimbriae of UPEC; aryl galactoside and GalNAc, high-affinity binders to FmlH designed through structure-guided analyses, inhibit the binding of UPEC to human kidney tissue [76,77]. Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, oral pathogens capable of causing severe periodontitis, secrete sialidases as a major virulence factor(s) that remove sialic acids from sialylated glycoproteins expressed on host cells. Consistently, Zanamivir, an FDA-approved influenza drug that inhibits the enzymatic activity of sialidases, effectively inhibited P. gingivalis sialidase SiaPG, resulting in the inhibition of biofilm formation, initial attachment, and invasion into oral epithelial cells [78]. Glycan mimicry has also been effective against bacterial toxins that use host glycans as cellular receptors. Shiga-like toxin 1 (STL-1) of E. coli, an AB5 toxin, contains the characteristic B5 homopentamer that recognizes its glycan receptor globotriaosylceramide (Gb3), whose interactions were effectively interrupted by a pentavalent, water-soluble glycan mimicry dubbed STARFISH [79]. The homopentameric receptor-binding B subunits (CTB5) of cholera toxin utilize sialylated, fucosylated N-linked glycans expressed on intestinal epithelial cells; L-fucose analogs were able to inhibit the binding of CTB to host cells, indicating the potential of a non-natural fucose-containing polymer as an antitoxin strategy [80].
Vaccines and antibodies targeting bacterial glycans (Table 1)
Antibodies designed to target specific bacterial cell-surface glycans are shown to be effective against bacterial pathogens, including drug-resistant bacteria. For example, two anti-CPS IgG monoclonal antibodies (MAbs) against carbapenem-resistant Klebsiella pneumoniae ST258, capable of binding to the CPS of the clinically relevant clade 2, promoted opsonophagocytic killing and protected mice infected intratracheally with the bacteria, demonstrating passive immunotherapy potential [81]. An MAb recognizing the ketodeoxyoctanoate (KDO) within the LOS of the respiratory pathogen nontypeable Haemophilus influenzae (NTHi) was able to kill the bacteria, providing insights into the development of a multivalent vaccine(s) against NTHi using a glycoconjugate made of multiple KDOs or a KDO-N-acetyllactosamine conjugated to an immunogenic protein [82]. Also, MAbs recognizing the glycan receptor-binding pockets of Salmonella typhoid toxin neutralize the toxin with various efficacy depending on their epitope locations [83,84].
Glycan-based vaccines against the CPSs of S. pneumoniae and S. Typhi are perhaps best known. S. pneumoniae vaccines that target the CPS afford serotype-specific protection [85]. Unfortunately, yet not unexpectedly, there are 98 recognized polysaccharide serotypes among S. pneumoniae; the wide use of vaccines has prompted bacterial evolution to overcome vaccine-induced immune responses, suggesting necessary refinement of pneumococcal vacci-nation strategies [85]. Coformulation of licensed pneumococcal vaccines with synthetic glycoconjugates representing serotypes not covered by existing vaccines has shown potential in multivalent vaccine production [86]. Subunit vaccines against S. Typhi are based on the CPS Vi antigen, which affords serotype-specific protection. Similar to pneumococcal CPS vaccines, S. Typhi strains that lack Vi have emerged in endemic areas [87].
Similar to the previously-mentioned examples, glycan-mediated vaccine development against many other bacteria is in progress, including bioconjugate vaccines against hypervirulent K. pneumoniae serotypes [88] and polyvalent pneumococcal bioconjugate vaccines [89]. Additional promising examples include glycoconjugate vaccine candidates against C. difficile GI infection designed to resemble surface glycans PS-I, PS-II, and PS-III using four synthetic antigens ranging in size from disaccharides to hexasaccharide [90] and a peptide mimotope vaccine candidate of the glycan epitope on the LOS of N. gonorrhoeae against gonococcal genital tract infection [91].
The development of glycan-mediated vaccines would likely be accelerated as relevant technologies advance. Some of the aforementioned vaccines have been produced using glycoengineered E. coli cells (rather than conventional chemical methods), highlighting alternative vaccine production platforms. Also, newly characterized capsular polymerases that synthesize GBS capsule polymers could serve as another attractive tool to produce capsule polymers useful in glycoconjugate vaccine formulations [92]. Likewise, the recent development of a robust exponential glycan synthesis model, already shown to be capable of synthesizing 128-mer and creating a product relevant to the O-antigen of Bacteroides vulgatus, could serve as a tool for obtaining long, specific glycans [93], suggesting its broader application in multivalent glycan-based vaccine synthesis.
Technological advances in glycoscience
High-throughput approaches
Glycan microarrays are a high-throughput means of analyzing glycan-binding profiles of microbes, protein virulence factors, and others. A range of mammalian glycan microarrays has been instrumental in assigning carbohydrate specificities to several glycan-binding proteins ranging from microbial agents, such as bacterial toxins and viruses, to mammalian surface receptors for cell-to-cell interactions [94–96]. Advancements in bacterial glycan microarrays have been hampered by the bacterial glycome diversity that is even more diverse than mammalian counterparts [1]. Recent efforts have been directed towards constructing microbe-focused glycan arrays. For instance, combined complementary glycan synthesis strategies were used to create a pathogen-focused valuable platform for future investigations of serum antiglycan antibodies, innate immune receptors, and bacterial virulence factors [97]. Besides conventional glycan microarrays, electrospray ionization (ESI)-mass spectrometry (MS) has been demonstrated to be suitable for providing the interactions of glycan-binding proteins with their ligands in a high-throughput, quantitative manner. The use of an oligosaccharide library with common affinity tags against a series of glycan-binding proteins allowed for rapid identification and quantification of ligands [98]. Glycomics uses mass spectrometry to gain insight into the repertoire of oligosaccharides present, while computational analytic tools are essential for analyzing and quantifying glycans [99,100]. E-infrastructure has been established to overcome deficits in data and experimental transparency by enabling a workflow implementing the standardized submission of MS-based glycomics information into the public repository UniCarb-DR, MIRAGE (Minimum Requirement for A Glycomics Experiment) reporting guidelines, storage of unprocessed MS data in the GlycoPOST repository, and glycan structure registration using the GlyTouCan registry [99].
Mechanistic and translational studies
Lectins are glycan-binding proteins specifically recognizing one or more glycan structures. Many bacterial, viral, plant, and animal lectins are characterized, collectively serving as a valuable research tool. Lectins are particularly useful for mapping glycan structures expressed on cells and tissues and the tropism of particular pathogens, critical for understanding glycan-mediated host–pathogen interactions [1]. Glycosidases are one group of carbohydrate-active enzymes (CAEs) which hydrolyze the glycosidic bond between two or more glycans in a substrate-specific manner. For instance, sialic acids, often the terminal glycan of various glycoconjugates, are very diverse in structure, evident by more than 30 variants of sialic acids known thus far, which plays a critical role in the cell, tissue, or host tropism of many pathogens [101,102]. Various sialidases serve as an important research tool for investigating the tropism of pathogens and virulence factors [1]. PNGase F, also commonly used, is specific to N-linked glycoproteins and allows for research tailored for N-linked glycoproteins [103]. Many in vitro and in vivo research tools, such as glycoengineered knockout and knockin cells and mouse lines, have been helpful for understanding structure–function correlations. Moreover, computational methods, including a deep-learning tool, help us to understand glycan function in the context of host–pathogen interactions by using a large, curated glycan dataset in predicting glycan immunogenicity and the pathogenicity of bacterial strains [104]. Web resources such as GlycoSuiteDB and ProGlycProt V2.0 (for prokaryotic origin) have been designed to consolidate findings from the scientific literature on glycoprotein-derived glycan structures, their biological sources, the references in which the glycan was described, and the methods used to determine the glycan structure [105,106]. Furthermore, we predict that the expansion and streamlining of glycan synthesis methods would offer more readily accessible glycan products useful for in-depth structure–function studies, glycan arrays, vaccine development, and therapeutic development. For instance, streamlined one-pot procedures with automatic and programmable potential that integrate chemical, enzymatic, or chemoenzymatic synthesis methods have been established for producing complex glycans in a more effective manner [107,108].
Concluding remarks
Understanding the glycobiology of host–pathogen interactions is important and offers insights into antibacterial strategies. Successful interventions using glycan-based vaccines and glycan mimicry against several bacteria or bacterial toxins promise the fruitful outcomes of similar interventions against many other bacterial infections. Therefore, further collective efforts in this research area are expected to continually tackle clinical challenges posed by the global spread of multidrug-resistant pathogens (see Outstanding questions).
Outstanding questions.
Is there any way to improve the overall efficacies of glycan-mediated vaccines and/or glycan mimics?
Is there any way to slow down the emergence of bacterial pathogen variants that can evade vaccines targeting bacterial glycans and/or glycan mimics?
Is there any cost-effective way to implement a regular surveillance program to monitor the emergence of bacterial pathogen variants that can evade vaccines targeting bacterial glycans and/or glycan mimics?
Is there any cost-effective way to implement a basic research pipeline to rapidly determine newly emerged glycointeractions between bacterial pathogen variants and host?
Is there any cost-effective way to implement a translational pipeline to rapidly develop intervening strategies targeting newly emerged glycointeractions between bacterial pathogen variants and host?
Would the use of strategies targeting glycointeractions between pathogen and host in combination with other antibacterial strategies result in a better outcome in treating infection?
Highlights.
Glycans are common on the cell surface of host and bacterial cells, and not surprisingly, glycan-mediated molecular interactions play a critical role in bacterial pathogenesis and host responses against pathogens.
Bacterial glycans play a vital role in bacterial colonization, invasion, and/or immune evasion.
Many bacterial pathogens and their virulence factors exploit host glycans for pathogenesis and virulence.
Host glycans and lectins play a crucial role in inducing bactericidal immune responses, although many pathogens have developed evasion mechanisms.
Targeting glycan-mediated interactions between host and pathogen has emerged as an effective antibacterial strategy.
Technological advances in glycoscience have helped accelerate research progress in the field.
Acknowledgments
We acknowledge that we could not include and cite many other important papers in this review due to space constraints. This work was supported in part by NIH R01 AI137345, AI139625, AI141514, R03 AI135767, and the USDA/NIFA Hatch project 1017170 to J.S.
Footnotes
Declaration of interests
There are no interests to declare.
References
- 1.Varki A et al. (2017) Essentials of Glycobiology (3rd edn), Cold Spring Harbor Laboratory Press; [PubMed] [Google Scholar]
- 2.Poole J et al. (2018) Glycointeractions in bacterial pathogenesis. Nat. Rev. Microbiol 16, 440–452 [DOI] [PubMed] [Google Scholar]
- 3.Wolf AJ and Underhill DM (2018) Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol 18, 243–254 [DOI] [PubMed] [Google Scholar]
- 4.Wessels MR (2019) Capsular polysaccharide of group A Streptococcus. Microbiol. Spectr 7. 10.1128/microbiolspec.GPP3-0050-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henningham A et al. (2018) Virulence role of the GlcNAc side chain of the Lancefield cell wall carbohydrate antigen in non-M1-serotype group A Streptococcus. mBio 9, e02294–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgar RJ et al. (2019) Discovery of glycerol phosphate modification on streptococcal rhamnose polysaccharides. Nat. Chem. Biol 15, 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollmer W et al. (2019) The cell wall of Streptococcus pneumoniae. Microbiol. Spectr 7. 10.1128/microbiolspec.GPP3-0018-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avilés-Reyes A et al. (2018) Characterization of the pgf operon involved in the posttranslational modification of Streptococcus mutans surface proteins. Sci. Rep 8, 4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobbs AH et al. (2009) Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev 73, 407–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerlach D et al. (2018) Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 563, 705–709 [DOI] [PubMed] [Google Scholar]
- 11.Turner RD et al. (2018) Molecular imaging of glycan chains couples cell-wall polysaccharide architecture to bacterial cell morphology. Nat. Commun 9, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yakhnina AA and Bernhardt TG (2020) The Tol-Pal system is required for peptidoglycan-cleaving enzymes to complete bacterial cell division. Proc. Natl. Acad. Sci. U. S. A 117, 6777–6783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiyoshi H et al. (2018) Mechanisms to evade the phagocyte respiratory burst arose by convergent evolution in typhoidal Salmonella serovars. Cell Rep. 22, 1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huszczynski SM et al. (2019) Unique regions of the polysaccharide copolymerase Wzz(2) from Pseudomonas aeruginosa are essential for O-specific antigen chain length control. J. Bacteriol 201, e00165–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado RF et al. (2016) Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev 40, 480–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffatt JH et al. (2010) Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother 54, 4971–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelletier MR et al. (2013) Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 57, 4831–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lees-Miller RG et al. (2013) A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol. Microbiol 89, 816–830 [DOI] [PubMed] [Google Scholar]
- 19.Harding CM et al. (2018) Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol 16, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wangdi T et al. (2014) The Vi capsular polysaccharide enables Salmonella enterica serovar typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 10, e1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiebig T et al. (2020) Structural and mechanistic basis of capsule O-acetylation in Neisseria meningitidis serogroup A. Nat. Commun 11, 4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geisinger E et al. (2019) Acinetobacter baumannii: envelope determinants that control drug resistance, virulence, and surface variability. Annu. Rev. Microbiol 73, 481–506 [DOI] [PubMed] [Google Scholar]
- 23.Suwandi A et al. (2019) Std fimbriae-fucose interaction increases Salmonella-induced intestinal inflammation and prolongs colonization. PLoS Pathog. 15, e1007915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiralongo J et al. (2018) YesU from Bacillus subtilis preferentially binds fucosylated glycans. Sci. Rep 8, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De S et al. (2018) Structural basis of mammalian glycan targeting by Vibrio cholerae cytolysin and biofilm proteins. PLoS Pathog. 14, e1006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar P et al. (2018) Enterotoxigenic Escherichia coli–blood group A interactions intensify diarrheal severity. J. Clin. Invest 128, 3298–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F-Y et al. (2019) Helicobacter pylori induces intracellular galectin-8 aggregation around damaged lysosomes within gastric epithelial cells in a host O-glycan-dependent manner. Glycobiology 29, 151–162 [DOI] [PubMed] [Google Scholar]
- 28.Madison B et al. (1994) Type 1 fimbrial shafts of Escherichia coli and Klebsiella pneumoniae influence sugar-binding specificities of their FimH adhesins. Infect. Immun 62, 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hultgren SJ et al. (1991) Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu. Rev. Microbiol 45, 383–415 [DOI] [PubMed] [Google Scholar]
- 30.Semchenko EA et al. (2019) Glycointeractome of Neisseria gonorrhoeae: identification of host glycans targeted by the gonococcus to facilitate adherence to cervical and urethral epithelial cells. mBio 10, e01339–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lujan AL et al. (2018) Glycosylation-dependent galectin–receptor interactions promote Chlamydia trachomatis infection. Proc. Natl.Acad. Sci. U. S. A 115, E6000–E6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Guennec L et al. (2020) Receptor recognition by meningococcal type IV pili relies on a specific complex N-glycan. Proc. Natl. Acad. Sci. U. S. A 117, 2606–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bensing BA et al. (2019) Recognition of specific sialoglycan structures by oral streptococci impacts the severity of endocardial infection. PLoS Pathog. 15, e1007896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Oliveira DM et al. (2019) Human glycan expression patterns influence Group A streptococcal colonization of epithelial cells. FASEB J. 33, 10808–10818 [DOI] [PubMed] [Google Scholar]
- 35.Tong J et al. (2018) Sialic acid-dependent interaction of group B streptococci with influenza virus-infected cells reveals a novel adherence and invasion mechanism. Cell. Microbiol 20, e12818. [DOI] [PubMed] [Google Scholar]
- 36.Andreassen PR et al. (2020) Host-glycan metabolism is regulated by a species-conserved two-component system in Streptococcus pneumoniae. PLoS Pathog. 16, e1008332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen P et al. (2020) A unique combination of glycoside hydrolases in Streptococcus suis specifically and sequentially acts on host-derived αGal-epitope glycans. J. Biol. Chem 295, 10638–10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman CL et al. (2020) Host mucin is exploited by Pseudomonas aeruginosa to provide monosaccharides required for a successful infection. mBio 11, e00060–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beddoe T et al. (2010) Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci 35, 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J et al. (2013) Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature 499, 350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang YA et al. (2018) In vivo tropism of Salmonella Typhi toxin to cells expressing a multiantennal glycan receptor. Nat. Microbiol 3, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S et al. (2020) Salmonella typhoid toxin PltB subunit and its non-typhoidal Salmonella ortholog confer differential host adaptation and virulence. Cell Host Microbe 27, 937–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen T et al. (2020) The role of 9-O-acetylated glycan receptor moieties in the typhoid toxin binding and intoxication. PLoS Pathog. 16, e1008336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowler CC et al. (2019) Alternate subunit assembly diversifies the function of a bacterial toxin. Nat. Commun 10, 3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng L et al. (2014) Host adaptation of a bacterial toxin from the human pathogen Salmonella typhi. Cell 159, 1290–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byres E et al. (2008) Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature 456, 648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spanò S et al. (2008) Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe 3, 30–38 [DOI] [PubMed] [Google Scholar]
- 48.Haghjoo E and Galán JE (2004) Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. U. S. A 101, 4614–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang SJ et al. (2016) Receptor-mediated sorting of typhoid toxin during its export from Salmonella Typhi-infected cells. Cell Host Microbe 20, 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaji T et al. (2019) A CRISPR Screen using subtilase cytotoxin identifies SLC39A9 as a glycan-regulating factor. iScience 15, 407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wands AM et al. (2015) Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife 4, e09545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heim JB et al. (2019) Crystal structures of cholera toxin in complex with fucosylated receptors point to importance of secondary binding site. Sci. Rep 9, 12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roderer D et al. (2020) Glycan-dependent cell adhesion mechanism of Tc toxins. Nat. Commun 11, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shewell LK et al. (2020) All major cholesterol-dependent cytolysins use glycans as cellular receptors. Sci. Adv 6, eaaz4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaFrance ME et al. (2015) Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc. Natl. Acad. Sci. U. S. A 112, 7073–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao L et al. (2019) Sulfated glycosaminoglycans and low-density lipoprotein receptor contribute to Clostridium difficile toxin A entry into cells. Nat. Microbiol 4, 1760–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson DM et al. (2020) Structural insights into the transition of Clostridioides difficile binary toxin from prepore to pore. Nat. Microbiol 5, 102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prado Acosta M and Lepenies B (2019) Bacterial glycans and their interactions with lectins in the innate immune system. Biochem. Soc. Trans 47, 1569–1579 [DOI] [PubMed] [Google Scholar]
- 59.Coumou J et al. (2019) The role of mannose binding lectin in the immune response against Borrelia burgdorferi sensu lato. Sci. Rep 9, 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mnich ME et al. (2019) The C-type lectin receptor MGL senses N-acetylgalactosamine on the unique Staphylococcus aureus ST395 wall teichoic acid. Cell. Microbiol 21, e13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen WS et al. (2015) Fingerprinting of galectins in normal, P. aeruginosa-infected, and chemically burned mouse corneas. Invest. Ophthalmol. Vis. Sci 56, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li FY et al. (2020) Galectins in host defense against microbial infections. Adv. Exp. Med. Biol 1204, 141–167 [DOI] [PubMed] [Google Scholar]
- 63.Jia J et al. (2020) Galectin-3 coordinates a cellular system for lysosomal repair and removal. Dev. Cell 52, 69–87.e68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroeder BO (2019) Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep 7, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wheeler KM et al. (2019) Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol 4, 2146–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erturk-Hasdemir D et al. (2019) Symbionts exploit complex signaling to educate the immune system. Proc. Natl. Acad. Sci. U. S. A 116, 26157–26166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramakrishna C et al. (2019) Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun 10, 2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sirin S and Aslim B (2020) Characterization of lactic acid bacteria derived exopolysaccharides for use as a defined neuroprotective agent against amyloid beta(1–42)-induced apoptosis in SH-SY5Y cells. Sci. Rep 10, 8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huus KE et al. (2020) Commensal bacteria modulate immunoglobulin A binding in response to host nutrition. Cell Host Microbe 27, 909–921 e905 [DOI] [PubMed] [Google Scholar]
- 70.Briliute J et al. (2019) Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat. Microbiol 4, 1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapebie P et al. (2019) Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat. Commun 10, 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mortales CL et al. (2020) N-glycan branching is required for development of mature B cells. J. Immunol 205, 630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sepuru KM and Rajarathnam K (2019) Structural basis of chemokine interactions with heparan sulfate, chondroitin sulfate, and dermatan sulfate. J. Biol. Chem 294, 15650–15661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang MM et al. (2019) Small-molecule control of antibody N-glycosylation in engineered mammalian cells. Nat. Chem. Biol 15, 730–736 [DOI] [PubMed] [Google Scholar]
- 75.Martinez DR et al. (2019) Fc characteristics mediate selective placental transfer of IgG in HIV-infected women. Cell 178, 190–201.e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouckaert J et al. (2005) Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol 55, 441–455 [DOI] [PubMed] [Google Scholar]
- 77.Spaulding CN et al. (2017) Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 546, 528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frey AM et al. (2019) Characterization of Porphyromonas gingivalis sialidase and disruption of its role in host-pathogen interactions. Microbiology (Reading) 165, 1181–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kitov PI et al. (2000) Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 403, 669–672 [DOI] [PubMed] [Google Scholar]
- 80.Wands AM et al. (2018) Fucosylated molecules competitively interfere with cholera toxin binding to host cells. ACS Infect. Dis 4, 758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diago-Navarro E et al. (2018) Novel, broadly reactive anticapsular antibodies against carbapenem-resistant Klebsiella pneumoniae protect from infection. mBio 9, e00091–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apicella MA et al. (2018) Nontypeable Haemophilus influenzae lipooligosaccharide expresses a terminal ketodeoxyoctanoate in vivo, which can be used as a target for bactericidal antibody. mBio 9, e01401–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahn C et al. (2021) Mechanisms of typhoid toxin neutralization by antibodies targeting glycan receptor binding and nuclease subunits. iScience 24, 102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen T et al. (2021) The structural basis of Salmonella A2B5 toxin neutralization by antibodies targeting the glycan-receptor binding subunits. Published online December 8, 2021. 10.2139/ssrn.3745287 [DOI] [PMC free article] [PubMed]
- 85.Paton JC and Trappetti C (2019) Streptococcus pneumoniae Capsular Polysaccharide. Microbiol. Spectr 7. 10.1128/microbiolspec.GPP3-0019-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaplonek P et al. (2018) Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proc. Natl. Acad. Sci. U. S. A 115, 13353–13358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wain J et al. (2005) Vi antigen expression in Salmonella enterica serovar Typhi clinical isolates from Pakistan. J. Clin. Microbiol 43, 1158–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feldman MF et al. (2019) A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. U. S. A 116, 18655–18663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harding CM et al. (2019) A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun 10, 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Broecker F et al. (2019) Synthetic oligosaccharide-based vaccines protect mice from Clostridioides difficile infections. ACS Chem. Biol 14, 2720–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gulati S et al. (2019) Preclinical efficacy of a lipooligosaccharide peptide mimic candidate gonococcal vaccine. mBio 10, e02552–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Litschko C et al. (2018) A new family of capsule polymerases generates teichoic acid-like capsule polymers in Gram-negative pathogens. mBio 9, e00641–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Q et al. (2020) Chemical synthesis of glycans up to a 128-mer relevant to the O-antigen of Bacteroides vulgatus. Nat. Commun 11, 4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paulson JC et al. (2006) Sweet spots in functional glycomics. Nat. Chem. Biol 2, 238–248 [DOI] [PubMed] [Google Scholar]
- 95.Song X et al. (2014) Chemistry of natural glycan microarrays. Curr. Opin. Chem. Biol 18, 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Briard JG et al. (2018) Cell-based glycan arrays for probing glycan–glycan binding protein interactions. Nat. Commun 9, art. no. 880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geissner A et al. (2019) Microbe-focused glycan array screening platform. Proc. Natl. Acad. Sci. U. S. A 116, 1958–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitov PI et al. (2019) A quantitative, high-throughput method identifies protein-glycan interactions via mass spectrometry. Commun. Biol 2, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rojas-Macias MA et al. (2019) Towards a standardized bioinformatics infrastructure for N- and O-glycomics. Nat. Commun 10, 3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abrahams JL et al. (2020) Recent advances in glycoinformatic platforms for glycomics and glycoproteomics. Curr. Opin. Struct. Biol 62, 56–69 [DOI] [PubMed] [Google Scholar]
- 101.Wasik BR et al. (2016) Effects of sialic acid modifications on virus binding and infection. Trends Microbiol 24, 991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Angata T and Varki A (2002) Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem. Rev 102, 439–469 [DOI] [PubMed] [Google Scholar]
- 103.Suzuki T et al. (2002) Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. FASEB J 16, 635–641 [DOI] [PubMed] [Google Scholar]
- 104.Bojar D et al. (2021) Deep-learning resources for studying glycan-mediated host–microbe interactions. Cell Host Microbe 29, 132–144.e133 [DOI] [PubMed] [Google Scholar]
- 105.Cooper CA et al. (2003) GlycoSuiteDB: a curated relational database of glycoprotein glycan structures and their biological sources. 2003 update. Nucleic Acids Res. 31, 511–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choudhary P et al. (2019) ProGlycProt V2.0, a repository of experimentally validated glycoproteins and protein glycosyltransferases of prokaryotes. Glycobiology 29, 461–468 [DOI] [PubMed] [Google Scholar]
- 107.Li W et al. (2019) Strategies for chemoenzymatic synthesis of carbohydrates. Carbohydr. Res 472, 86–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheng C-W et al. (2018) Hierarchical and programmable one-pot synthesis of oligosaccharides. Nat. Commun 9, art. no. 5202 [DOI] [PMC free article] [PubMed] [Google Scholar]


