Abstract
Basic research on hemoglobin has been essential for understanding the origin and treatment of many hematological disorders due to abnormal hemoglobins. The most important of the hemoglobinopathies is sickle cell disease - Linus Pauling’s “molecular disease” that gave birth to molecular medicine. In this review, I will describe the contributions of basic biophysical research on normal and sickle cell hemoglobin (HbS) to understanding the molecular pathogenesis of the disease and providing the conceptual basis for the various approaches to drug therapy that target HbS polymerization. Most prominent among these are the allosteric model of Monod, Wyman, and Changeux and the Gill-Wyman thermodynamic linkage relation for describing how oxygen pressure determines HbS polymerization at equilibrium and the importance of the highly unusual kinetics of HbS polymerization to the pathophysiology, clinical course, and drug discovery for treating this common and severe disease. The article focuses primarily on experimental and theoretical results from my lab, so it is not a comprehensive review of the subject.
Keywords: hemoglobin, sickle, protein aggregation, kinetics, MWC, thermodynamic linkage
1. Introduction.
The era of molecular medicine began with Linus Pauling’s 1949 discovery that sickle cell disease is caused by an abnormal hemoglobin.(Eaton, 2003; Pauling et al., 1949) Research on sickle cell disease has again taken center stage in hematology because of new drug therapies, cure by stem cell transplantation, and the advent of gene therapy as a promising way of curing the disease. This inherited disorder is caused by a mutation of the codon for glutamate in the two β globin genes from to GAG to GTG. This single nucleotide causes a change to valine at the 6th position from the N-terminus (Fig. 1). The substitution of a negatively charged residue with a neutral hydrophobic one on the molecular surface creates a sticky patch that causes polymerization of sickle hemoglobin upon deoxygenation in the tissues to form multi-stranded fibers that distort (“sickle”) red cells. The less flexible red cells can cause obstruction in the microcirculation, the smallest vessels of the tissues. Obstruction in the microvasculature results in decreased oxygen delivery to the tissues, chronic damage to almost every organ of the body, a shortened life span for both the red cells and the patient, and episodes so painful that they are called sickle cell crises. Because of improvements in management and treatment most of the 100,000 patients in the United states have considerably increased life expectancy. However, many millions suffer from the disease in under-resourced countries where the disease is devastating (Piel et al, 2013). In sub-Saharan Africa, 50–90% of children with sickle cell disease die before age 5.(McGann, 2014)
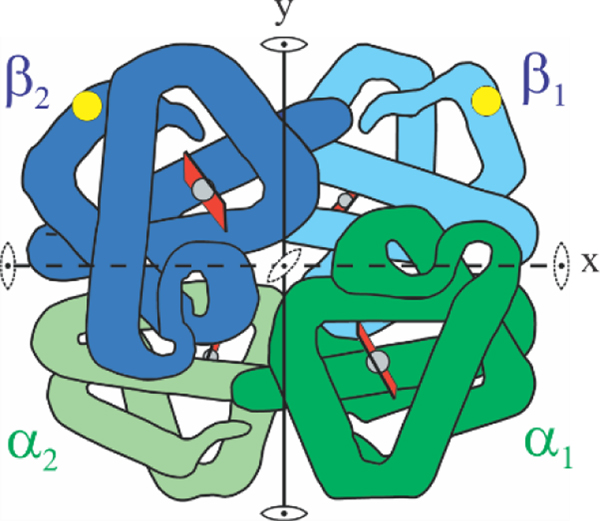
Figure 1.
Schematic structure of hemoglobin molecule. The molecule consists of 2 α and 2 β subunits. The yellow circle indicates the location of the β6 glu to val mutation on the molecular surface.
The physical chemistry of normal (HbA) and sickle cell (HbS) hemoglobin has turned out to be very important for understanding the molecular pathogenesis of the disease and providing the conceptual basis for current approaches to drug therapy. This review covers these topics, which have been the focus of much of my research for the past 50 years.(Eaton, 2018, 2020; Eaton, 2021) During this period, I and many other biophysical scientists have been highly influenced by the work and intellectual leadership in the hemoglobin field by the “Rome group”, especially by Maurizio Brunori, coauthor with Eraldo Antonini of : “Hemoglobin and Myoglobin in their Reaction with Ligands”, the famous research textbook that has motivated this special issue.(Antonini and Brunori, 1970) I will therefore begin with some history of my connection to Maurizio.
2. Some history
The work of Alessandro Rossi Fanelli, Eraldo Antonini and Maurizio Brunori established the standard of excellence in research on heme proteins for decades, from the 1950’s onward. In addition, the “Rome group” provided the intellectual leadership worldwide on studies of myoglobin, hemoglobin, and cytochrome oxidase. Their leadership and universal admiration was based on their published work, the textbook of Antonini and Brunori,(Antonini and Brunori, 1970) on numerous visits by researchers to La Sapienza to learn about their latest results, and on Brunori’s organization of numerous important international conferences that defined the outstanding problems in these fields. I had become a fan of the work from Rome while I was a medical student, when I was a close friend of Abel Schejter, a post-doctoral fellow in the laboratory of Philip George, one of the the leading scientists of the day on the thermodynamics of heme protein ligand binding reactions. Abel had a deep interest in hemoglobin and discussions with him on the latest papers from Rome were always highly stimulating and educational, a welcome reprieve from medical school. In retrospect, these discussions not only sparked my lasting interest in hemoglobin, but they were a major influence on my decision not to continue with medicine after graduating from medical school, but to work for a PhD and pursue a career of research on heme proteins
I first met Maurizio Brunori in 1970 when I had just started making making measurments of spectra on single crystals of normal hemoglobin in linearily-polarized light using a micropsectrophotometer that I had assembled, similar to the one I used in my PhD thesis work with the legendary spectroscopist, Robin Hochstrasser.(Eaton and Hochstrasser, 1967, 1968; Eaton and Trommsdorff, 2013; Eaton and Zewail, 1996) Maurizio was attending an international conference on hemoglobin organized by John Edsall and Jeffries Wyman at at the Stone House Mansion on the NIH campus, where they resided as Fogarty International Scholars-in-Residence. (Maurizio was later to also become a Fogarty International Scholar-in-Residence, spending 3 periods hosted by me at NIH between 1986 and 1990.) Wyman was a member of the Rome group from 1962 to 1985 and an important mentor to Maurizo. During the meeting, Maurizio and Antonini visited my lab and seemed impressed by my microspectrophtometric measurements. Hearing compliments from such famous scientists at the beginning my career as an independent scientist was a big morale booster for me. It was not until 1975 that Maurizio and I became friends, when, with Kurt Wüthrich, we were the foreign participants at the first Taniguchi Biophysics Symposium held at Lake Biwa near Kyoto. I have been deeply indebted to Maurizio ever since for his friendship, for the many enlightening discussions with him, and for his continuous wise advice and brilliant insights on all matters of science.
3. Physical Chemistry of HbS polymerization
3.1. Polymerization at equilibrium: MWC model and Gill-Wyman thermodynamic linkage
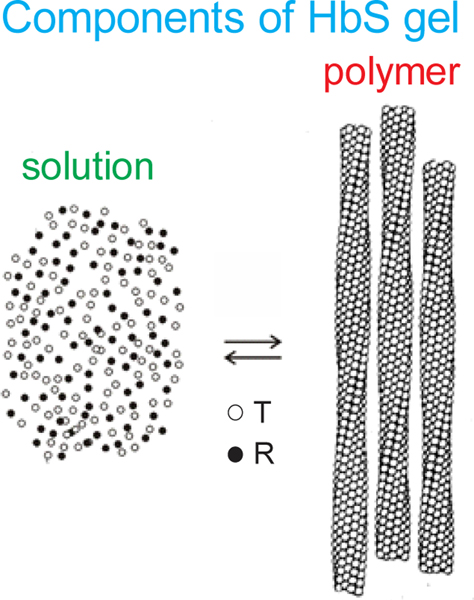
One of the major results from my first equilibrium studies, carried out in collaboration with James Hofrichter, an outstanding experimentalist, and Philip Ross, a skilled calorimetrist, showed that the mixture of free and polymerized hemoglobin molecules, referred to as a gel, behaved very much like a crystal-solution equilibrium.(Ross et al., 1977) As in crystallization, the solubility measures the thermodynmic stability of the fiber. It corresponds to the concentration of hemoglobin in the supernatant after sedimenting the fibers by ultracentirfuging the gel (Fig. 2). Both the fraction polymerized and the kinetics of fiber formation depend on the ratio of the initial concentration to the solubility, the so-called the supersaturation ratio.(Cellmer et al., 2016; Hofrichter et al., 1976)
Fig. 2.
Schematic showing that a gel consists of an equilibrium between Hb molecules free in solution and polymerized Hb. (Left image) The concentration of hemoglobin in the supernatant after sedimenting the polymers by ultracentrifugation is the solubility. It shows that only the T (deoxy) conformation of hemoglobin can polymerize, while the R (oxy) conformation is excluded at all oxygen pressures.
The most important factor that controls polymerization and sickling in vivo is of course the oxygen pressure. The two-state model of Jacque Monod, Jefferies Wyman, and Jean-Pierre Changeux (MWC) together with the thermodynamic linkage relation developed by Wyman and Stanley Gill, a Brunori collaborator, have provided the theoretical explanation.(Gill et al., 1979; Henry et al., 2020a; Monod et al., 1965; Sunshine et al., 1982) Wyman was an effectively permanent visitor of Antonini and Brunori while working on these two landmark projects. An interesting aspect of the MWC model is that it contains some very sophisticated and subtle features that were not fully understood by many scientists working full-time on hemoglobin. Misunderstanding of MWC contributed to a long period of controversy beginning in about 1970 concerning the relative merits of MWC and the KNF sequential model(Koshland et al., 1966) and the dismissal of MWC by Gary Ackers based on his tetramer-dimer dissociation measurements and a difficult to penetrate thermodynamic analysis.(Ackers, 1998; Ackers et al., 1992) The controversy persisted into the early 2000’s when the last remnants of doubt concerning the correctness of MWC for explaining cooperativity were finally eliminated.(Eaton et al., 1999; Levantino et al., 2012; Shibayama et al., 1998; Shulman, 2001; Yun et al., 2002)
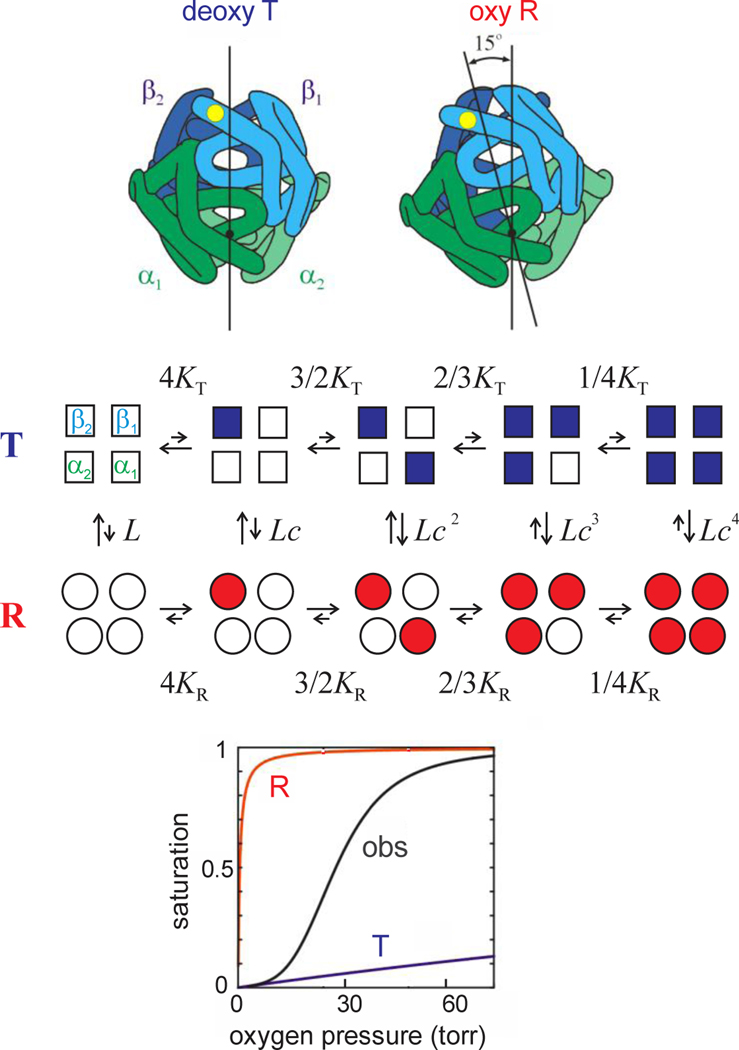
The basic idea of MWC is that hemoglobin exists in a reversible equilbrium between a low and high affinity conformation, called “T” for tense and “R” for relaxed (Fig. 3). These two conformations correspond to the arrangement of the 4 subunits in the X-ray-determined structures of fully deoxygenated and fully oxgenated hemoglobin, respectfully – so-called quaternary structures.(Perutz, 1970) In the MWC model, oxygen binding to both quaternary structures is non-cooperative. Cooperativity, as reflected in the sigmoid shape of the binding curve, is the result of the shift from the T to R quaternary structure as succesive molecules of oxygen bind. The overall affinity is decreased by preferential binding of the allosteric effector, 2,3-diphosphoglycerate (2,3-DPG), in the cleft between the β subunits of the T quaternary structure that shifts the T-R equilibrium toward T.(Benesch and Benesch, 1969) (Bunn and Briehl, 1970; Perutz, 1970).
Fig. 3.
The MWC two-state allosteric model. (Upper panel, quaternary structures) The main difference between the 2 quaternary structures is rotation of the symmetrically related αβ dimers of about 15 degrees. (Middle panel, model) Empty symbols designate deoxygenated subunits and filled symbols oxygenated subunits. KT (~0.001 torr−1) and KR (~1 torr−1) are the association constants for binding to the T and R quaternary structures, respectively, L (~5×105) is the ratio of the T to R population at zero oxygen pressure, and c = KT/KR. The approximate magnitude of the equilibrium constants is indicated schematically by the relative length of the arrows for the forward and backward reaction rates. (Lower panel, binding curves) The oxygen binding curve for both the R and T conformations is non-cooperative. The shift from the T to R conformation as successive molecules of oxygen bind results in the observed (obs) sigmoid shape, signifying cooperativity.
The MWC model has been quite successfully used to explain a wide range of kinetic experiments, beginning with the work of John Hopfield, Seiji Ogawa, and Robert Shulman who assigned Quentin Gibson’s fast-reacting hemoglobin (Hb*) to the MWC R conformation.(Gibson, 1959; Hopfield et al., 1971) They have also led to new kinds of information about mechanism, such as the demonstration that the transition state along the free energy path between the quaternary conformations is much closer to the R than the T conformation. This was done by comparing equilibrium and activation enthalpy and entropy parameters on Brunori’s trout I hemoglobin in collaboration with Brunori and Massimo Coletta, a Brunori student, (Hofrichter et al., 1991) and by the application of a linear free energy relation to human hemoglobin data.(Eaton et al., 1991) Kinetic studies also have played the dominant role in extending the MWC model to include tertiary conformational changes.(Eaton et al., 2007; Henry et al., 2002; Henry et al., 2015; Viappiani et al., 2014; Viappiani et al., 2004). In our a two-state (TTS) allosteric model, there are 2 conformations of the individual subunits that are present in both the R and T conformations, called r and t. The key feature of the model, verified on rather demanding experiments by Cristiano Viappiani, Andrea Mozzarelli and coworkers,(Viappiani et al., 2014; Viappiani et al., 2004) is that the high oxygen affinity of r is the same in the R and T conformations and the low affinity of t is the same in R and T. The model is also supported by Resonance Raman experiments of Thomas Spiro and quantum mechanical/molecular mechanics computer simulations by Bringas et al.(Bringas et al., 2017; Jones et al., 2014)
The first lucid description of the MWC model and how it compares to other models for explaining cooperative oxygen binding by hemoglobin is to be found in the last chapter of the Antonini and Brunori book.(Antonini and Brunori, 1970) Careful study of that chapter, together with many enlightening discussions with Maurizio were responsible for keeping me from being one of the hemoglobin researchers who did not fully understand MWC. I and my colleagues were therefore able to improve on the MWC model by extending it with the TTS model.
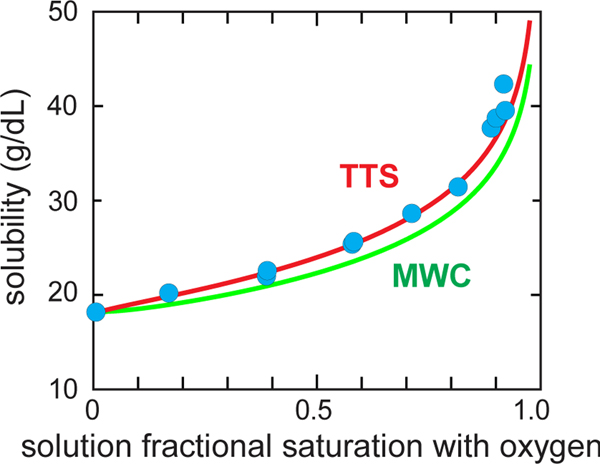
The MWC model together with an extremely important thermodynamic relation derived by Gill and Wyman that allows the calculation of the solubility from the free and polymerized HbS oxygen binding curves provides a remarkably satisfying explanation of the experimentally measured solubilities as a function of oxygen saturation. As shown in Figure 4, assuming that only the T quaternary structure can polymerize and has the same oxygen affinity in the fiber as the T conformation of free hemoglobin, the predicted solubility from the Gill-Wyman thermodynamic relation (the “polyphasic linkage relation”) comes very close to the experimentally measured solubilities with no adjustable parameters. We have very recently shown that the solubility predicted by our TTS model gives almost perfect agreement with the experimental data (Fig. 4), but it does require an adjustable parameter.(Henry et al., 2020a)
Fig. 4.
Measured solubility of HbS as a function of the fractional saturation with oxygen of the free hemoglobin molecules. The blue circles are the measured solubilities.(Sunshine et al., 1982) The green (MWC) curve utilizes the Gill-Wyman polyphasic linkage relation to calculate the solubility from the free HbS cooperative binding curve and the polymer binding curve assumed to be the non-cooperative binding curve of the T quaternary structure of free hemoglobin.(Sunshine et al., 1982) The red (TTS) curve is the predicted solubility of the TTS model calculated in the same way, after adjusting one parameter of the TTS model that lowers the affinity of polymerized HbS compared to T, i.e. l, the ratio of t tertiary conformations to r tertiary conformations at zero oxygen pressure in the polymerized T conformation.
The description of a gel of HbS at equilibrium at all oxygen pressures is then quite simple; it is a mixture of T and R conformations in solution, with only T conformations contained in the fiber (Fig. 2). The exclusion of the R conformation from the fiber has motivated the development of drugs that preferentially bind to R to reduce sickling, a controversial approach to treating the disease discussed below.
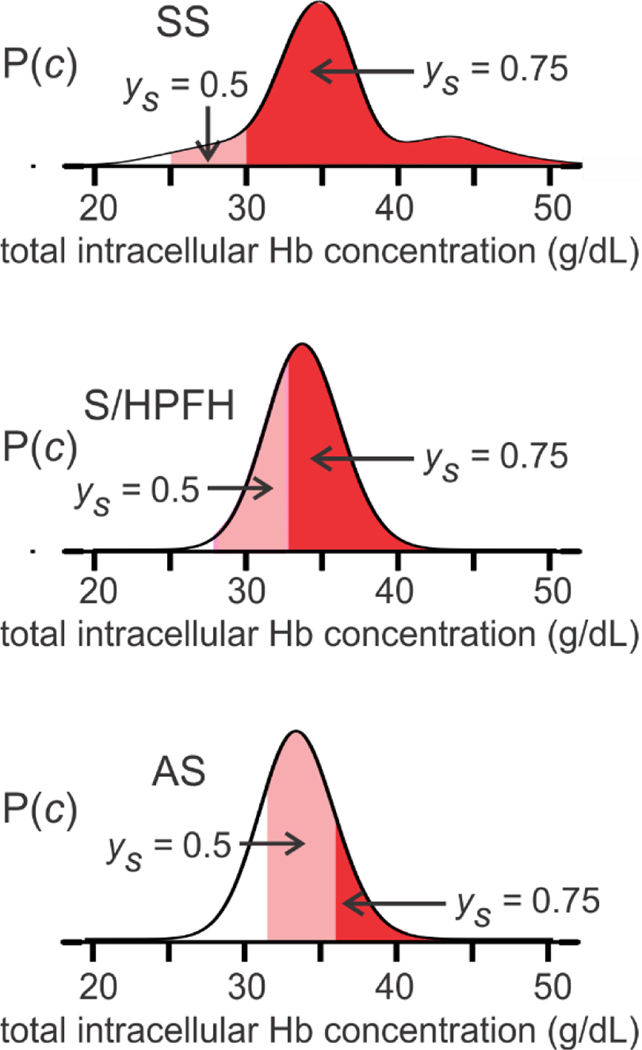
In Figure 5, the MWC model is used to determine whether or not fibers form at equilibrium for a given intracellular Hb concentration and fractional saturation with oxygen of free hemoglobin molecules for 3 conditions - homozygous sickle cell disease (SS), sickle cell disease with pancellular hereditary peristence of fetal hemoglobin (S/HPFH), and sickle trait (AS).(Henry et al., 2020a)
Fig. 5.
Intracellular concentration distributions and fraction sickled at equilibrium in SS disease, S/HPFH, and sickle trait (AS). The ordinate is the probability of a red cell containing a total hemoglobin concentration (c) indicated on the abscissa. The dark red area shows cells where the solution in the cell is supersaturated at 75% saturation with oxygen of the free hemoglobin molecules (i.e., the initial Hb concentration is greater than the equilibrium solubility), while the light red area shows the cells that are superstaurated at 50% saturation (p50) with oxygen of the free hemoglobin. See (Henry et al., 2020a) for details of the concentration distributions and sickling calculations, which also include the large non-ideality from molecular crowding in these concentrated protein soluitons, as well as copolymerization of HbF in SS disease (~90% HbS, ~10% HbF) and in S/HPFH (~70% HbS, ~30% HbF), and HbA in sickle trait (~40% HbS, ~60% HbA).(Eaton and Hofrichter, 1990; Ross and Minton, 1977). The larger portion of cells at lower and higher hemoglobin concentrations in SS disease compared to S/HPFH and sickle trait correspond to an increase in reticulocyte production responding to the anemia and dehydration from mutiple sickling/unsickling cycles. respectively.
3.2. Discovery of HbS polymerization kinetics and mechanism
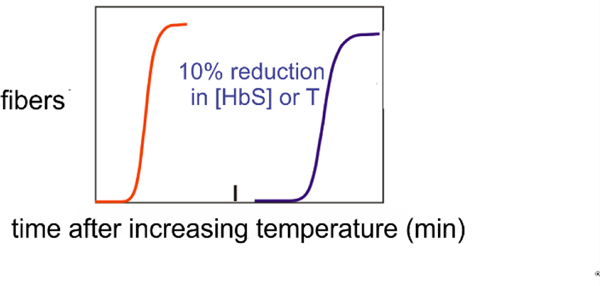
The kinetics of HbS polymerization are highly unusual with a delay period (lag phase) prior to the appearance of fibers that is enormously sensitive to HbS concentration, depending on the inverse 30th power, and to temperature (activation energy = 90 kcal/mol) (Fig. 6).(Hofrichter et al., 1974; Malfa and Steinhardt, 1974; Moffat and Gibson, 1974)
Fig. 6.
HbS polmerization kinetics. Schmatic of kinetic progress curves after transferring a completely deoxygenated HbS solution from 0°C, where it is a liquid solution, to room temperature where fibers form to produce a viscous gel. The delay times are identical when the kinetics are monitored by birefringence, calorimetry, turbidity/light scattering, or nuclear magnetic resonance.(Eaton et al., 1976b; Hofrichter et al., 1974) A reduction in HbS concentration by 10% or in temperature by 5°C increases the delay time by an order of magnitude.
Although we proposed a nucleation/growth mechanism in the oroiginal paper on the kinetics, a satisfactory mechanism was not developed until the arrival of Frank Ferrone in 1976 as a postdoctoral fellow. Frank brought considerable expertise to the lab on laser photolysis of the hemoglobin carbon monoxide complex from his PhD thesis research with Hopfield. Antonini and Brunori had previously shown that CO photodissociation is a powerful method to initiate polymerization by rapidly creating deoxyHbS in single red cells using a microspectrophotometer.(Antonini et al., 1983; Antonini et al., 1978) The temperature jump method we had been using to initiate polymerization of deoxyHbS had a time resolution of a little less than a minute. Ferrone used the photolysis method to vastly improve the time resolution by initiating polymerization in milliseconds. The increased time resolution allowed us to study polymerization and sickling on the in vivo time scale of subseconds and seconds.(Ferrone et al., 1985a; Ferrone et al., 1980) and in individual red cells in work by Brunori’s student, Massimo Coletta. Importantly, Coletta et al. showed that polymerization inside red cells is the same as in purifiied HbS solutions.(Coletta et al., 1982)
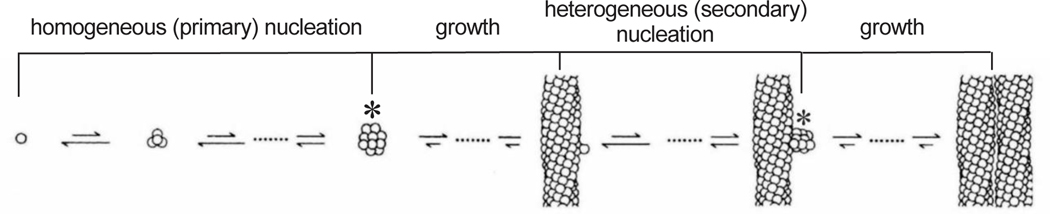
During Ferrone’s postdoctoral period, we developed the double nucleation mechanism (Fig. 7). According to this mechanism, there are 2 nucleation processes, a primary and a secondary one, which we called homogeneous and heterogeneous nucleation, respectively. The mechanism explained the delay period (lag phase), the high concentration and temperature dependence of the delay time, the stochastic variation in the delay time when measured on small volumes of HbS solutions or on red cells (~10−10 cc), and the observation that concentration dependence for the homogeneous nucleation rate is twice that of the delay time – 50th and 25th power, respectively, in the Ferrone experiments (Cao and Ferrone, 1996) and 80th and 40th power in the experiments of Christoph et al. (Christoph et al., 2005; Eaton and Hofrichter, 1990; Ferrone et al., 1985b; Ferrone et al., 1980; Ferrone et al., 2002; Hofrichter, 1986; Szabo, 1988; Weng et al., 2008) The mechanism also explained the wide variety of shapes of sickled cells as resulting from the number of homogeneously nucleated fibers, with the classic sickle shape containing a single homogeneously nucleated fiber.(Eaton and Hofrichter, 1987) In a totally different context, the double nucleation mechanism is being used to successfully explain the aggregation kinetics of the Alzheimer’s peptide to form amyloid fibrils.(Cohen et al., 2015; Cohen et al., 2013; Cohen et al., 2011a, b; Cohen et al., 2011c; Tornquist et al., 2018)
Fig. 7.
The double nucleation mechanism for HbS polymerization (Eaton and Hofrichter, 1990; Ferrone et al., 1985b; Ferrone et al., 1980; Ferrone et al., 2002; Weng et al., 2008). The first fiber in any given volume forms by the classical Osawa nucleation growth model,(Osawa and Sakura, 1975) called homogeneous because it occurs in the solution bulk without any contact to other fibers or surfaces. As indicated by the arrows, the initial aggregation steps are thermodynamically unfavorable because the overall reaction is uphill in free energy until a critical nucleus (*) is formed. The vast majority of fibers are formed by heterogeneous nucleation on the surface of preexisting fibers, which provides additional stability to the nucleus from contacts with the fiber surface. As more fibers form there is more surface for this secondary nucleation process, providing an autocatalytic mechanism that produces the delay period.
4. Clinical relevance of biophysical studies
4.1. HbS polymerization and clinical severity
We recognized in our very first study of the kinetics that the delay time should be a major factor in determining the clinical course of the disease. (Bunn, 1997; Eaton et al., 1976a; Ferrone, 2015; Hofrichter et al., 1974) The most important consequence of the delay time is that it allows patients to survive the disease because most cells can escape the microcirculation before HbS polymerization begins. This was first shown in CO photolysis experiments on single red cells by Mozzarelli when he was on sabbatical leave in my lab (Mozzarelli et al., 1987) and has been re-investigated in detail quite recently with considerably more experimental information and the ability to predict sickling on the seconds time scale as occurs in vivo.(Henry et al., 2020a). The diagram in Figure 5 shows that, were polymerization at equilibrium, every cell of patients with homozygous sickle cell (SS) disease would be sickled at tissue oxygen pressures. It is unlikely that individuals born with SS disease could survive the equilbrium condition for more than about one year when the replacement of fetal hemglobin (HbF) by HbS ceases to continue. Interestingly, in the double heterozygous condition of sickle cell disease with pancellular persistence of HbF (S/HPFH), most cells would also be sickled at equilibrium even at venous oxygen saturations of 75%, while in sickle trait most cells would be sickled at 50% saturation (Fig. 5). Yet, both S/HPFH and sickle trait are benign disorders.
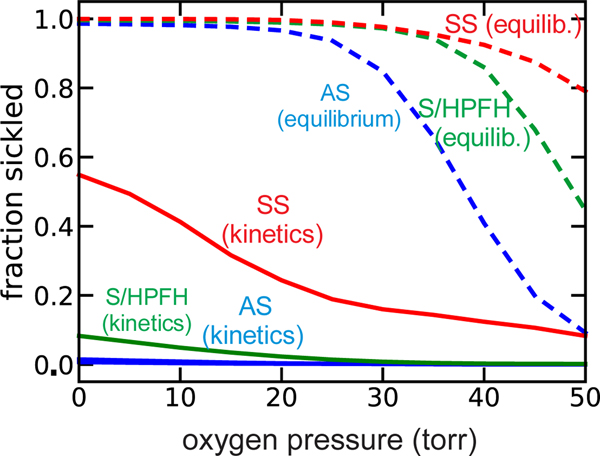
Again, the reason that that both S/HPFH and sickle trait are benign is the kinetics of polymerization. Our recent calculations of sickling times at the rapid rate of oxygen decrease as cells pass from the arterial circulation through the tissues to the venous circulation, show that the delay time in SS individuals allows most cells to escape microcirculation without sickling even when the oxygen pressures drops below 10 torr. Moreover, sickling in vivo is enormously reduced in S/HPFH, and rare in sickle trait (Fig. 8), except in the hypertonic, acidotic renal medulla where osmotic shrinkage of the red cell volume to increase the hemoglobin concentration and reduce the delay time is responsible for impairment of kidney function.(Bunn and Forget, 1986; Xu and Thein, 2019)
Figure 8.
Comparision of equilibrium and in vivo sickling. The kinetics curves for whole blood are calculated at a rate of oxygen pressure decrease of 40 torr/sec from 100 torr to the indicated final oxygen pressure. The fraction sickled depends on the rate of the oxygen pressure decrease and is slightly less 80 torr/sec and slightly more at 20 torr/sec. (Henry et al., 2020a). Details of calculations are given in (Henry et al., 2020a).
The concept that the delay time relative to the transit time through the microcirculation is a major determinant of clinical severity creates a coherent explanation of clinical observations.(Eaton and Hofrichter, 1987) Factors that decrease the delay time or increase the transit time increase clinical severity and vice versa. Consequently, increased adherence of cells to the vascular endothelium, which slows transit, contributes to the probability of vaso-occluion, as originally pointed out by Robert Hebbel.(Hebbel et al., 1980; Hebbel et al., 2004). Adherence as an additional determinant of clinical severity is perfectly consistent with this dynamical picture. Thus, the enormous sensitivity of the delay time to intracellular Hb concentration, temperature, and pH readily explains why fever, red cell dehydration, or acidosis can trigger a sickle cell crisis. In the case of infection, slowing transit times from increased adherence due to elevated white cells should also contribute. The stochastic nature of HbS polymerization in the small volume of a red cell may also play a role in the episodic and often unpredictable onset of sickle cell crises. Moreover, as already implied in discussing S/HPFH and sickle trait, there is a close correlation of predicted supersaturation and therefore delay times with disease severity among the various sickle syndromes.(Brittenham et al., 1985; Bunn et al., 1982; Sunshine et al., 1978).
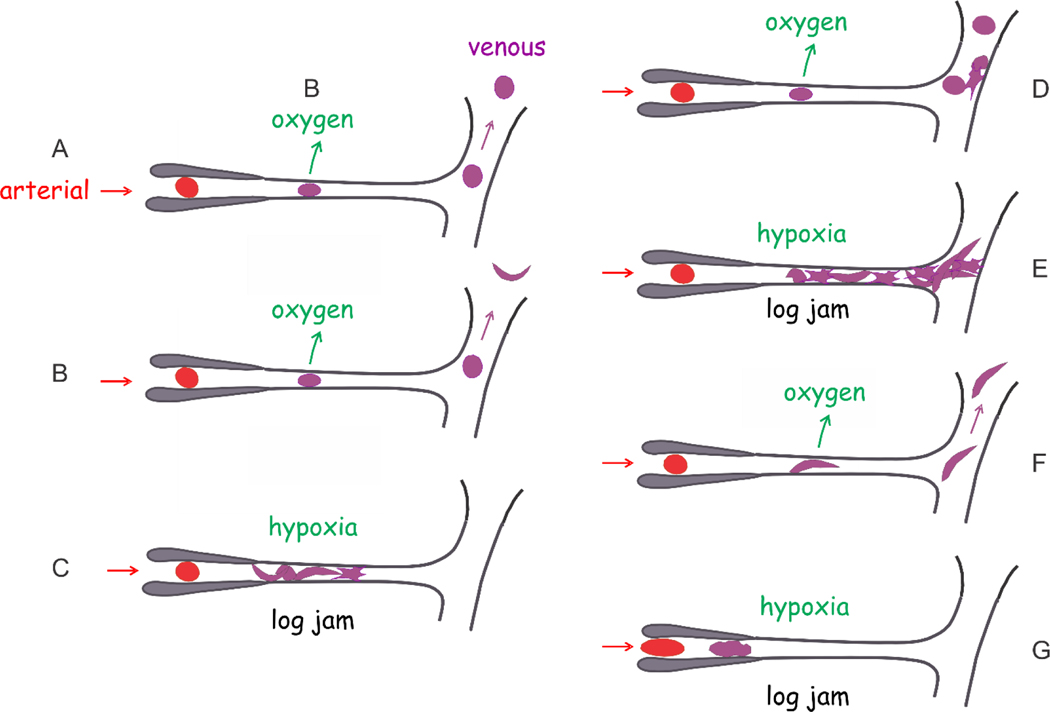
Multiple scenarios have been proposed for the predomaint sites of in vivo sickling and obstruction of the microvasculature (Fig. 9). So while the scenarios A and B depicted in Figure 9 are the most frequent in S/HPFH and sickle trait, the distribution of these scenarios in SS is yet to be determined. Differences in the distribition of the scenarios is most probably responsible for the wide range of clinical severity in SS disease.
Figure 9.
In vivo sickling scenarios.(Henry et al., 2020a) Schematic of microcirculation shows an arteriole, a capillary and venule. (A) The delay time is so long that cells do not sickle in the microcirculation and may even return to the lungs without sickling. (B) Sickling only occurs after the cell escapes the microcirculation and is unsickled upon oxygenation in the lungs. (C) The delay time is so short that polymerization occurs in the capillary to block further passage of cells, called a “log jam.” The surrounding tissues are adversely affected by decreased oxygen delivery. (D) Cells escape both the capillary and blockage by adherent sickled cells in the post-capillary venule. (E) Cells sickle and adhere to the endothelium of the post-capillary venule to cause a log jam. (F) Cell sickles in capillary but nevertheless escapes the microcirculation to the larger vessels. (G) Because the concentration of intracellular hemoglobin is so high that fibers are not completely melted in the lungs, the delay time is eliminated.(Mozzarelli et al., 1987) In this case, cells sickle before entering the microcirculation and may initiate a log jam in the arteriole.
5.1. Treating sickle cell disease
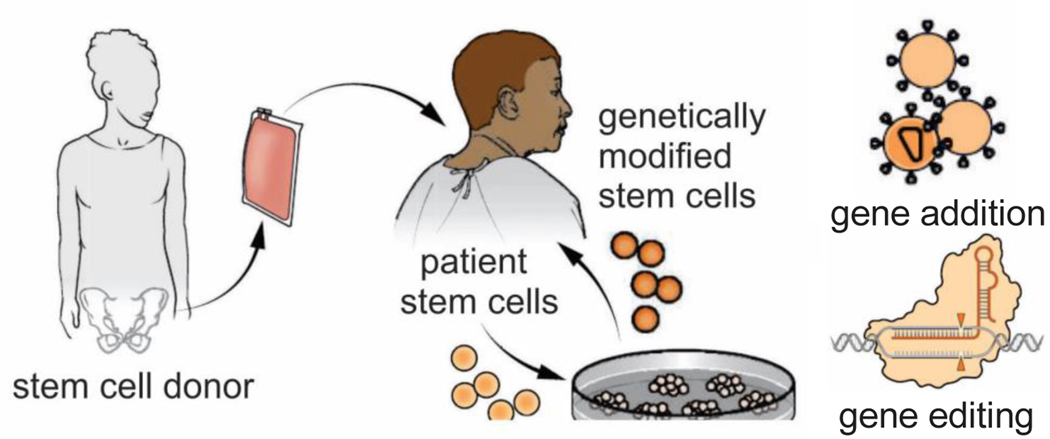
Sickle cell disease has been cured by hematopoietic stem cell transplantation in over 95% of both children and adults that have undergone this procedure.(Cisneros and Thein, 2020; Tisdale et al., 2020) In this treatment, stem cells in the bone marrow of the patient are largely eliminated by myelosuppressive therapy and replaced with bone marrow stem cells from an unaffected, tissue matched sibling donor (Fig. 10, center-left).(Eapen et al., 2019) About 15% of sickle cell patients in this country have a sibling-matched donor and can therefore be cured. Promising new curative therapies are also currently being developed using genetic approaches (Fig. 10, center-right).(Drysdale et al., 2021) A Modified HIV is being used to transfer globin genes that code for a non-polymerizing hemoglobin to the patient’s own stem cells, which after gene transfer are reinjected into the patient, avoiding the possibility of graft versus host disease. One such gene is a mutated β globin gene for HbA, in which the threonine in position 87 is converted to an asparagine.(Demirci et al., 2020) Position 87 is in a lateral intermolecular contact in the sickle fiber and is responsible for much of the highly reduced co-polymerization of HbF. Another genetic approach is based on the discovery that BCL11A down regulates HbF synthesis, so inhibiting it would be therapeutic.(Menzel et al., 2007; Sankaran et al., 2009) In this approach, reduction of BCL11A expression is achieved either by gene addition or by gene editing with Crispr-Cas9 technology and is currently being tested in clinical trials.(Cisneros and Thein, 2020; Orkin and Bauer, 2019)
Figure 10.
Sickle cell cures (Tisdale et al., 2020). Allogeneic stem cell transplantation is an established curative strategy that uses bone marrow stem cells from an immunlogically matched normal or sickle trait donor (left image). In new and very promising approaches currently being developed, the patient’s own bone marrow cells are modified either with the addition of a β-globin gene that codes for a polymerization inhibitory β globin or by using Crispr-Cas technology to edit the β6 locus or reactivate HbF synthesis.
Although the curative therapies described above are or will soon be available to most patients in this country, they are expensive and can only be administered at advanced medical facilities. More than 95% of patients suffering from sickle cell disease live in under-resourced countries. So, these curative therapies will not be available to the vast majority of sickle cell patients in the world for many years to come. What is urgently needed now for these individuals is an inexpensive, oral drug that decreases severity by inhibiting sickling. This is an area where biophysical science can be of great help.
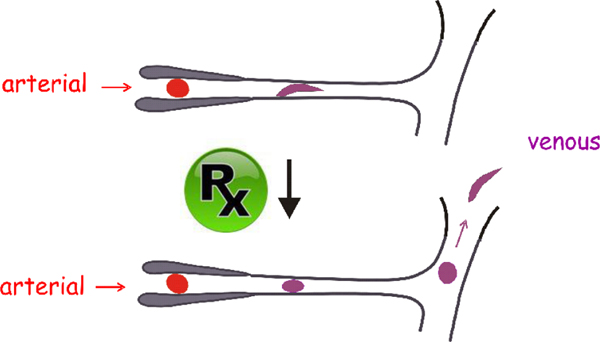
Current standard treatment includes blood transfusions and pain medication. In keeping with the importance of intracellular hemoglobin concentration (MCHC), patients are advised to keep well-hydrated to avoid red cell shrinkage. The immediate goal of drug therapy is not to completely inhibit HbS polymerization, but to partially inhibit polymerization so as to increase the delay time that will allow more cells to escape the microcirculation before sickling begins. (Fig. 11) The most successful drug so far is hydroxyurea, which has proven to be effective in reducing the frequency of vaso-occlusive pain crises and improving survival. Unfortunately, it is considerably underutilized.(Ware and Dertinger) The major effect of hydroxyurea is to stimulate the production of HbF, which replaces HbS. HbF has numerous differences in amino acids with HbS, one of the most important being at position 87 as mentioned above. As a result HbF, copolymerizes with HbS only weakly, so the overall effect is a dilution of HbS that increases the delay time for cell sickling.(Eaton and Bunn, 2017) It would be much more effective if HbF were increased in all cells, which has motivated considerable efforts by the hematology community and Pharma to find ways that mimic S/HPFH, in which HbF is evenly distributed in all cells.
Figure. 11.
Drug therapy. Drugs can be therapeutic without completely inhibiting polymerization. They need only increase the delay time of HbS polymerization to allow more cells to escape the microcirculation without sickling.
In addition to increasing HbF synthesis, 4 other anti-sickling strategies for drug therapy have been proposed that target HbS polymerization.(Eaton and Bunn, 2017) These include (i) blocking an intermolecular contact site. (ii) preferential binding of a drug to the non-polymerizing R conformation, (iii) reducing the concentration of 2,3-DPG, and (iv) reducing the intracellular Hb concentration. Drug development by Pharma most frequently begins with identifying the relevant target. In sickle cell disease, the target has been known since the first Hb structures were determined by Max Perutz in the early 1960’s. The problem with this approach has been that it is very different than finding a compound that can non-covalently bind to an enzyme’s active or allosteric site, which is generally a pocket in the protein that can provide multiple, non-covalent interactions to stabilize the drug-protein complex. The hemoglobin surface in the region of β6 and other intermolecular contact sites in the fiber is smooth with no such pockets. An additional difficulty is that the drug should not preferentially bind to either quaternary structure, as a shift of the equilibrium to R will produce an unwanted increase in oxygen affinity, as discussed next, and a shift toward T would promote polymerization. There have been no successes yet and, currently very little effort to search for compounds that work by this mechanism.
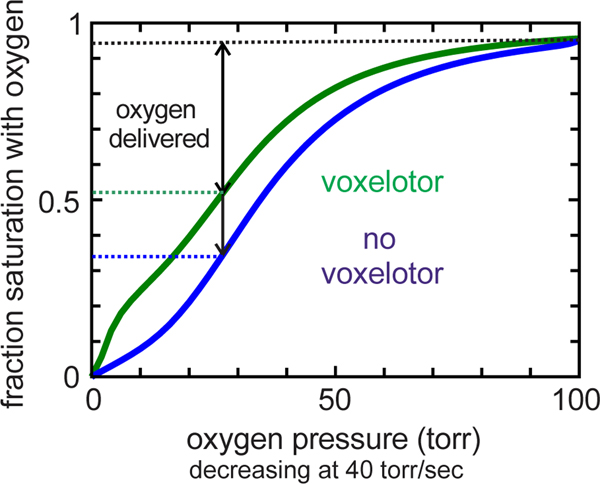
A new anti-sickling drug that works by mechanism (ii), voxelotor, was approved in 2019 by the FDA, only the second FDA approved anti-sickling drug since hydroxyurea in 1998. Voxelotor forms a reversible covalent Schiff base to the N-terminus in the pocket between the αchains of the R conformation to shift the T-R equilibrium toward R. It has been shown to inhibit in vitro sickling and increase blood hemoglobin levels by 1–2 g/dL, but there is no evidence as yet that it reduces either crisis frequency or organ damage. The downside of increasing R is that it produces an increase in oxygen affinity with a concomitant left shift in the oxygen dissociation curve that decreases oxygen delivery (Fig. 12). Oxygen delivery can be predicted using a modified MWC model in which drug binding and dissociation rates are added to the standard MWC. (Henry et al., 2020b) Our recent calculations suggest that in spite of reduced sickling and increase in Hb levels by voxelotor, the net effect is that there is no increase in oxygen delivery. (Henry et al., 2020a)
Figure 12.
Whole blood oxygen dissociation curves (ODC’s) at in vivo rate of oxygen pressure decrease (ODC).(Henry et al., 2020a) The in vivo ODC is different from the equilibrium ODC and the ODC measured in the laboratory at the much lower rate of oxygen pressure decrease (~0.3 torr/sec). (Henry et al., 2020b) Blue curve: ODC for SS blood. Green curve: ODC for SS blood with 26% of hemoglobin modified with voxelotor, the average modification in treated patients.(Vichinsky et al., 2019), The biphasic green curve is the result of the drug binding tightly to the R conformation of HbS.(Henry et al., 2020b)
The rationale for reducing the concentration of 2,3-DPG is more complicated because of multiple effects, as shown in experiments by William Poillon.(Poillon and Kim, 1990; Poillon et al., 1995) The therapeutic effect of decreasing 2,3-DPG is that it increases deoxyHbS solubility by destablizing the fiber, increasing intracellular pH, and may even increase red cell volume to decrease the intracellular HbS concentration. The negative effect of decreasing 2,3-DPG is that it will increase oxygen affinity, although the decrease in 2,3-DPG that increases solubility of deoxyHbS sufficient to be therapeutic may not increase oxygen affinity as much as the tight binding voxelotor(Henry et al., 2020b). The net effect has not yet been determined. The interest in reducing 2,3-DPG is based on the clinical observation of the symptoms of sickle cell disease in individuals with sickle trait, who also have a deficiency in pyruvate kinase, the enzyme that catalyzes ATP formation at the end of the glycolysis pathway. The reduction in the rate of ATP formation causes a buildup of precursors in the pathway, one of which is 2,3-DPG that increases sickling. It does so by stabilizing the HbS fiber, decreasing intracellular pH, and shifting the T-R equilibrium toward the lower affinity and polymerizing T conformation – all effects that would contribute to producing symptoms of sickle cell disease. Currently under investigation is a drug that increases ATP and decreases 2,3-DPG by activating pyruvate kinase.(Rab et al., 2021)(Xu et al., 2020).
A promising approach to drug therapy that has no counter-acting effects is to reduce the intracellular hemoglobin concentration to take advantage of its enormous effect on the delay time. This could be accomplished by a small increase in red cell volume. We have recently predicted that as little as a 10% increase in red cell volume sufficiently increases the delay time of sickling to have a major therapeutic effect.(Li et al., 2017) Another approach is to decrease heme synthesis, which would be expected to decrease the intracellular Hb concentration as it does in iron deficiency anemia. Interestingly, sickle cell patients with iron deficiency and a decreased MCHC experience an increase in Hb and a decrease in hemolysis.(Castro et al., 1994)
I have only discussed treatment of sickle cell disease with drugs that are directly anti-sickling. Two other drugs have been approved by the FDA for treating the disease – crizanlizumab and glutamine. Crizanlizumab is an antibody that reduces adherence by binding to P-selectin, a protein that binds cells to the vascular endothelium.(Karki and Kutlar, 2021) It presumably acts by decreasing transit times and therefore the probability of sickling in the microvasculature. Glutamine’s therapeutic effect is the reduction of oxidative stress,(Cox et al., 2020) which along with inflammation is one the multiple sequelae of HbS polymerization. There are many other compounds in clinical trials that focus on sequelae. Accounts of these therapeutic efforts and a more comprehensive discussion of all drugs being considered to treat SS disease can be found in several recent reviews.(Ballas, 2020; Cisneros and Thein, 2020; Nardo-Marino et al., 2020; Osunkwo et al., 2020; Pace et al.; Telen et al., 2019)
Given the multiple strategies for inhibiting sickling in addition to increasing HbF, there is cause for optimism. Moreover, there are now large libraries of compounds that have been tested in humans, such as the more than 12,000 compound “ReFrame” library of the California Institute of Biomedical Research.(Janes et al., 2018) Compounds in these libraries that exhibit therapeutically significant anti-sickling at concentrations known to be non-toxic can be very rapidly approved for clinical trials. My lab has been testing these compounds using a robust and sensitive high throughput kinetic assay for sickling(Dunkelberger et al., 2018) and we will be reporting results of our screen in the very near future.
ACKNOWLEDGEMENT
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, Bethesda. The author thanks H. Franklin Bunn and Attila Szabo for many helpful comments on the manuscript and H. Franklin Bunn for his constant help and advice on biochemical and clinical aspects of sickle cell disease since the very beginning of my sickle cell project. All of the research described here in which I was directly involved was carried out in collaboration with a group of talented post-doctoral fellows and senior investigators in the Laboratory of Chemical Physics, especially James Hofrichter, Eric R. Henry, Philip D. Ross, Frank A. Ferrone, and Helen R. Sunshine, Andrea Mozzarelli on sabbatical leave from the University of Parma, and H. Franklin Bunn on sabbatical leave from Harvard Medical School. I have also profited enormously from discussions with Swee Lay Thein and John F. Tisdale, my current sickle cell collaborators in the National Heart, Lung and Blood Institute.
Footnotes
Declaration of Conflict of Interest. There are none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackers GK (1998). Deciphering the molecular code of hemoglobin allostery. Adv Prot Chem 51, 185–253. [DOI] [PubMed] [Google Scholar]
- Ackers GK, Doyle ML, Myers D, and Daugherty MA (1992). Molecular code for cooperativity in hemoglobin. Science 255, 54–63. [DOI] [PubMed] [Google Scholar]
- Antonini E, Benedetti PA, Brunori M, Coletta M, Eaton WA, Giardina B, and Hofrichter J. (1983). The use of a microspectrophotometer in the study of the physiological and pathological occurrenecs in hemoglobin. Ricerca in Clinica E in Laboratorio 13, 127–139. [PubMed] [Google Scholar]
- Antonini E, and Brunori M. (1970). Hemoglobin and Myoglobin in their Reaction with Ligands, Vol 21 (Lonadon and New York: North Holland publishin Company; ). [Google Scholar]
- Antonini E, Brunori M, Giardina B, Benedetti PA, Bianchini G, and Grassi S. (1978). Single cell observations of gas reactions and shape changes in normal and sickling erythrocytes. Biophys J 24, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas SK (2020). The evolving pharmacotherapeutic landscape for the treatment of sickle cell disease. Medit J Hematol Infec Dis 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R, and Benesch RE (1969). Intracellular organic phospates as regulators of oxygen rel;ease by hemoglobbin. Nature 221, 618-&. [DOI] [PubMed] [Google Scholar]
- Bringas M, Petruk AA, Estrin DA, Capece L, and Marti MA (2017). Tertiary and quaternary structural basis of oxygen affinity in human hemoglobin as revealed by multiscale simulations. Sci Rep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittenham GM, Schechter AN, and Noguchi CT (1985). Hemoglobin S polymerization. Primary determinant of the hemolytic and clinical severeity of the scikle syndromes. Blood 65, 183–189. [PubMed] [Google Scholar]
- Bunn HF (1997). Mechanisms of disease - Pathogenesis and treatment of sickle cell disease. New Engl J Med 337, 762–769. [DOI] [PubMed] [Google Scholar]
- Bunn HF, and Briehl RW (1970). Interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest 49, 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn HF, and Forget BG (1986). Hemoglobin: Molecular, Genetic, and Clinical Aspects (Saunders, New York, N.Y.). [Google Scholar]
- Bunn HF, Noguchi CT, Hofrichter J, Schechter GP, Schechter AN, and Eaton WA (1982). Molecular and cellular pathogenesis of hemoglobin SC disease. Proc, Natl Acad Sci USA 79, 7527–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZQ, and Ferrone FA (1996). A 50th order reaction predicted and observed for sickle hemoglobin nucleation. J Mol Biol 256, 219–222. [DOI] [PubMed] [Google Scholar]
- Castro O, Poillon WN, Finke H, Massac E, and Kim BC (1994). Improvement of sickle cell anemia ny iron-limited erythropoiesis. Amer J Hematol 47, 74–81. [DOI] [PubMed] [Google Scholar]
- Cellmer T, Ferrone FA, and Eaton WA (2016). Universality of supersaturation in protein-fiber formation. Nat Struct and Mol Biol 23, 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph GW, Hofrichter J, and Eaton WA (2005). Understanding the shape of sickled red cells. Biophys J 88, 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros GS, and Thein SL (2020). Recent advances in the treatment of sickle cell disease. Front Physiol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SIA, Arosio P, Presto J, Kurudenkandy FR, Biverstal H, Dolfe L, Dunning C, Yang XT, Frohm B, Vendruscolo M, et al. (2015). A molecular chaperone breaks the catalytic cycle that generates toxic A beta oligomers. Nature Struct Mol Biol 22, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SIA, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, and Knowles TPJ (2013). Proliferation of amyloid-beta 42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci USA 110, 9758–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SIA, Vendruscolo M, Dobson CM, and Knowles TPJ (2011a). Nucleated polymerization with secondary pathways. II. Determination of self-consistent solutions to growth processes described by non-linear master equations. J Cgem Phys 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SIA, Vendruscolo M, Dobson CM, and Knowles TPJ (2011b). Nucleated polymerization with secondary pathways. III. Equilibrium behavior and oligomer populations. J Chem Phys 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SIA, Vendruscolo M, Welland ME, Dobson CM, Terentjev EM, and Knowles TPJ (2011c). Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments. J Chem Phys 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta M, Hofrichter J, Ferrone FA, and Eaton WA (1982). Kinetics of sickle cell hemoglobin polymerization in single red cells. Nature 300, 194–197. [DOI] [PubMed] [Google Scholar]
- Cox SE, Hart E, Kirkham FJ, and Stotesbury H. (2020). L-Glutamine in sickle cell disease. Drugs of Today 56, 257–268. [DOI] [PubMed] [Google Scholar]
- Demirci S, Gudmundsdottir B, Li Q, Haro-Mora JJ, Nassehi T, Drysdale C, Yapundich M, Gamer J, Seifuddin F, Tisdale JF, et al. (2020). beta T87Q-globin gene therapy reduces sickle hemoglobin production, allowing for ex vivo anti-sickling Activity in human erythroid cells. Mol Therap Meth Clin Devel 17, 912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale CM, Nassehi T, Gamer J, Yapundich M, Tisdale JF, and Uchida N. (2021). Hematopoietic stem cell-targeted gene-addition and gene-editing strategies for beta-Hemoglobinopathies. Cell Stem Cell 28, 191–208. [DOI] [PubMed] [Google Scholar]
- Dunkelberger EB, Metaferia B, Cellmer T, and Henry ER (2018). Theoretical simulation of red cell sickling upon deoxygenation based on the physical chemistry of sickle hemoglobin fiber formation. J Phys Chem B (Eaton Festschrift) 122, 11579–11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen M, Brazauskas R, Walters MC, Bernaudin F, Bo-Subait K, Fitzhugh CD, Hankins JS, Kanter J, Meerpohl JJ, Bolanos-Meade J, et al. (2019). Effect of donor type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: a retrospective multicentre, cohort study. Lancet Haematol 6, E585–E596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WA (2003). Linus Pauling and sickle cell disease. Biophys Chem 100, 109–116. [DOI] [PubMed] [Google Scholar]
- Eaton WA (2018). Autobiography of William A. Eaton. J Phys Chem B 122, 10974–10980. [DOI] [PubMed] [Google Scholar]
- Eaton WA (2020). Hemoglobin S polymerization and sickle cell disease: A retrospective on the occasion of the 70th anniversary of Pauling’s Science paper. Amer J Hematol 95, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WA (2021). Impact of conformational substates and enrgy landscapes on understanding hemoglobin kinetics and function. J Biol Phys in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WA, and Bunn HF (2017). Treating sickle cell disease by targeting HbS polymerization. Blood 129, 2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WA, Henry ER, and Hofrichter J. (1991). Appilcation of linear-free energy relations to protein conformational changes: the quaternary structural change of hemoglobin. Proc Natl Acad Scis USA 88, 4472–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WA, Henry ER, Hofrichter J, Bettati S, Viappiani C, and Mozzarelli A. (2007). Evolution of allosteric models for hemoglobin. Iubmb Life 59, 586–599. [DOI] [PubMed] [Google Scholar]
- Eaton WA, Henry ER, Hofrichter J, and Mozzarelli A. (1999). Is cooperative oxygen binding by hemoglobin really understood? Nat Struct Biol 6, 351–358. [DOI] [PubMed] [Google Scholar]
- Eaton WA, and Hochstrasser RM (1967). Electronic spectrum of single crystals of ferricytochrome c. J Chem Phys 46, 2533–2539. [DOI] [PubMed] [Google Scholar]
- Eaton WA, and Hochstrasser RM (1968). Single crystal spectra of ferrmyoglobin complexes in polarized light. J Chem Phys 49, 985–995. [DOI] [PubMed] [Google Scholar]
- Eaton WA, and Hofrichter J. (1987). Hemoglobin S gelation and sickle cell disease. Blood 70, 1245–1266. [PubMed] [Google Scholar]
- Eaton WA, and Hofrichter J. (1990). Sickle cell hemoglobin polymerization. Adv Prot Chem 40, 63–279. [DOI] [PubMed] [Google Scholar]
- Eaton WA, Hofrichter J, and Ross PD (1976a). Delay time of gelation: possible determinant of clinical severity in scikle cell disease. Blood 47, 621–627. [PubMed] [Google Scholar]
- Eaton WA, Hofrichter J, Ross PD, Tschudin RG, and Becker ED (1976b). Comparison of sickle cell hemoglobin gelation kinetics when measured by NMR and optical methods. Biochem Biophys Res Comm 69, 538–547. [DOI] [PubMed] [Google Scholar]
- Eaton WA, and Trommsdorff HP (2013). Robin Main Hochstrasser (1931–2013), giant of physical chemistry. Proc Natl Acad Sci USA 110, 9189–9190. [Google Scholar]
- Eaton WA, and Zewail AH (1996). Scientific contributions of Robin M. Hochstrasser. J Phys Chem 100, 11791–11805. [Google Scholar]
- Ferrone FA (2015). The delay time in sickle cell disease after 40 years: A paradigm assessed. Amer J Hematol 90, 438–445. [DOI] [PubMed] [Google Scholar]
- Ferrone FA, Hofrichter J, and Eaton WA (1985a). Kinetics of sickle hemoglobin polymerization. 1. Studies using tempertaure jump and laser photolysis techniques. J Mol Biol 183, 591–610. [DOI] [PubMed] [Google Scholar]
- Ferrone FA, Hofrichter J, and Eaton WA (1985b). Kinetics of sickle hemoglobin polymerization. 2. A double nucleation mechanism. J Mol Biol 183, 611–631. [DOI] [PubMed] [Google Scholar]
- Ferrone FA, Hofrichter J, Sunshine HR, and Eaton WA (1980). Kinetic studies on photolysis induced gelation of sickle cell hemoglobin suggest a new mechanism. Biophys J 32, 361–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone FA, Ivanova M, and Jasuja R. (2002). Heterogeneous nucleation and crowding in sickle hemoglobin: An analytic approach. Biophys J 82, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson QH (1959). Photochemical formation of a quickly reacting form of haemoglobin. Biochem J 71, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SJ, Benedict RC, Fall L, Spokane R, and Wyman J. (1979). Oxygen binding to sickle cell hemoglobin. J Mol Biol 130, 175–189. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Boogaerts MAB, Eaton JW, and Steinberg MH (1980). Erythrocyte adherence to endothelium in sickle cell anemia. A possible determinant of clinical severity. New Eng J Med 302, 992–995. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Osarogiagbon R, and Kaul D. (2004). The endothelial biology of sickle cell disease: Inflammation and a chronic vasculopathy. Microcirc 11, 129–151. [PubMed] [Google Scholar]
- Henry ER, Bettati S, Hofrichter J, and Eaton WA (2002). A tertiary two-state allosteric model for hemoglobin. Biophys Chem 98, 149–164. [DOI] [PubMed] [Google Scholar]
- Henry ER, Cellmer T, Dunkelberger EB, Metaferia B, Hofrichter J, Li Q, Ostrowski D, Ghirlando R, Louis JM, Moutereau S, et al. (2020. a). Allosteric control of hemoglobin S fiber formation by oxygen and its relation to the pathophysiology of sickle cell disease. Proc Natl Acad, Sci USA 117, 15018–15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ER, Harper J, Glass KE, BB M, Louis JM, and Eaton WA. (2020b). MWC allosteric model explains unusual oxygen binding curves for drug-bound hemoglobin. Bophys J in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ER, Mozzarelli A, Viappiani C, Abbruzzetti S, Bettati S, Ronda L, Bruno S, and Eaton WA (2015). Experiments on hemoglobin in single crystals and silica gels distinguish among allosteric models. Biophys J 109, 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J. (1986). Kinetics of sickle hemoglobin polymerization 3. Nucleation rates detremined from stochastic fluctuations in polymerization kinetics. J Mol Biol 189, 553–571. [DOI] [PubMed] [Google Scholar]
- Hofrichter J, Henry ER, Szabo A, Murray LP, Ansari A, Jones CM, Coletta M, Falcioni G, Brunori M, and Eaton WA (1991). Dynamics of the quaternary conformational change in trout hemoglobin. Biochemistry 30, 6583–6598. [DOI] [PubMed] [Google Scholar]
- Hofrichter J, Ross PD, and Eaton WA (1974). Kinetics and mechanism of deoxyhemoglobin S gelation: A new approach to understanding sickle cell disease. Proc Natl Acad Sci USA 71, 4864–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J, Ross PD, and Eaton WA (1976). Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad, Sci 73, 3035–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ, Shulman RG, and Ogawa S. (1971). Allosteric model of hemoglobin, 1, kinetics. J Mol Biol 61, 425–443. [DOI] [PubMed] [Google Scholar]
- Janes J, Young ME, Chen E, Rogers NH, Burgstaller-Muehlbacher S, Hughes LD, Love MS, Hull MV, Kuhen KL, Woods AK, et al. (2018). The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc Natl Acad Sci USA 115, 10750–10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Monza E, Balakrishnan G, Blouin GC, Mak PJ, Zhu QH, Kincaid JR, Guallar V, and Spiro TG (2014). Differential control of heme reactivity in alpha and beta subunits of hemoglobin: a combined raman spectroscopic and computational study. J Amer Chem Soc 136, 10325–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki NR, and Kutlar A. (2021). P-Selectin blockade in the treatment of painful vaso-occlusive crises in sickle Cell disease: a spotlight on crizanlizumab. J Pain Res 14, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland DE, Nemethy G, and Filmer D. (1966). Comparison of experimental binding data and theoretical models in protein containing subunits. Biochemistry 5, 365–385. [DOI] [PubMed] [Google Scholar]
- Levantino M, Spilotros A, Cammarata M, Schiro G, Ardiccioni C, Vallone B, Brunori M, and Cupane A. (2012). The Monod-Wyman-Changeux allosteric model accounts for the quaternary transition dynamics in wild type and a recombinant mutant human hemoglobin. Proc Natl Acad, Sci USA 109, 14894–14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Henry ER, Hofrichter J, Smith JF, Cellmer T, Dunkelberger EB, Metaferia BB, Jones-Straehle S, Boutom S, Christoph GW, et al. (2017). Kinetic assay shows that increasing red cell volume could be a treatment for sickle cell disease. ProcNatl Acad Sci 114, E689–E696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfa R, and Steinhardt J. (1974). Temperature dependent latent period in aggregation of sickle cell hemoglobin. Biochem Biophys Res Comm 59, 887–893. [DOI] [PubMed] [Google Scholar]
- McGann PT (2014). Sickle cell anemia: An underappreciated and unaddressed contributor to global childhood mortality. J Pediat 165, 18–22. [DOI] [PubMed] [Google Scholar]
- Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H, et al. (2007). A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nature Genet 39, 1197–1199. [DOI] [PubMed] [Google Scholar]
- Moffat K, and Gibson QH (1974). Kinetics of polymerization and depolymerization of sickle cell hemoglobiun. Biochem Biophys Res Comm 61, 237–242. [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, and Changeux JP (1965). On the nature of allosteric transitions: a plausible model. J Mol Biol 12, 88–118. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A, Hofrichter J, and Eaton WA (1987). Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science 237, 500–506. [DOI] [PubMed] [Google Scholar]
- Nardo-Marino A, Brousse V, and Rees D. (2020). Emerging therapies in sickle cell disease. Brit J Haematol 190, 149–172. [DOI] [PubMed] [Google Scholar]
- Orkin SH, and Bauer DE (2019). Emerging genetic therapy for sickle cell disease. Ann Rev Med 70, 257–271. [DOI] [PubMed] [Google Scholar]
- Osawa F, and Sakura S. (1975). The Thermodynamic of the Polymerization of Protein, (New York: Academic Press; ). [Google Scholar]
- Osunkwo I, Manwani D, and Kanter J. (2020). Current and novel therapies for the prevention of vaso-occlusive crisis in sickle cell disease. Therap Adv Hematol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace BS, Starlard-Davenport A, and Kutlar A. Sickle cell disease: progress towards combination drug therapy. Brit J Haemotol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L, Itano HA, Singer SJ, and Wells IC (1949). Sickle cell anemia, a molecular disease. Science 110, 543–548. [DOI] [PubMed] [Google Scholar]
- Perutz MF (1970). Stereochemistry of cooperative effects in haemoglobin. Nature 228, 726-&. [DOI] [PubMed] [Google Scholar]
- Poillon WN, and Kim BC (1990). 2,3-Diphosphoglycerate and intracelluar pH as determinats of the physiologival solubility of deoxyhemoglobin S. Blood 76, 1028–1036. [PubMed] [Google Scholar]
- Poillon WN, Kim BC, Labotka RJ, Hicks CU, and Kark JA (1995). Antisickling effects of 2,3-diphosphoglycerate depletion. Blood 85, 3289–3296. [PubMed] [Google Scholar]
- Rab MAE, van Oirschot BA, Kosinski PA, Hixon J, Johnson K, Chubukov V, Dang L, Pasterkamp G, van Straaten S, van Solinge WW, et al. (2021). AG-348 (mitapivat), an allosteric activator of red blood cell pyruvate kinase, increases enzymatic activity, protein stability, and adenosine triphosphate levels over a broad range of PKLR genotypes. Haematologica 106, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PD, Hofrichter J, and Eaton WA (1977). Thermodynamics of gelation of deoxyhemoglobin S. J Mol Biol 115, 111–134. [DOI] [PubMed] [Google Scholar]
- Ross PD, and Minton AP (1977). Analysis of Non-ideal Behavior in Concentrated Hemoglobin Solutions. J Mol Biol 112, 437–452. [DOI] [PubMed] [Google Scholar]
- Sankaran VG, Xu J, Ragoczy T, Ippolito GC, Walkley CR, Maika SD, Fujiwara Y, Ito M, Groudine M, Bender MA, et al. (2009). Developmental and species-divergent globin switching are driven by BCL11A. Nature 460, 1093–U1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama N, Morimoto H, and Saigo S. (1998). Asymmetric cyanomet valency hybrid hemoglobin, (alpha(+CN-)beta(+CN-))(alpha beta): The issue of valency exchange. Biochemistry 37, 6221–6228. [DOI] [PubMed] [Google Scholar]
- Shulman RG (2001). Spectroscopic contributions to the understanding of hemoglobin function: Implications for structural biology. Iubmb Life 51, 351–357. [DOI] [PubMed] [Google Scholar]
- Sunshine HR, Hofrichter J, and Eaton WA (1978). Requirements for therapeutic inhibition of sickle hemoglobin gelation. Nature 275, 238–240. [DOI] [PubMed] [Google Scholar]
- Sunshine HR, Hofrichter J, Ferrone FA, and Eaton WA (1982). Oxygen binding by sickle hemoglobin polymers. J Mol Biol 158, 251–273. [DOI] [PubMed] [Google Scholar]
- Szabo A. (1988). Fluctuations in the polymerization of sickle hemoglobin: A simple analytic model. J Mol Biol 199, 539–542. [DOI] [PubMed] [Google Scholar]
- Telen MJ, Malik P, and Vercellotti GM (2019). Therapeutic strategies for sickle cell disease: towards a multi-agent approach. Nature Rev Drug Disc 18, 139–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale JF, Thein SL, and Eaton WA (2020). Treating sickle cell anemia. Science 367, 1198–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornquist M, Michaels TCT, Sanagavarapu K, Yang XT, Meisl G, Cohen SIA, Knowles TPJ, and Linse S. (2018). Secondary nucleation in amyloid formation. Chem Comm 54, 8667–8684. [DOI] [PubMed] [Google Scholar]
- Viappiani C, Abbruzzetti S, Ronda L, Bettati S, Henry ER, Mozzarelli A, and Eaton WA (2014). Experimental basis for a new allosteric model for multisubunit proteins. Proc Natl Acad Sci USA 111, 12758–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viappiani C, Bettati S, Bruno S, Ronda L, Abbruzzetti S, Mozzarelli A, and Eaton WA (2004). New insights into allosteric mechanisms from trapping unstable protein conformations in silica gels. Proc Natl Acad Sci USA 101, 14414–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, Hassab H, Achebe MM, Alkindi S, Brown RC, et al. (2019). A phase 3 randomized trial of voxelotor in sickle cell disease. New Engl J Med 381, 509–519. [DOI] [PubMed] [Google Scholar]
- Ware RE, and Dertinger SD (2021). Absence of hydroxyurea-induced mutational effects supports higher utilisation for the treatment of sickle cell anaemia. Brit J Haemotol in press. [DOI] [PubMed] [Google Scholar]
- Weng WJ, Aprelev A, Briehl RW, and Ferrone FA (2008). Universal metastability of sickle hemoglobin polymerization. J Mol Biol 377, 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JZ, and Thein SL (2019). The carrier state for sickle cell disease is not completely harmless. Haematologica 104, 1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun KM, Morimoto H, and Shibayama N. (2002). The contribution of the asymmetric alpha 1 beta 1 half-oxygenated intermediate to human hemoglobin cooperativity. J Biol Chem 277, 1878–1883. [DOI] [PubMed] [Google Scholar]