Abstract
Objectives
C-reactive protein (CRP) has been associated with adiposity and cardiometabolic disease risk in many populations but remains remarkably understudied in Pacific Islander populations. Here, we provide the first examination of correlates of CRP in adult Samoans (n=108, ages 35–55 years) to test the hypotheses that CRP exhibits sex-dependent associations with measures of BMI, adiposity, and cardiometabolic disease risks.
Methods
We analyzed associations between measures of adiposity (total fat mass, visceral fat mass, percent total body fat), body mass index (BMI), cardiometabolic risks, behaviors, demographics, and CRP. Unadjusted analyses of CRP were undertaken using Pearson’s Pairwise, and Spearman’s Rank Correlations; one-way analysis of variance and Kruskal-Wallis tests assessed variables by CRP quartiles. Adjusted analyses of CRP correlates were examined using generalized linear regression.
Results
Serum CRP ranged from 0.08 mg/L to 13.3 mg/L (median 1.4 mg/L) and varied significantly by sex t (108) =−2.47, p=0.015. CRP was weakly to moderately associated with measures of adiposity and BMI (r and ρ ranged between 0.25–0.50, p<0.05) and some cardiometabolic markers (including HbA1c, fasting insulin, and insulin resistance). CRP was significantly associated with percent body fat in women and men, adjusting for other variables.
Conclusions
These data are the first to demonstrate CRP correlates in a sample of adult Samoans. CRP differed by sex and was associated with BMI, adiposity, and some cardiometabolic risk markers. These data align with findings in other populations.
Keywords: Samoa, C-reactive Protein (CRP), inflammation
Objectives
C-Reactive Protein (CRP), an acute phase reactant regulated by pro-inflammatory cytokines, is a useful predictor of systemic inflammation (Tonstad & Cowan, 2009), a physiological state associated with many poor cardiometabolic health outcomes including cardiovascular disease (CVD), hypertension, and Type 2 Diabetes Mellitus (T2D) (Chen, Chen, Wang, & Liang, 2015). CRP values > 2 mg/L are considered indicative of ‘metabolic inflammatory’ states (Markanday, 2015), and the American Heart Association identifies individuals with CRP > 3mg/L as having high risk of CVD (Ridker Paul, 2003; Tonstad & Cowan, 2009). However, associations between CRP and cardiometabolic health in low- and middle-income countries, and specifically Pacific Islander populations, are not well described. Thus, there is a critical need to determine the extent to which CRP may also be a correlate of cardiometabolic health and disease in these less well-studied populations.
CRP was positively associated with increased adiposity and body mass index (BMI, kg/m2) both risk factors for cardiometabolic disease, in studies of US (Rexrode, Pradhan, Manson, Buring, & Ridker, 2003), Israeli (Aronson et al., 2004), European (Gentile et al., 2010; Timpson et al., 2011), Japanese (Saijo et al., 2004; Tsuriya et al., 2011), and Korean adults (Park, Park, & Yu, 2005). Positive associations between CRP levels and BMI (which we describe here as a measure of ‘body size’ to differentiate it from the direct measurement of fat using DXA, or ‘adiposity’) were observed in Japanese adults < 75 years old (individuals with BMI ≥ 25 kg/m2 were more likely to have CRP levels ≥ 1 mg/L compared to individuals with lower BMIs) (Kawamoto, Kusunoki, Abe, Kohara, & Miki, 2013), and US adults > 17 years old (individuals with BMI ≥ 30 kg/m2 had 2.13–6.21 the odds of CRP levels > 2.2 mg/L, and women who were obese were four times as likely to have CRP levels > 10 mg/L compared to women with lower weight) (Visser, Bouter, McQuillan, Wener, & Harris, 1999). Similarly, visceral adiposity (as recorded by Computed Tomography) was positively associated with CRP in Japanese adults with mild obesity and impaired glucose tolerance (Tsuriya et al., 2011), in Japanese adults without diabetes (Saijo et al., 2004), and in Korean adults without inflammatory disease (Park et al., 2005). Additionally, positive associations between CRP and additional cardiometabolic diseases/risks were observed in other adult populations (Table 1). Higher CRP levels in women, compared to men, have also been observed in multiple studies (Blaha et al., 2011; Cartier et al., 2009; Visser et al., 1999), with increased accumulation of subcutaneous fat in women posited as one explanation for those differences (Cartier et al., 2009).
Table 1:
Cardiometabolic Diseases and Risk positively associated with CRP in adults.
| Cardiometabolic disease/risk | Location/population | Studies |
|---|---|---|
| Microvascular dysfunction | Australia | (Tong et al., 2018) |
| Arterial disease | The Netherlands | (Faber, van der Graaf, Westerink, & Visseren, 2010) |
| Dyslipidemia | Pakistan, Sudan, Mongolia | (Dongway, Faggad, Zaki, & Abdalla, 2015; Tang et al., 2014; Waheed, Naveed, & Farooq, 2009) |
| Hypertension | Europe | (Carbone et al., 2019) |
| Hyperglycemia | United States | (de Rekeneire et al., 2006; King, Mainous, Buchanan, & Pearson, 2003; Pradhan, Manson, Rifai, Buring, & Ridker, 2001) |
| Insulin resistance | United States | (Festa et al., 2000) |
| Diabetes incidence | Scotland, United States (Japanese Americans) | (Freeman et al., 2002; Nakanishi, Yamane, Kamei, Okubo, & Kohno, 2003) |
| Smoking | Japan | (Fröhlich et al., 2003; Ohsawa et al., 2005) |
| Reduced physical activity | United States | (Loprinzi et al., 2013; Plaisance & Grandjean, 2006) |
| Alcohol consumption (as related to severe cirrhosis) | France | (Cervoni et al., 2012) |
Examining the association among CRP, adiposity, BMI, and cardiometabolic disease risk in Pacific Islanders is of interest due to the high prevalence of obesity and related non-communicable diseases (NCDs) in the region (Hawley & McGarvey, 2015; McLennan & Ulijaszek, 2015). In Samoa specifically, the prevalence of obesity increased from 27.7% to 53.1% and 44.4% to 76.7% in adult men and women respectively, between 1978–2013. In a global compilation, mean age-standardized BMI in 2019 among Samoan men was 33.9 kg/m2 (24–44.9 kg/m2), and 37.4 kg/m2 (27.2–47.4 kg/m2) among women (Murray et al., 2020). Samoan adult BMI levels exceed those of many populations in which the association between CRP and adiposity has been previously studied. Additionally, over the same period (1978–2013), diabetes prevalence in Samoa increased from 1.2% to 19.6% and 2.3% to 19.5% in adult men and women respectively (Lin et al., 2017).
Pacific Islanders are not well represented in studies of CRP; thereby, this study provides an opportunity to advance understandings of global variation in CRP levels as well as explore the utility of using CRP as a clinical predictor of risk across different populations. Previous studies of CRP in the Pacific Islands provide preliminary support for positive associations between CRP and body size, and for the presence of elevated CRP levels in the Pacific Islands populations; however, these studies are limited by a focus on younger age groups (Brown et al., 2010; McDade, 2001) or small sample sizes (Sukala et al., 2012). For example, Brown et al. (2010) found positive associations between CRP and measures of body size (BMI, waist circumference, and waist-to-hip ratio) and blood pressure in Hawaiian children (n=66, mean age 8.5 years ± 0,4; cohort included 50% Native Hawaiians). McDade et al. (2001) found that among n=760 Samoan children and young adults (ages 2–20 years), 6.2% had CRP levels > 5 mg/L, and Sukala et al. (2012) found baseline CRP levels ≥ 3mg/L in 67% of Pacific Islander and Maori adult participants (n=18) enrolled in an exercise intervention living in New Zealand (n=2 participants were Samoan). The purpose of this report is to 1) determine CRP levels in a sample of adult Samoans (n=108); 2) to determine the extent to which associations, observed elsewhere, between CRP and measures of BMI, adiposity, and cardiometabolic risk, are present; and 3) to determine if CRP differs by sex.
Methods
Here we present a secondary, cross-sectional analysis of correlates of CRP in a sample of adult Samoans (ages 35–55 years). Participants were a subsample (n=108, 50.9% female) of adults who participated in the Soifua Manuia (“Good Health”) study of adiposity between 2017–2019 (Hawley et al., 2020). The Soifua Manuia study followed up with n=519 individuals who had participated in a 2010 genome-wide association study that identified a missense variant in the CREBRF gene associated with increased BMI (Minster et al., 2016). The Soifua Manuia study aimed to characterize the association of the CREBRF variant with metabolic (body composition, glucose homeostasis) and behavioral traits (dietary intake, physical activity, sleep, weight control behaviors) that influence energy homeostasis (Hawley et al., 2020). Given the study objectives, participants in the Soifua Manuia study were purposively recruited based on their CREBRF genotype; inclusion criteria included agreement in 2010 to be re-contacted for future studies, age between 32–72 years old, no current pregnancy, no physical or cognitive disabilities precluding participation, and resident on the island of Upolu (due to the nature of measurement protocols). Because the CREBRF genotype was not associated with CRP levels, it is not included in the following analyses.
The study was approved by the Health Research Committee of the Samoan Ministry of Health, and the Institutional Review Board of Yale University (who had an IAA with Brown University #18–97). All participants provided written informed consent.
Setting
Samoa is an independent Polynesian island nation located in the South Pacific with a population of approximately 203,774 (CIA, 2020), the majority of whom live on the islands of Upolu and Savaiì. The country is divided into four census regions: the Apia Urban Area (AUA; Apia is the capital of Samoa), the Northwest Region of Upolu (NWU), the Rest of Upolu (ROU), and Savai’i (SAV). Classified as an upper middle-income country, the Samoan economy is largely dependent on agriculture and fishing, tourism, development aid, and remittances from abroad (CIA, 2020).
Data collection
Study assessments took place at the Obesity Lifestyle and Genetic Adaptations (OLaGA, n. Life) Research Center located in the Ministry of Health, Apia, Samoa. Physical measurements and questionnaire data were collected by trained Samoan research assistants.
Anthropometrics and Blood Pressure
Participant weight and height were measured in duplicate using a digital scale (Tanita HD 351: Tanita Corporation of America and portable stadiometer (SECA 213, Seca GmbH & Co), respectively. BMI (kg/m2) was calculated based on average measures of weight and height. Following a 10-minute seated rest period, blood pressure was measured three times, with a 3-minute rest period between measurements, using an Omron HEM-907XL automated blood pressure monitor (Omron HEM-907XL, Omron Healthcare). The last two measures were averaged for analyses.
Body Composition
Total body composition, including fat mass (kg), visceral adipose tissue mass (VAT; kg; estimated using the CoreScan application, GE Healthcare Medicine), percent body fat, and lean mass (kg) were assessed using dual-energy X-ray absorptiometry (DXA; Lunar iDXA, Encore version 17, GE Healthcare Medicine). Because additional eligibility criteria were applied to DXA measurements (participants with recent X-Ray or CT exposure did not undergo the DXA screening), analyses using DXA-derived outcomes were restricted to n=99 participants. A further n=3 individuals are missing visceral adipose tissue mass measures due to challenges accommodating their body width within the scan area.
Questionnaire
Questionnaire data included demographic information (age and sex), current smoking status (yes/no), alcohol consumption (ever consumed alcohol; yes/no), self-report of health status, self-report of having received a physician’s diagnosis of hypertension or diabetes (yes/no) and use (yes/no) and type of diabetes medications. Additionally, women were asked if they were currently using contraceptive methods (i.e., pills, injections, vaginal ring, implants, or intrauterine devices) some of which have been found elsewhere to be positively associated with CRP (Buchbinder et al., 2008; de Medeiros, Medeiros, Santos, Barbosa, & Yamamoto, 2018; Divani, Luo, Datta, Flaherty, & Panoskaltsis-Mortari, 2015). Only one participant reported current use; as the individual was using a contraceptive injection that is not known to be significantly associated with CRP (B. Al-Youzbaki, 2011; Fajumi, 1984), her data were retained in the analyses. Study data were collected and managed using Research electronic data capture (REDCap) electronic data capture tools (Harris et al., 2019; Harris et al., 2009) hosted at Yale University.
Biochemical data
Glycated hemoglobin A1c (HbA1c) was collected via capillary finger stick and assayed using a DCA Vantage analyzer (Siemens Healthcare GmbH). Participants provided fasting venous blood samples into vacutainer tubes containing the appropriate additive for collection of plasma or serum. Tubes to measure glucose included the glycolytic inhibitor sodium fluoride. Blood was immediately centrifuged, aliquoted in to 2mL cryovials, and stored at −80°F on site in Samoa until transport on dry ice to the US for assay. Plasma glucose was determined in triplicate using an Analox GM9 Glucose Analyzer in triplicate. Plasma insulin was determined in triplicate using Crystal Chem Human Insulin ELISA Kit (Catalog #DRP300). Serum lipid panel (Total Cholesterol and triglycerides) were determined in the CLIA-certified clinical lab at the University of Pittsburgh Medical Center in accordance with clinical standards.
Fasting serum samples were also assayed for high-sensitivity CRP (hsCRP, mg/L; hsCRP can detect low levels of CRP; the paper subsequently refers to hsCRP as CRP). CRP was assayed at the Yale University Reproductive Ecology Laboratory using the CRP (human) ELISA kit from Enzo Life Sciences (ENZ-KIT102). Samples were diluted at least 1:4 in assay buffer to remove matrix interference and were assayed following the manufacturer’s protocol. Optical densities were read on an 800 TS Absorbance Reader from BioTek Instruments and Gen5 software was used for data reduction. All samples were assayed in duplicate, and we report the mean concentration of each set of duplicates in mg/L. As a quality control measure, we reran all samples for which duplicates had a coefficient of variation (CV) >20. The mean inter-assay CV for CRP assays was 13.6% (19.3 = high control; 8.0 = low control).
CRP values were initially measured on a randomly selected subsample of participants (n=118, 50.0% female) who were between the ages of 35–55 (individuals younger than 35 and older than 55 from the larger cohort were excluded for these analyses due to the positive associations between age and inflammation, (Wyczalkowska-Tomasik, Czarkowska-Paczek, Zielenkiewicz, & Paczek, 2016)). Of these individuals, five who reported using diabetes medication (either pills or insulin) were excluded from analyses as insulin supplementation (n=1 user) can artificially influence fasting insulin levels, and metformin (n=4 users) can reduce circulating CRP levels (Chait & den Hartigh, 2020; S. N. Li et al., 2009). Using the IQR method of outlier detection due to the right skewing of the CRP data, we identified an additional n=5 extreme outliers occurring at > 13.9 mg/L that were removed from analyses. Seven individuals with CRP values between 8.95 mg/L to 13.9 mg/L were identified as mild outliers and retained for analyses for a total sample size of n=108 individuals. Sensitivity analyses were conducted excluding the n=7 individuals with outlying values (supplementary Table 3 and Supplementary Table 5) and demonstrate that the retention of these n=7 individuals did not substantially or significantly change the results. Additionally, while CRP > 10 mg/L has previously been used an indicator of obvious inflammation or infection (Aronson et al., 2004), the latter is less likely (but not entirely ruled out) in this sample due to a) low prevalence of macro-parasitic infections in humans in this setting and b) participants self-reported health statuses did not indicate acute illness.
Physical Activity
Objectively measured physical activity was assessed using a wrist worn ActiGraph GT3X+ (ActiGraph Corporation, Pensacola, FL). GT3X+ were placed on the participant’s non-dominant wrist and worn for 7–10 days. Data were cleaned, and non-wear time was determined using the Choi algorithm (Choi, Liu, Matthews, & Buchowski, 2011). A valid day of accelerometer wear was defined as ≥ 10 hours of wear time. Using the ‘TwoRegression’ R package (P. Hibbing & van Hees, 2018), raw csv files were used to estimate time spent in moderate to vigorous physical activity (MVPA, ≥ 3.0 metabolic equivalents; METs) using the Hibbing 2-regression adult non-dominant wrist algorithm (P. R. Hibbing, Lamunion, Kaplan, & Crouter, 2018). Number of valid days among the sample ranged from 0–11 days. Only participants with at least 3 days minimum of valid wear days were included in these analyses. Accelerometer analyses were conducted on n=89 participants, as n=15 participants did not have data, and n=4 participants registered fewer than 3 days of valid wear time. All MVPA analyses in this paper control for number of valid minutes of wear time.
Cardiometabolic disease definitions
The frequency of type 2 diabetes was assessed based on participants’ self-report of taking medication for diabetes and/or having a fasting plasma glucose (FPG) level ≥ 126mg/dL and/or HbA1c values of ≥ 6.5% at time of data collection. Insulin resistance was calculated using the Homeostatic Model Assessment for Insulin Resistance: HOMA-IR [(fasting glucose (mg/dL) / fasting insulin (mU/L)/405 (Rivas-Crespo, 2015)]. Hypertension was defined as elevated blood pressure [systolic blood pressure (BP) ≥ 140 mmHg and/or diastolic BP ≥ 90mmHg] or reported hypertension medication use. We acknowledge that hypertension characterization based on BP measures at only one timepoint may be problematic (Whelton et al., 2018).
Statistical Analyses
CRP
CRP level was examined as a continuous variable, and quartiles (based on score distribution), and adjusted in several ways in response to raw data distribution and variance: 1) Due to the (expected) right skewing of the CRP data (Kushner, Rzewnicki, & Samols, 2006), CRP median values and interquartile ranges are presented in the descriptive statistics; 2) For correlation analyses CRP was normalized using log-transformation to examine associations between CRP and total fat mass, total lean mass, total cholesterol, systolic BP, and diastolic BP. For all other variables (which did not meet normality assumptions), untransformed CRP data were used for Spearman Rank Correlation calculations; 3) For scatter plots, CRP was left as raw data; 4) Following previous studies with similar aims (Ayas et al., 2019; Hayashino, Mashitani, Tsujii, & Ishii, 2014; Ye et al., 2007), CRP values were divided into quartiles for unadjusted analyses of associations between BMI, measures of adiposity, cardiometabolic markers, and behaviors by CRP quartile; finally, 5) Analyses controlling for sex and adjusting for adiposity, BMI and other predictors of CRP were undertaken using generalized linear regressions with CRP as the outcome variable.
Descriptive Statistics and exploratory analyses
Sample characteristics are presented in the following manner: based on the distribution of data, continuous variables are expressed as either median and interquartile range (75–25), or mean and standard deviation (SD); minimum and maximum values are presented for each variable. Categorical variables are expressed as percentages. Normality was assessed using the Shapiro Wilk test. Comparisons between the sexes for all measured variables were undertaken using Mann-Whitney U tests and independent samples two-tailed T-Tests (for variables that met, or could be statistically transformed to meet, normality assumptions).
The relationships between CRP and measures of adiposity (Total fat mass, percent body fat, and visceral adipose tissue mass), BMI, and select cardiometabolic risks (HbA1c, fasting insulin, systolic BP, total cholesterol) by sex, were visualized with scatterplots (Figure 1a–d; supplementary figure 1a–d). Correlation analyses were used to test associations between continuous measures of CRP, adiposity (Total fat mass, percent body fat, visceral adipose tissue mass), BMI, total lean mass, cardiometabolic risk markers (HbA1c, FPG, fasting insulin (FI), total cholesterol, triglycerides, systolic BP, and diastolic BP), and average minutes of MVPA. Spearman correlations were used to assess measures that did not meet normality assumptions or could not be normalized; Pearson’s pairwise correlations were used to calculate associations between those variables that met normality assumptions (log-transformed CRP, log-transformed BMI, total fat mass, log-transformed total lean mass, total cholesterol, log-transformed systolic BP, and diastolic BP). Partial correlations, controlling for accelerometer wear time, were used to examine associations between log transformed MVPA and the other variables (Full correlation analyses presented in Supplementary Table 1).
Figure 1: Scatterplots of CRP and selected adiposity, BMI, and cardiometabolic measures.
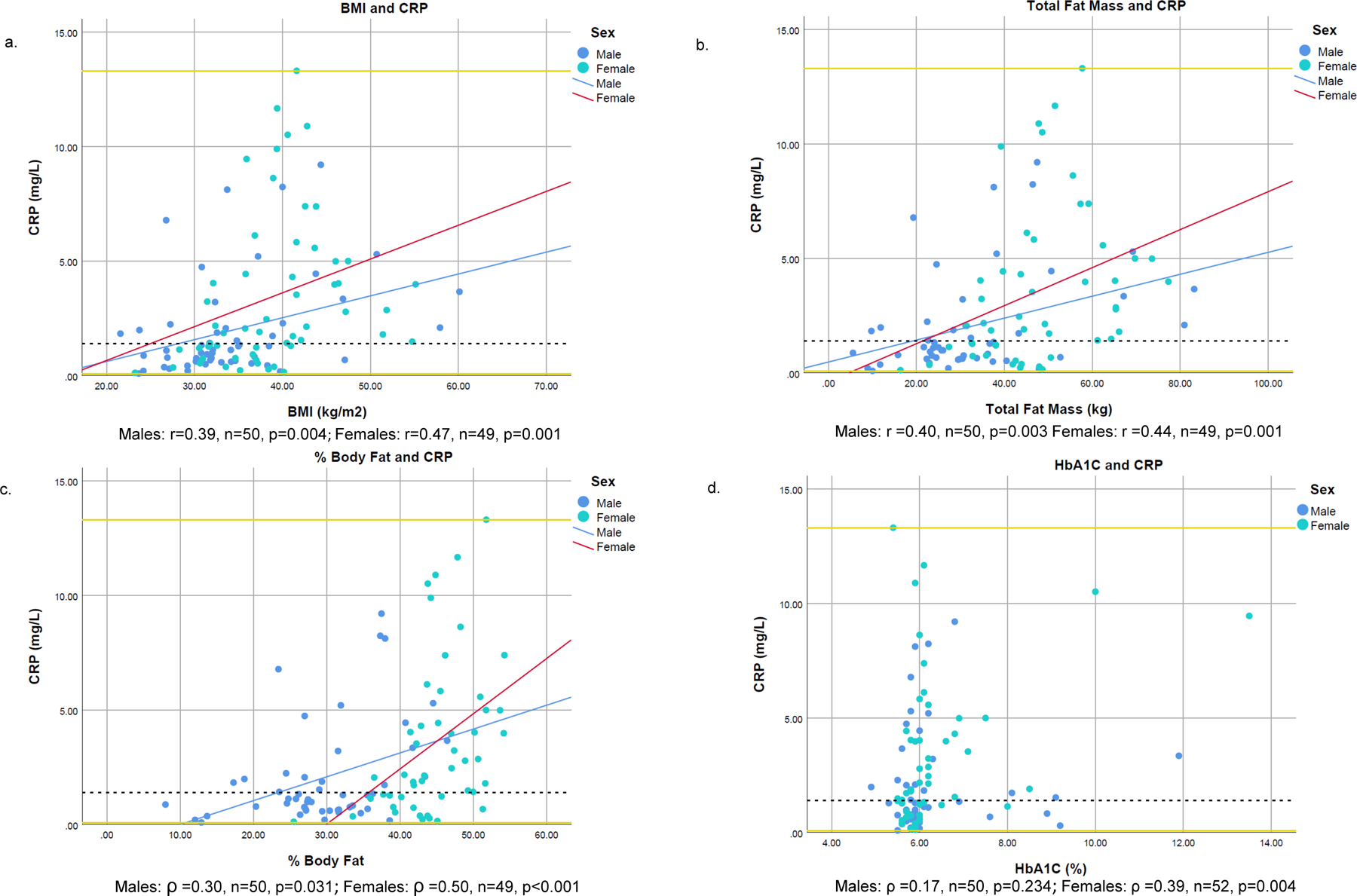
1BMI was logarithmically transformed before analysis.
2Scatter plots created with raw data; correlation analyses for BMI and Total Fat Mass, were analyzed using Pearson’s correlation; all other variables analyzed via Spearman Rank correlation.
3Reference lines are placed at minimum (0.08 mg/L), median (1.4 mg/L), and maximum (13.3 mg/L) values of CRP
Unadjusted analyses
Unadjusted associations between CRP quartiles and participant measures of BMI, adiposity, cardiometabolic risks, and behaviors were tested with non-parametric Kruskal Wallis (for non-normally distributed variables), one-way analysis of variance (ANOVA) (for normally distributed variables), and chi-squared (for categorical variables) tests of association. Mirroring prior studies with similar aims (Ayas et al., 2019; Hayashino et al., 2014), CRP was separated into data-driven quartiles as follows: quartile 1 (0.08≤ 0.68 mg/L), quartile 2 (0.73− ≤ 1.42 mg/L), quartile 3 (1.48− ≤ 3.97 mg/L), and quartile 4 (3.98 – ≤ 13.3 mg/L). Due to the right skewing of the CRP data, median values best represented the centrality measure for CRP in the unadjusted analyses. Sample characteristics were compared by CRP quartile (means or medians presented based on distribution of each variable); total sample (n=108) and sex-stratified analyses were completed. Sex-stratified analyses were conducted using Kruskal-Wallis tests due to reduced sample sizes resulting from stratification. Associations between CRP quartiles and markers associated with diabetes risk and incidence (HbA1c, FPG, FI, and HOMA-IR) were examined within the total sample, and among individuals without diabetes (HbA1c < 6.5% and/or FPG <126 mg/L, n =82) due to reported elevations of CRP levels in individuals living with diabetes (Ebrahimi et al., 2016; Freeman et al., 2002; King, Mainous, Buchanan, & Pearson, 2003). Additionally, associations between CRP quartiles and BP values were examined within the total sample, and among individuals without hypertension (n=80), due to reported elevations of CRP in individuals living with hypertension (Carbone et al., 2019). Post-hoc assessments of significant results from the total sample were undertaken using Bonferroni’s correction and Dunn’s test (for non-parametric analyses); boxplots visualizing the results can be found in the supplementary section (Supplementary Figure 2a–g and Supplementary Table 2a–g). Analyses excluding individuals living with diabetes or hypertension can be found in Supplementary Table 4a–d.
Multiple Regression
Variables that demonstrated significant associations (p< 0.05) or associations that trended towards significance with CRP quartiles (p<0.1) in the unadjusted analyses (in either the total sample or within the sex-stratified analyses) were included as predictors in sex-stratified linear multiple regression models where log-transformed CRP was the outcome. Models were sex-stratified based on the significant difference observed between CRP and sex in the unadjusted analyses. Additionally, due to the strong associations observed in the unadjusted analyses between CRP and adiposity, sex-stratification facilitated the identification of CRP correlates while controlling for the biologically intrinsic higher fat mass found in women (Blaak, 2001). The female model was reduced to n=45, and male model to n=50 participants (models were reduced based on missing data and the reduced number of individuals who had completed DXA body scans). Due to the absence of observed significance between CRP levels and diabetes in the unadjusted models, individuals living with diabetes or hypertension were not excluded from the regression analyses. Stepwise backwards linear regression procedures (using p<0.2 as criterion for removal from the model) were used to identify the most parsimonious models. The models were assessed for multiple regression assumptions, specification errors, and multicollinearity, and adjusted as needed; the outcome CRP was natural log transformed to normalize both models’ residuals. Non-significant correlates of CRP were retained in the model following backwards stepwise procedures if shown to improve each model’s explanatory power, and age was retained in all models. Unstandardized and standardized model coefficients are presented.
Analyses and figures were conducted using STATA v 15 (Stata Corp, College Station, TX) and IBM SPSS v. 26 (IBM, Armonk, NY).
Results
The distribution of CRP values in the sample ranged from 0.08 mg/L to 13.3 mg/L, with a median value of 1.4 mg/L, and mean value of 2.7 mg/L (Table 2). CRP differed by sex with women having higher median (2.0 mg/L ± 3.8 vs. 1.1 mg/L ± 1.4) and mean values (3.4 mg/L ± 3.3 vs. 1.9 mg/L ± 2.2) compared to men. The sample was characterized by high BMI (mean 36.5 kg/m2 ± 7.6), and high levels of adiposity as measured by visceral adipose tissue mass (median 1.5 kg ± 0.8), total fat mass (mean 39.8 kg ±16.9), and percent body fat (median 38.6% ± 15.8%). Men and women differed in their measures of body composition (i.e., total fat mass, percent body fat, and Total Lean Mass, but excluding visceral adipose tissue mass) and BMI; women had statistically significant higher BMI, total fat mass, percent body fat, and lower lean mass than men. Diabetes frequency was 24.1% in the sample (27.3% among women and 20.7% among men) and did not significantly differ between sexes. Median Hba1c and fasting glucose values fell within the prediabetes range. Average total cholesterol levels were within elevated risk ranges (Goff David et al., 2014) and hypertension was present in 24.5% of the sample. Systolic BP values within the sample had a mean value of 125.8 mmHg ± 18.6, and diastolic BP value of 79.7 mmHg ± 13.2. Smoking and alcohol consumption were significantly more prevalent among men compared to women, and men recorded statistically significant higher minutes of MVPA than women.
Table 2:
Descriptive characteristics of the sample (n=108)
| Total Sample (n =108) | Women (n = 55) | Men (n = 53) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | Md (IQR)/% | Mean (SD) | Min, Max | Md (IQR) | Mean (SD) | Md (IQR) | Mean (SD) |
Mann-W U†
p value |
T-test
p value |
| Age (years) | 45.9 (7.8) | - | 35.2, 55.0 | 46.0 (7.7) | - | 45.8 (8.1) | - | 0.606 | - |
| CRP (mg/L) | 1.4 (3.3) | 2.7 (2.9) | 0.08, 13.3 | 2.0 (3.8) | 3.4 (3.3) | 1.1 (1.4) | 1.9 (2.2) | - | 0.015 |
|
Body Size and Adiposity Measures
| |||||||||
| BMI (kg/m2) | - | 36.5 (7.6) | 21.6, 60.1 | - | 38.8 (6.7) | - | 34.2 (7.9) | - | <0.001 |
| Visceral adipose tissue (kg)§ | 1.5 (0.9) | - | 0.9, 5.7 | 1.5 (0.8) | - | 1.5 (1.0) | - | 0.837 | - |
| Total Fat mass (kg) | - | 39.8 (16.9) | 5.6, 83.2 | - | 47.2 (13.4) | - | 32.4 (16.9) | - | <0.001 |
| Total body fat (%) | 38.6 (15.8) | - | 8.0, 54.2 | 44.2 (7.0) | - | 29.4 (9.7) | - | <0.001 | - |
| Total Lean Mass (kg) | - | 61.2 (11.4) | 36.5, 101.4 | - | 54.2 (6.9) | - | 68.0 (10.7) | - | <0.001 |
|
Cardiometabolic characteristics
| |||||||||
| Diabetes (a1c ≥ 6.5% and/or FPG ≥ 126mg/dL) (%) | 24.1% | - | - | 27.3% | - | 20.7% | - | 0.428 | - |
| Mean HbA1c (%) | 5.9 (0.4) | - | 4.9, 13.5 | 6.0 (0.4) | - | 5.9 (0.3) | - | 0.258 | - |
| Mean FBG (mg/dL) | 100.9 (29.0) | - | 69.4, 285.5 | 102.0 (30.1) | - | 99.8 (23.9) | - | 0.475 | - |
| Insulin (mU/L) | 5.9 (5.2) | - | 1.9, 118.1 | 6.6 (5.2) | - | 5.6 (3.1) | - | 0.224 | - |
| HOMA-IR | 1.6 (1.5) | - | 0.4, 28.9 | 2.0 (1.7) | - | 1.4 (1.2) | - | 0.165 | - |
| Total Cholesterol (mg/dL) | - | 201.5 (33.2) | 116.0, 311.0 | - | 201.4 (34.5) | - | 201.6 (32.0) | - | 0.976 |
| Triglycerides (mg/dL) | 111.5 (67.0) | - | 45.0, 489.0 | 107.0 (45.0) | - | 132.0 (84.0) | - | 0.005 | - |
| Hypertension/BP ≥ 140/90 mmHg (yes) | 24.5% | - | - | 31.5% | - | 17.3% | - | 0.090 | - |
| Systolic BP (mmHg) | - | 125.8 (18.6) | 89.0, 175.0 | - | 125.8 (22.8) | - | 125.8 (13.1) | - | 0.734 |
| Diastolic BP (mmHg) | - | 79.7 (13.2) | 45.5, 124.5 | - | 80.7 (14.2) | - | 78.5 (12.1) | - | 0.400 |
|
Behaviors
| |||||||||
| Smoking (%) (yes) | 36.1% | - | - | 21.8% | - | 50.9% | - | 0.002 | - |
| Ever consumed alcohol (%) (yes) | 40.2% | - | - | 12.9% | - | 67.9% | - | <0.001 | - |
| Average MVPA (min)¶ | - | 105.3 (7.2) | 91.0, 119.7 | 86.7 (9.9) | - | 117.6 (10.2) | - | 0.031 | |
CRP: C-Reactive Protein, BMI: Body Mass Index: HbA1c: glycated hemoglobin; FBG: fasting blood glucose; HOMA-IR: homeostatic model assessment of insulin resistance; HDL: high-density lipoprotein cholesterol, LDL: low-density lipoprotein cholesterol, MVPA: moderate to vigorous physical activity
Categorical variables assessed via Chi-squared tests of association
CRP, BMI, lean mass, and systolic BP were natural log transformed to meet normality assumptions; Total Fat Mass, Total Cholesterol, and Diastolic BP had normal distributions; These variables were analyzed with independent sample T tests.
VAT was measured on n=96 participants (n=48 female), Total Fat Mass, % Body Fat, and Total Lean Mass was measured on n=99 participants (n=49 female)
Accelerometer data is from n=89 participants. Estimated marginal means are presented based on general linear models that controlled for accelerometer wear time. Statistical difference analyzed by LSD on estimated marginal means
CRP was weakly to moderately correlated (r and ρ ranged between 0.25–0.50, p<0.001) with measures of adiposity and BMI in both men and women (Figure 1; see supplementary table 1 for correlation statistics for all variables). In women, CRP demonstrated the strongest correlations with percent body fat (ρ =0.50, p<0.001), followed by BMI (r=0.47, <=0.001), total fat mass (r=0.44, p=0.001), and visceral adipose tissue mass (ρ =0.35, p=0.013). In men, CRP was correlated most strongly with total fat mass (r=0.40, p=0.003), BMI (r=0.39, p=0.004), percent body fat (ρ =0.30, p=0.031) and visceral adipose tissue mass (ρ =0.26, p=0.077). Stronger, but moderate, correlations were observed between CRP and HbA1c in women (ρ =0.39, p=0.004) compared to men (ρ =0.17, p=0.234); stronger correlations between fasting insulin values and CRP were observed in men (ρ =0.34, p=0.012) compared to women (ρ =0.25, p=0.071). Other notable differences between the sexes occurred in associations between CRP and HOMA-IR values (in women, ρ =0.34, p=0.012, and in men, ρ =0.21, p=0.131), diastolic BP (in women, r=0.05, p=0.723 and in men, r=0.35, p=0.009), and systolic BP (in women r=0.03, p=0.833 and in men, r=0.31, p=0.025) (Supplementary table 1). CRP was not significantly correlated with total cholesterol or average minutes of MVPA.
Unadjusted analyses
In unadjusted analyses among the total sample, there was a positive association between CRP quartiles and BMI (F(3,98) =9.90, p <0.001), total fat mass (F(3, 98)=10.88, p<0.001), visceral adipose tissue mass (X2(3,96) = 8.55, p =0.035), percent body fat (X2(3,99) = 24.6, p<0.001), HbA1c (X2(3,102)= 8.41, p=0.038), fasting insulin (X2(3,107) = 15.45, p=0.001), and HOMA-IR (X2(3,106) = 10.68, p=0.013) (Table 3). As supported by post-hoc examination and visualized through box plots (Supplementary figure 2a–g and Supplementary table 2a–g), significant variation within measures of adiposity, BMI, and some cardiometabolic risk markers (i.e., fasting insulin and HOMA-IR) were most evident between individuals who fell into the lowest (< median of 1.4 mg/L, Q1-Q2) and the highest (≥ 1.4 mg/L, Q3-Q4) CRP quartiles. In sex-stratified analyses (Table 3), significant differences in BMI, total fat mass, percent body fat, diabetes prevalence, HbA1c, and triglycerides were observed by CRP quartile in women. In men, significant differences in diastolic BP by CRP quartiles were observed. Of note, associations between CRP quartiles and total fat mass, fasting insulin, and minutes of MVPA in men, and associations between smoking and CRP quartiles in women trended towards statistical significance at the p<0.1 level.
Table 3:
Unadjusted associations between CRP quartiles, and measures of body size, adiposity, cardiometabolic risks, and behaviors
| CRP Quartile 1 (n=27) | CRP quartile 2 (n= 27) | CRP quartile 3 (n=27) | CRP quartile 4 (n =27) | Kruskal-Wallis† | 1-way ANOVA‡ | Kruskal-Wallis sex-stratified | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Md (IQR)/Mean (SD) | Md (IQR)/Mean (SD) | Md (IQR)/Mean (SD) | Md (IQR)/Mean (SD) | Total p value | Total p value | Women p value | Men p value | |
| CRP (mg/L) median (IQR) | 0.4 (0.3) | 1.1 (0.4) | 2.1 (1.0) | 6.1 (4.4) | <0.001 | <0.001 | <0.001 | |
| CRP (mg/L) Minimum, maximum | 0.08 – 0.68 | 0.73–1.42 | 1.48–3.97 | 3.98–13.3 | ||||
|
| ||||||||
| Female (%) | 39.3% | 39.3% | 60.7% | 62.9% | 0.057 | - | - | - |
| Age (years) | 47.5 (6.0) | 47.0 (8.5) | 44.6 (5.2) | 46.3 (5.7) | 0.398 | - | 0.514 | 0.348 |
|
Body Size and Adiposity
| ||||||||
| BMI (kg/m2) | 32.9 (5.7) | 32.7 (4.0 | 39.9 (9.8) | 40.7 (6.1) | - | <0.001 | <0.001 | 0.123 |
| Visceral adipose tissue mass (kg)§ | 1.4 (1.0) | 1.3 (0.7) | 1.8 (0.9) | 1.9 (0.9) | 0.035 | - | 0.057 | 0.239 |
| Total fat mass (kg) | 32.8 (13.4) | 29.8 (10.4) | 45.3 (19.6) | 50.3 (14.3) | - | <0.001 | 0.006 | 0.082 |
| Total Body fat (%) | 31.6% (13.8%) | 32.6% (12.1%) | 41.8% (15.4%) | 44.6% (9.4%) | <0.001 | - | 0.005 | 0.130 |
| Total lean mass (kg) | 62.2 (11.1) | 8.4 (43.1) | 63.2 (15.3) | 59.9 (9.7) | - | 0.773 | 0.186 | 0.447 |
|
Cardiometabolic Markers
| ||||||||
| Diabetes (a1c ≥ 6.5% and/or FPG ≥ 126 mg/dL) | 7.1% | 14.8% | 21.4% | 29.6% | 0.118 | - | 0.046 | 0.811 |
| Mean HbA1c (%) | 5.8 (0.2) | 5.9 (0.4) | 6.0 (0.5) | 6.1 (0.7) | 0.038 | - | 0.031 | 0.796 |
| Mean FBG (mg/dL) | 99.0 (30.2) | 100.9 (30.0) | 100.3 (23.7) | 101.8 (36.4) | 0.799 | - | 0.212 | 0.429 |
| Insulin (mU/L) | 5.2 (2.4) | 5.1 (2.5) | 8.7 (7.5) | 7.1 (6.5) | 0.001 | - | 0.109 | 0.077 |
| HOMA-IR | 1.4 (0.8) | 1.3 90.9) | 2.4 (1.8) | 2.4 (2.5) | 0.013 | - | 0.104 | 0.342 |
| Total Cholesterol (mg/dL) | 199.7 (37.2) | 201.7 (26.4) | 197.7 (28.6) | 207.1 (39.8) | - | 0.761 | 0.249 | 0.586 |
| Triglycerides (mg/dL) | 110.0 (62.0) | 111.0 (64.0) | 135.0 (75.0) | 110.0 (61.0) | 0.238 | - | 0.040 | 0.565 |
| Systolic BP (mmHg) | 125.1 (20.3) | 125.5 (21.8) | 127.1 (14.5) | 125.6 (18.7) | - | 0.945 | 0.899 | 0.349 |
| Diastolic BP (mmHg) | 77.9 (11.4) | 76.2 (14.3) | 83.4 (11.6) | 81.2 (14.5) | - | 0.187 | 0.979 | 0.028 |
| Hypertension (yes) | 18.5% | 22.2% | 30.8% | 26.9% | 0.745 | - | 0.627 | 0.189 |
|
Behaviors
| ||||||||
| Smoking (%) (yes) | 40.7% | 33.3% | 33.3% | 37.0% | 0.932 | - | 0.075 | 0.209 |
| Ever consumed alcohol (%) (yes) | 40.7% | 50.0% | 40.7% | 29.6% | 0.512 | - | 0.618 | 0.321 |
| Average MVPA (min)¶ | 97.9 (14.2) | 131.0 (14.2) | 87.1 (14.5) | 105.9 (14.9) | 0.171 | - | 0.202 | 0.083 |
Categorical variables assessed via Chi-squared tests of association
BMI, lean mass, and systolic BP were natural log transformed to meet normality assumptions; Total Fat Mass, Total Cholesterol, and Diastolic BP had normal distributions.
VAT was measured on n=96 participants (n=48 female), Total Fat Mass, % Body Fat, and Total Lean Mass was measured on n=99 participants (n=49 female)
Accelerometer data is from n=89 participants. Estimated marginal means are presented based on general linear models that controlled for accelerometer wear time. Statistical difference analyzed by LSD on estimated marginal means
Sensitivity Analyses
In sensitivity analyses conducted in n=101 individuals who had CRP values < 8.95 mg/L, associations between CRP quartiles and visceral adipose tissue (p=0.151) in the total sample, and diastolic BP and CRP quartiles in men (p=0.159) were no longer statistically significant. Associations between CRP quartiles and HOMA-IR (p=0.084) in the total sample, and triglycerides in women (p=0.095) trended towards statistical significance (see Supplementary table 3).
In analyses removing individuals living with diabetes, CRP quartiles remained significantly associated with insulin (p=0.015) and borderline associated with HOMA-IR (p=0.094) but were no longer associated with HbA1c. When the sample was stratified by presence or absence of diabetes, differences in HbA1c by CRP quartiles were not observed among either group. Unadjusted analyses of CRP quartiles excluding individuals living with hypertension, demonstrated again an absence of significant statistical association with systolic BP; diastolic BP values were only significantly associated with CRP in men (p =0.039). Similarly, when the sample was stratified by presence or absence of hypertension, systolic and diastolic BP values did not vary by CRP quartile among either group (Supplementary Table 4a–d).
Adjusted models
Total fat mass, BMI, percent body fat, HbA1c, fasting insulin, HOMA-IR, MVPA, diastolic BP, and triglycerides were entered in sex-stratified linear regression models (based on their significant associations with CRP quartiles in the unadjusted analysis), along with age, with logarithmically transformed CRP as the outcome variable (Table 4). Following backwards stepwise regression procedures, the multivariable models produced similar results relative to the unadjusted results. In finalized models that control for age, percent body fat, HbA1c, and HOMA-IR were retained in the women’s model, with percent body fat and HbA1c found to be significant correlates of CRP; in men, only percent body fat was retained and found to be a significant correlate of CRP. In women, holding all other variables constant, a one unit (%) increase in percent body fat among women was associated with 11.6% higher CRP values, and a one unit (%) increase in HbA1c among women was associated with 52.2% higher CRP values. The standardized regression coefficients demonstrated that in women, a change of one standard deviation in percent body fat was associated with a change of 0.51 standard deviations of CRP, holding other variables constant, whereas a change of one standard deviation in HbA1c was associated with a change of 0.29 standard deviations of CRP. Percent body fat demonstrated a relatively stronger magnitude of effect on CRP levels compared to the other predictors in the model. In men, a one unit (%) increase in percent body fat was associated with 5.1% higher CRP values, when controlling for age. By standardizing the variables, we observed that a change of one standard deviation in percent body fat was associated with a change of 0.43 standard deviations of CRP, holding age constant. In sensitivity analyses of individuals with CRP < 8.95 (see supplementary table 5), percent body fat, but not HbA1c, remained a statistically significant predictor of CRP in the women’s model. In the men’s sensitivity analyses, percent body fat again was retained and remained significantly associated with CRP in the final model.
Table 4:
Sex-stratified adjusted analyses using linear regression to identify associations between logarithmically transformed CRP and measures of adiposity and cardiometabolic markers
| a. Women: n = 45†, Adjusted R2 = 0.35 | b. Men: n= 50, Adjusted R2 = 0.15 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Parameter | Unstandardized Coefficients | Std. Error | Standardized coefficients | t | p value | 95% Confidence intervals | Parameter | Unstandardized Coefficients | Std. Error | Standardized coefficients | t | p value | 95% Confidence intervals |
| HbA1c | 0.42 | 0.18 | 0.29 | 2.30 | 0.027 | 0.05–0.79 | % Body Fat | 0.05 | 0.01 | 0.43 | 3.27 | 0.002 | 0.02–0.08 |
| HOMA-IR | 0.04 | 0.03 | 0.16 | 1.32 | 0.196 | −0.02–0.11 | Age | 0.01 | 0.02 | 0.02 | 0.20 | 0.845 | −0.05–0.06 |
| % Body Fat | 0.11 | 0.02 | 0.51 | 4.11 | <0.001 | 0.05–0.16 | |||||||
| Age | −0.03 | 0.03 | −0.12 | −0.98 | 0.332 | −0.09–0.03 | |||||||
Sample size decreased due to missing data in variables included in model entry, and the reduced number of participants with DXA scan measurements.
Discussion
Overall, we found average CRP levels in this sample (median 1.4 mg/L ± 3.3 and mean 2.7 mg/L ± 2.9) to be comparable to those recorded in large cohort studies in the US and Europe. For example, in n=2514 US adults ≥ 20 years old (mean age of 44.7 years) and living without diabetes measured as part of the National Health and Nutrition Examination Survey (NHANES) 1999–2000, mean CRP was 1.61 mg/L± 0.05 (Meng et al., 2007). In n=390 post-menopausal Italian women (mean age of 63.1 years), mean CRP was 2.6 mg/L ± 3.8 (Gentile et al., 2010)], and in the Framingham Heart Study, mean CRP ranged from 3.6–7.3 mg/L in n=3173 adults (mean age of 57.1 years) with < 25% and ≥ 25% stenosis (Wang Thomas et al., 2002). Notably, average CRP levels in this sample were low compared to other studied individuals with high/higher levels of BMI. CRP levels in adult Pima Indians (mean age of 32 years) with mean BMI of 36.3 kg/m2 were 4.5 mg/L and 4.9 mg/L among individuals that did and did not develop diabetes respectively (Krakoff et al., 2003). In a study of adult Saudi Arabian men and women with Class II (BMI: ≥ 35 kg/m2 and < 40 kg/m2) or Class III (BMI ≥ 40 kg/m2) (mean BMI 50.9 kg/m2 ± 13.2) obesity pre-and post -laparoscopic gastrectomy surgery, mean CRP levels were 12.4 mg/L (Hakeam, O’Regan, Salem, Bamehriz, & Jomaa, 2009), and 6 months post-operation, (mean BMI 35.1 kg/m2 ± 6.85) mean CRP was 5.6 mg/L ±4.2. Additionally, in individuals with BMI levels between 35 kg/m2 and < 70 kg/m2 undergoing bariatric surgery in France, mean CRP levels were between 7.8 mg/L and 12.7 mg/L (Paepegaey et al., 2015). This comparative approach is limited, especially with the last two examples, due to potential unknown additional comorbidities, environmental exposures, or cardiometabolic conditions these groups may have had influencing their CRP levels. However, it suggests that other unconsidered factors, beyond BMI or adiposity, may be influencing or mitigating CRP production in these participants.
CRP levels in this Samoan sample were positively but only weakly-to-moderately associated with adiposity (i.e., total fat mass, percent body fat, visceral adipose tissue mass) and BMI, mirroring the results found in other studies [(Hak et al., 1999; Krakoff et al., 2003; Lemieux et al., 2001; Park et al., 2005; Weyer et al., 2002; Yudkin John, Stehouwer, Emeis, & Coppack, 1999), see introduction] despite the higher adiposity and BMI levels observed among Samoans compared to many other studied populations. In contrast, in n=32 Pima Native Americans without diabetes and similar levels of adiposity (mean percent body fat and CRP in women was 39.0% ± 1.0 and 7.9 mg/L ± 1.6, and in men 28.0% ± 2.0 and 5.5 mg/L ±1.9), CRP demonstrated stronger associations with percent body fat (r =0.71, p<0.001) and BMI (r=0.75, p<0.001) than we observed here (Weyer et al., 2002). However, in a larger study of Pima participants (n=126), the association between CRP and BMI (r=0.53, p=0.001) (Krakoff et al., 2003) was similar to our findings. This suggests that higher levels of CRP may generally be associated with higher adiposity or BMI, but that other factors may constrain the strength of correlations in each population or context.
The greatest differences among adiposity and BMI levels were observed between participants with CRP levels below and participants with CRP levels above the median value (1.4 mg/L) (see supplementary figure 2a–d). For example, we observed mean BMI values of 32.9kg/m2 and 32.7 kg/m2 in participants with CRP levels within quartile 1 and 2 respectively, but BMI was significantly greater (39.9 kg/m2 and 40.7 kg/m2 respectively) in participants with CRP levels falling within quartiles 3 and 4. In the adiposity measures, we see a similar trend of significant upward shifts in mass and percent body fat in participants with CRP values within the two highest CRP quartiles in contrast to participants with CRP levels falling below the median. Notably, the results demonstrate that the variation in CRP is occurring between individuals who, based on their mean BMI values, can all be classified as obese [(using Polynesian cutoffs: ‘normal’ < 26 kg/m2, ‘overweight’ ≥ 26 to ≤ 32 kg/m2, and > 32 kg/m2; (Swinburn, Ley, Carmichael, & Plank, 1999)], and lends support to regarding obesity physiology as variable and heterogenous rather than a homogenous state.
The results also replicated sex differences in CRP, with higher levels observed in women compared to men, as recorded in other studies (Blaha et al., 2011; Visser et al., 1999); more measures of adiposity were also significantly associated with CRP quartiles in the unadjusted analyses in women compared to men. In both sexes, the adjusted models showed that percent body fat had a greater effect on CRP levels compared to other retained variables and percent body fat was a significant predictor of CRP. This indicates that even when adjusting for other variables, associations are maintained between the two measures (CRP and percent body fat) for both sexes; this suggests that percent body fat may be a useful predictor of inflammatory levels for both men and women in this sample.
Our findings demonstrated that CRP was associated with several markers of diabetes risk including: HbA1c, fasting insulin, and HOMA-IR values in unadjusted analyses of the total sample, HbA1c in women in the adjusted model, and fasting insulin in individuals without diabetes. Other studies demonstrate correlations between fasting insulin, insulin resistance and CRP. In the Women’s Health Study, increased CRP was associated with increased likelihood of elevated fasting insulin levels in adult women without diabetes (Pradhan, Manson, Rifai, Buring, & Ridker, 2001). Additionally, associations between CRP, fasting insulin, and insulin resistance have been demonstrated in Peruvian adults (Gelaye et al., 2010) and between CRP and insulin resistance across racial and ethnic groups in US adults measured in NHANES (1999–2002) (Meng et al., 2007). CRP has also been found to be associated with insulin sensitivity in adults without diabetes (Festa et al., 2000), and it has been suggested that decreased insulin sensitivity may enhance CRP expression (Festa et al., 2000) (Haffner, 2003).
However, the mechanisms directing many of these associations remain unclear (Chen et al., 2015; Festa et al., 2000), and the confounding effects of body fat on CRP levels, and other cardiometabolic risk factors in individuals with hyperinsulinemia and/or insulin resistance may be contributing to observed associations between CRP and insulin/insulin resistance (Chen et al., 2015; Festa et al., 2000). Other studies also demonstrate that associations between CRP and diabetes risk may be inconsistent (Freeman et al., 2002; Krakoff et al., 2003; Nakanishi, Yamane, Kamei, Okubo, & Kohno, 2003; Pradhan et al., 2001). Thereby, we advocate for further clarification of the relationship between CRP, diabetes risk markers, and BMI and adiposity, before determining the public health utility of CRP measures in Samoans, or other populations with high adiposity and BMI, as a predictor of diabetes risk.
We did not find a statistically significant association between CRP and many other known correlates including total cholesterol, hypertension and systolic BP, smoking, alcohol consumption, and physical activity in this sample; however, triglyceride levels in women, and diastolic BP values in men did demonstrate significant associations with CRP. The lack of associations observed between CRP and elevated lipid and blood pressure levels necessitate further study. There are several reasons potentially explaining the absence of these associations in this sample. For example, Shafi Dar et al. (2010) showed that CRP was associated with hypertension in Indian adults, but that CRP also varied by hypertension stage and duration of disease (Shafi Dar et al., 2010). These factors were not captured in this analysis. Additionally, as hypertension is a disease most associated with older age, the sampling strategy for these analyses (excluding individuals > 55 years old) may have created some selection bias that inadvertently cut out individuals with advanced hypertensive disease duration or severity and reduced our abilities to observe associations between CRP and hypertension/BP values. Additionally, absent associations between smoking, alcohol consumption, and CRP warrant further investigation. A future study would benefit from: 1) matching those who smoke and/or consume alcohol by BMI and adiposity levels to better determine associations between these behaviors and inflammation, and 2) assessing the magnitude and frequency of consumption in association with inflammation.
Strengths and Limitations
Due to the paucity of existing data regarding CRP levels in Pacific Islanders, it is difficult to assess whether the observed CRP levels here fall within expected ranges for the population/region or are aberrant. Additionally, there are likely contributors to CRP that we were unable to include or consider in these analyses such as several genetic polymorphisms known to be associated with CRP levels (Miller et al., 2005; Vickers et al., 2002). Notably, in the multi-ethnic Women’s Health Initiative Observational cohort study, several single nucleotide polymorphisms (SNPs) associated with variable CRP levels were identified to be occurring more frequently in Asian/Pacific Islander participants (Lee et al., 2009). Early-life exposures may also influence CRP levels. For example, low birthweight and pathogen exposures during infancy were found to be associated with CRP levels in young adults participating in the Cebu Longitudinal Health and Nutrition Survey (McDade, Rutherford, Adair, & Kuzawa, 2010), and early-life social adversity was associated with CRP levels in Canadian adults (A. Li et al., 2015). The cross-sectional nature of this study precludes the examination of similar factors here. Participants were not asked whether they were taking non-steroidal anti-inflammatories (NSAIDs), some of which are known to influence CRP levels (Tarp et al., 2012); additionally, the prevalence of α-thalassemia [known to impact HbA1c measurement (Xu, Ji, Chen, Xia, & Zhou, 2016)] in this sample is unknown. However, a previous study conducted in Samoan adults demonstrate that the presence of α-thalassemia in the population is estimated to be low to absent (Lie-Injo, Pawson, & Solai, 1985). We cannot rule out that CRP levels in these participants may be explained by additional factors such as by pathogens (McDade, Rutherford, Adair, & Kuzawa, 2009) including lymphatic filariasis (Lau et al., 2020), particulate air pollution (Liu et al., 2019), menstruation (Chaireti, Lindahl, Byström, Bremme, & Larsson, 2016; Wander, Brindle, & O’Connor, 2008) and/or tissue injury (Kushner et al., 2006). We advocate for future studies to build upon our cross-sectional analyses of CRP correlates by using larger analytical sample sizes and longitudinal assessments. This study is strengthened by the assessment of multiple measures of adiposity, the use of multiple cardiometabolic risk correlates, and the integration of behavioral, biochemical, and demographic variables into the analyses. Additionally, it is among the first to assess CRP correlates in adults in Samoa.
Conclusion
Here we provide the first examination of CRP, an often-used marker of systemic inflammation, in an adult Samoan cohort, and suggest that levels of CRP in the sample are moderately correlated with measures of adiposity, BMI, and select cardiometabolic risk factors (i.e., Hba1c, fasting insulin, insulin resistance). These results help inform understandings of populational variation in CRP and provide insight into the large extent of CRP variation occurring within degrees of increased adiposity and BMI. We advocate for further investigation of pro-inflammatory biomarkers in the Pacific to inform the establishment of population-specific reference ranges and aid in the screening and diagnosis of cardiometabolic diseases and risk factors.
Supplementary Material
Acknowledgements
We wish to extend our gratitude to the Samoan Ministry of Health; the team of research assistants; and the participants who made this work possible. Additionally, we appreciate the support of the Global Health Equity Scholars program and National Institutes of Health: Fogarty International Center and National Institute of Diabetes and Digestive and Kidney Disease (D43TW010540), and the National Heart, Lung, and Blood Institute (R01HL093093; R01HL140570) which funded this work.
Sources of Support:
US National Institutes of Health and Fogarty International Center Global Health Equity Scholars Program and the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) D43TW010540; US National Institutes of Health grant R01HL093093 (PI: McGarvey), R01HL140570 (PI: McGarvey)
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Data Availability Statement
The data that support the findings of this study can be made available from the corresponding author and the PIs of the studies which funded this work, upon reasonable request.
References
- Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, … Levy Y (2004). Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord, 28(5), 674–679. doi: 10.1038/sj.ijo.0802609 [DOI] [PubMed] [Google Scholar]
- Ayas NT, Hirsch Allen AJ, Fox N, Peres B, Mehrtash M, Humphries KH, … van Eeden SF (2019). C-Reactive Protein Levels and the Risk of Incident Cardiovascular and Cerebrovascular Events in Patients with Obstructive Sleep Apnea. Lung, 197(4), 459–464. doi: 10.1007/s00408-019-00237-0 [DOI] [PubMed] [Google Scholar]
- B. Al-Youzbaki W (2011). C-reactive protein and lipid profile among depot- medroxyprogesterone acetate injections users. Annals of the College of Medicine, Mosul, 37(1), 48–56. doi: 10.33899/mmed.2011.34639 [DOI] [Google Scholar]
- Blaak E (2001). Gender differences in fat metabolism. Current Opinion in Clinical Nutrition & Metabolic Care, 4(6). Retrieved from https://journals.lww.com/co-clinicalnutrition/Fulltext/2001/11000/Gender_differences_in_fat_metabolism.6.aspx [DOI] [PubMed] [Google Scholar]
- Blaha MJ, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, O’Leary DH, … Nasir K (2011). Association between obesity, high-sensitivity C-reactive protein ≥2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology, 31(6), 1430–1438. doi: 10.1161/ATVBAHA.111.223768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Mautz WJ, Warrington M, Allen L, Tefft HA, Gotshalk L, & Katzmarzyk PT (2010). Relation between C-reactive protein levels and body composition in a multiethnic sample of school children in Hawaii. American journal of human biology : the official journal of the Human Biology Council, 22(5), 675–679. doi: 10.1002/ajhb.21064 [DOI] [PubMed] [Google Scholar]
- Buchbinder S, Kratzsch J, Fiedler GM, Yar V, Brügel M, Leichtle A, … Thiery J (2008). Body weight and oral contraceptives are the most important modulators of serum CRP levels. Scandinavian Journal of Clinical and Laboratory Investigation, 68(2), 140–144. doi: 10.1080/00365510701487727 [DOI] [PubMed] [Google Scholar]
- Carbone F, Elia E, Casula M, Bonaventura A, Liberale L, Bertolotto M, … Montecucco F (2019). Baseline hs-CRP predicts hypertension remission in metabolic syndrome. European Journal of Clinical Investigation, 49(8), e13128. doi: 10.1111/eci.13128 [DOI] [PubMed] [Google Scholar]
- Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, & Després J-P (2009). Sex differences in inflammatory markers: what is the contribution of visceral adiposity? The American Journal of Clinical Nutrition, 89(5), 1307–1314. doi: 10.3945/ajcn.2008.27030 [DOI] [PubMed] [Google Scholar]
- Chaireti R, Lindahl TL, Byström B, Bremme K, & Larsson A (2016). Inflammatory and endothelial markers during the menstrual cycle. Scand J Clin Lab Invest, 76(3), 190–194. doi: 10.3109/00365513.2015.1129670 [DOI] [PubMed] [Google Scholar]
- Chait A, & den Hartigh LJ (2020). Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Frontiers in cardiovascular medicine, 7, 22–22. doi: 10.3389/fcvm.2020.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen R, Wang H, & Liang F (2015). Mechanisms Linking Inflammation to Insulin Resistance. International Journal of Endocrinology, 2015, 508409. doi: 10.1155/2015/508409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi L, Liu Z, Matthews CE, & Buchowski MS (2011). Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc, 43(2), 357–364. doi: 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros SF, Medeiros M. A. S. d., Santos N. d. S., Barbosa BB, & Yamamoto MMW (2018). Combined Oral Contraceptive Effects on Low-Grade Chronic Inflammatory Mediators in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. International Journal of Inflammation, 2018, 9591509. doi: 10.1155/2018/9591509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divani AA, Luo X, Datta YH, Flaherty JD, & Panoskaltsis-Mortari A (2015). Effect of Oral and Vaginal Hormonal Contraceptives on Inflammatory Blood Biomarkers. Mediators of Inflammation, 2015, 379501. doi: 10.1155/2015/379501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, Mirhafez SR, Moohebati M, Esmaily H, … Ghayour-Mobarhan M (2016). Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. Journal of Clinical Laboratory Analysis, 30(5), 672–676. doi: 10.1002/jcla.21920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajumi JO (1984). The effects of Depo-Provera on serum protein levels in Nigerian women. J Steroid Biochem, 20(2), 581–583. doi: 10.1016/0022-4731(84)90127-4 [DOI] [PubMed] [Google Scholar]
- Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy Russell P, & Haffner Steven M (2000). Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome. Circulation, 102(1), 42–47. doi: 10.1161/01.CIR.102.1.42 [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GDO, … Sattar N (2002). C-Reactive Protein Is an Independent Predictor of Risk for the Development of Diabetes in the West of Scotland Coronary Prevention Study. Diabetes, 51(5), 1596. doi: 10.2337/diabetes.51.5.1596 [DOI] [PubMed] [Google Scholar]
- Gelaye B, Revilla L, Lopez T, Suarez L, Sanchez SE, Hevner K, … Williams MA (2010). Association between insulin resistance and c-reactive protein among Peruvian adults. Diabetology & Metabolic Syndrome, 2(1), 30–30. doi: 10.1186/1758-5996-2-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile M, Panico S, Rubba F, Mattiello A, Chiodini P, Jossa F, … Rubba P (2010). Obesity, overweight, and weight gain over adult life are main determinants of elevated hs-CRP in a cohort of Mediterranean women. European Journal of Clinical Nutrition, 64(8), 873–878. doi: 10.1038/ejcn.2010.69 [DOI] [PubMed] [Google Scholar]
- Goff David C, Lloyd-Jones Donald M, Bennett G, Coady S, D’Agostino Ralph B, Gibbons R, … Wilson Peter WF (2014). 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Journal of the American College of Cardiology, 63(25_Part_B), 2935–2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM (2003). Insulin resistance, inflammation, and the prediabetic state. The American Journal of Cardiology, 92(4, Supplement 1), 18–26. doi: 10.1016/S0002-9149(03)00612-X [DOI] [PubMed] [Google Scholar]
- Hak AE, Stehouwer Coen DA, Bots Michiel L, Polderman Kees H, Schalkwijk Casper G, Westendorp Iris CD, … Witteman Jacqueline CM (1999). Associations of C-Reactive Protein With Measures of Obesity, Insulin Resistance, and Subclinical Atherosclerosis in Healthy, Middle-Aged Women. Arteriosclerosis, thrombosis, and vascular biology, 19(8), 1986–1991. doi: 10.1161/01.ATV.19.8.1986 [DOI] [PubMed] [Google Scholar]
- Hakeam HA, O’Regan PJ, Salem AM, Bamehriz FY, & Jomaa LF (2009). Inhibition of C-Reactive Protein in Morbidly Obese Patients After Laparoscopic Sleeve Gastrectomy. Obesity Surgery, 19(4), 456–460. doi: 10.1007/s11695-008-9729-y [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, … Duda SN (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley NL, & McGarvey ST (2015). Obesity and Diabetes in Pacific Islanders: the Current Burden and the Need for Urgent Action. Current diabetes reports, 15(5), 29. doi: 10.1007/s11892-015-0594-5 [DOI] [PubMed] [Google Scholar]
- Hawley NL, Pomer A, Rivara AC, Rosenthal SL, Duckham RL, Carlson JC, … McGarvey ST (2020). Exploring the Paradoxical Relationship of a Creb 3 Regulatory Factor Missense Variant With Body Mass Index and Diabetes Among Samoans: Protocol for the Soifua Manuia (Good Health) Observational Cohort Study. JMIR Res Protoc, 9(7), e17329. doi: 10.2196/17329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashino Y, Mashitani T, Tsujii S, & Ishii H (2014). Serum High-Sensitivity C-Reactive Protein Levels Are Associated With High Risk of Development, Not Progression, of Diabetic Nephropathy Among Japanese Type 2 Diabetic Patients: A Prospective Cohort Study (Diabetes Distress and Care Registry at Tenri [DDCRT7]). Diabetes Care, 37(11), 2947. doi: 10.2337/dc14-1357 [DOI] [PubMed] [Google Scholar]
- Hibbing P, & van Hees V (2018). TwoRegression: Process Data from Wearable Research Devices Using Two-Regression Algorithms. Retrieved from https://cran.r-project.org/web/packages/TwoRegression/index.html
- Hibbing PR, Lamunion SR, Kaplan AS, & Crouter SE (2018). Estimating Energy Expenditure with ActiGraph GT9X Inertial Measurement Unit. Med Sci Sports Exerc, 50(5), 1093–1102. doi: 10.1249/mss.0000000000001532 [DOI] [PubMed] [Google Scholar]
- Kawamoto R, Kusunoki T, Abe M, Kohara K, & Miki T (2013). An association between body mass index and high-sensitivity C-reactive protein concentrations is influenced by age in community-dwelling persons. Ann Clin Biochem, 50(Pt 5), 457–464. doi: 10.1177/0004563212473445 [DOI] [PubMed] [Google Scholar]
- King DE, Mainous AG, Buchanan TA, & Pearson WS (2003). C-Reactive Protein and Glycemic Control in Adults With Diabetes. Diabetes Care, 26(5), 1535. doi: 10.2337/diacare.26.5.1535 [DOI] [PubMed] [Google Scholar]
- Krakoff J, Funahashi T, Stehouwer CDA, Schalkwijk CG, Tanaka S, Matsuzawa Y, … Lindsay RS (2003). Inflammatory Markers, Adiponectin, and Risk of Type 2 Diabetes in the Pima Indian. Diabetes Care, 26(6), 1745. doi: 10.2337/diacare.26.6.1745 [DOI] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D, & Samols D (2006). What Does Minor Elevation of C-Reactive Protein Signify? The American Journal of Medicine, 119(2), 166.e117–166.e128. doi: 10.1016/j.amjmed.2005.06.057 [DOI] [PubMed] [Google Scholar]
- Lau CL, Meder K, Mayfield HJ, Kearns T, McPherson B, Naseri T, … Graves PM (2020). Lymphatic filariasis epidemiology in Samoa in 2018: Geographic clustering and higher antigen prevalence in older age groups. PLOS Neglected Tropical Diseases, 14(12), e0008927. doi: 10.1371/journal.pntd.0008927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, You NC, Song Y, Hsu YH, Manson J, Nathan L, … Liu S (2009). Relation of genetic variation in the gene coding for C-reactive protein with its plasma protein concentrations: findings from the Women’s Health Initiative Observational Cohort. Clin Chem, 55(2), 351–360. doi: 10.1373/clinchem.2008.117176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux I, Pascot A, Prud’homme D, Alméras N, Bogaty P, Nadeau A, … Després J-P (2001). Elevated C-Reactive Protein. Arteriosclerosis, thrombosis, and vascular biology, 21(6), 961–967. doi: 10.1161/01.ATV.21.6.961 [DOI] [PubMed] [Google Scholar]
- Li A, Tu MT, Sousa AC, Alvarado B, Kone GK, Guralnik J, & Zunzunegui MV (2015). Early life adversity and C-reactive protein in diverse populations of older adults: a cross-sectional analysis from the International Mobility in Aging Study (IMIAS). BMC geriatrics, 15(1), 102. doi: 10.1186/s12877-015-0104-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SN, Wang X, Zeng QT, Feng YB, Cheng X, Mao XB, … Deng, H. P. (2009). Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels, 24(6), 446–453. doi: 10.1007/s00380-008-1137-7 [DOI] [PubMed] [Google Scholar]
- Lie-Injo LE, Pawson IG, & Solai A (1985). High frequency of triplicated alpha-globin loci and absence or low frequency of alpha thalassemia in Polynesian Samoans. Hum Genet, 70(2), 116–118. doi: 10.1007/bf00273068 [DOI] [PubMed] [Google Scholar]
- Lin S, Naseri T, Linhart C, Morrell S, Taylor R, McGarvey ST, … Zimmet P (2017). Trends in diabetes and obesity in Samoa over 35 years, 1978–2013. Diabetic medicine : a journal of the British Diabetic Association, 34(5), 654–661. doi: 10.1111/dme.13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Gu X, Deng F, Mu L, Baccarelli AA, Guo X, & Wu S (2019). Ambient particulate air pollution and circulating C-reactive protein level: A systematic review and meta-analysis. Int J Hyg Environ Health, 222(5), 756–764. doi: 10.1016/j.ijheh.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Markanday A (2015). Acute Phase Reactants in Infections: Evidence-Based Review and a Guide for Clinicians. Open forum infectious diseases, 2(3), ofv098–ofv098. doi: 10.1093/ofid/ofv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW (2001). Lifestyle incongruity, social integration, and immune function in Samoan adolescents. Soc Sci Med, 53(10), 1351–1362. doi: 10.1016/s0277-9536(00)00414-7 [DOI] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, Adair L, & Kuzawa CW (2010). Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proceedings of the Royal Society B: Biological Sciences, 277(1684), 1129–1137. doi: 10.1098/rspb.2009.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Rutherford JN, Adair L, & Kuzawa C (2009). Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr, 89(4), 1237–1245. doi: 10.3945/ajcn.2008.27080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan AK, & Ulijaszek SJ (2015). Obesity emergence in the Pacific islands: why understanding colonial history and social change is important. Public Health Nutrition, 18(8), 1499–1505. doi: 10.1017/S136898001400175X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y-X, Ford ES, Li C, Quarshie A, Al-Mahmoud AM, Giles W, … Strayhorn G (2007). Association of C-Reactive Protein with Surrogate Measures of Insulin Resistance among Nondiabetic US Adults: Findings from National Health and Nutrition Examination Survey 1999–2002. Clinical Chemistry, 53(12), 2152–2159. doi: 10.1373/clinchem.2007.088930 [DOI] [PubMed] [Google Scholar]
- Miller DT, Zee RYL, Suk Danik J, Kozlowski P, Chasman DI, Lazarus R, … Kwiatkowski DJ (2005). Association of Common CRP Gene Variants with CRP Levels and Cardiovascular Events. Annals of Human Genetics, 69(6), 623–638. doi: 10.1111/j.1529-8817.2005.00210.x [DOI] [PubMed] [Google Scholar]
- Minster RL, Hawley NL, Su C-T, Sun G, Kershaw EE, Cheng H, … McGarvey ST (2016). A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nature genetics, 48(9), 1049–1054. doi: 10.1038/ng.3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, … Lim SS (2020). Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396(10258), 1223–1249. doi: 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Yamane K, Kamei N, Okubo M, & Kohno N (2003). Elevated C-Reactive Protein Is a Risk Factor for the Development of Type 2 Diabetes in Japanese Americans. Diabetes Care, 26(10), 2754. doi: 10.2337/diacare.26.10.2754 [DOI] [PubMed] [Google Scholar]
- Paepegaey A-C, Genser L, Bouillot J-L, Oppert J-M, Clément K, & Poitou C (2015). High levels of CRP in morbid obesity: the central role of adipose tissue and lessons for clinical practice before and after bariatric surgery. Surgery for Obesity and Related Diseases, 11(1), 148–154. doi: 10.1016/j.soard.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Park HS, Park JY, & Yu R (2005). Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Research and Clinical Practice, 69(1), 29–35. doi: 10.1016/j.diabres.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, & Ridker PM (2001). C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA, 286(3), 327–334. doi: 10.1001/jama.286.3.327 [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Pradhan A, Manson JE, Buring JE, & Ridker PM (2003). Relationship of total and abdominal adiposity with CRP and IL-6 in women. Annals of Epidemiology, 13(10), 674–682. doi: 10.1016/S1047-2797(03)00053-X [DOI] [PubMed] [Google Scholar]
- Ridker Paul M (2003). C-Reactive Protein. Circulation, 108(12), e81–e85. doi: 10.1161/01.CIR.0000093381.57779.67 [DOI] [PubMed] [Google Scholar]
- Rivas-Crespo. (2015). Comment on Boyko and Jensen. Do We Know What Homeostatis Model Assessment Measures? If Not, Does It Matter? Diabetes Care 2007;30:2725–2728. Diabetes Care, 38(12), e213. doi: 10.2337/dc15-1172 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Kiyota N, Kawasaki Y, Miyazaki Y, Kashimura J, Fukuda M, & Kishi R (2004). Relationship between C-reactive protein and visceral adipose tissue in healthy Japanese subjects. Diabetes, Obesity and Metabolism, 6(4), 249–258. doi: 10.1111/j.1462-8902.2003.0342.x [DOI] [PubMed] [Google Scholar]
- Shafi Dar M, Pandith AA, Sameer AS, Sultan M, Yousuf A, & Mudassar S (2010). hs-CRP: A potential marker for hypertension in Kashmiri population. Indian J Clin Biochem, 25(2), 208–212. doi: 10.1007/s12291-010-0037-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukala WR, Page R, Rowlands DS, Krebs J, Lys I, Leikis M, … Cheema BS (2012). South Pacific Islanders resist type 2 diabetes: comparison of aerobic and resistance training. European Journal of Applied Physiology, 112(1), 317–325. doi: 10.1007/s00421-011-1978-0 [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Ley SJ, Carmichael HE, & Plank LD (1999). Body size and composition in Polynesians. International Journal of Obesity, 23(11), 1178–1183. doi: 10.1038/sj.ijo.0801053 [DOI] [PubMed] [Google Scholar]
- Tarp S, Bartels EM, Bliddal H, Furst DE, Boers M, Danneskiold-Samsøe B, … Christensen R (2012). Effect of nonsteroidal antiinflammatory drugs on the C-reactive protein level in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum, 64(11), 3511–3521. doi: 10.1002/art.34644 [DOI] [PubMed] [Google Scholar]
- Timpson NJ, Nordestgaard BG, Harbord RM, Zacho J, Frayling TM, Tybjærg-Hansen A, & Smith GD (2011). C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. International journal of obesity (2005), 35(2), 300–308. doi: 10.1038/ijo.2010.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonstad S, & Cowan JL (2009). C-reactive protein as a predictor of disease in smokers and former smokers: a review. International journal of clinical practice, 63(11), 1634–1641. doi: 10.1111/j.1742-1241.2009.02179.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuriya D, Morita H, Morioka T, Takahashi N, Ito T, Oki Y, & Nakamura H (2011). Significant Correlation Between Visceral Adiposity and High-sensitivity C-reactive Protein (hs-CRP) in Japanese Subjects. Internal Medicine, 50(22), 2767–2773. doi: 10.2169/internalmedicine.50.5908 [DOI] [PubMed] [Google Scholar]
- Vickers MA, Green FR, Terry C, Mayosi BM, Julier C, Lathrop M, … Keavney B (2002). Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma C-reactive protein. Cardiovasc Res, 53(4), 1029–1034. doi: 10.1016/s0008-6363(01)00534-x [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, & Harris TB (1999). Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA, 282(22), 2131–2135. doi: 10.1001/jama.282.22.2131 [DOI] [PubMed] [Google Scholar]
- Wander K, Brindle E, & O’Connor KA (2008). C-reactive protein across the menstrual cycle. American journal of physical anthropology, 136(2), 138–146. doi: 10.1002/ajpa.20785 [DOI] [PubMed] [Google Scholar]
- Wang Thomas J, Nam B-H, Wilson Peter WF, Wolf Philip A, Levy D, Polak Joseph F, … O’Donnell Christopher J (2002). Association of C-Reactive Protein With Carotid Atherosclerosis in Men and Women: The Framingham Heart Study. Arteriosclerosis, thrombosis, and vascular biology, 22(10), 1662–1667. doi: 10.1161/01.ATV.0000034543.78801.69 [DOI] [PubMed] [Google Scholar]
- Weyer C, Yudkin JS, Stehouwer CDA, Schalkwijk CG, Pratley RE, & Tataranni PA (2002). Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima Indians. Atherosclerosis, 161(1), 233–242. doi: 10.1016/S0021-9150(01)00626-8 [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, … Wright JT Jr. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 71(19), e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, & Paczek L (2016). Inflammatory Markers Change with Age, but do not Fall Beyond Reported Normal Ranges. Archivum immunologiae et therapiae experimentalis, 64(3), 249–254. doi: 10.1007/s00005-015-0357-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Ji L, Chen W, Xia Y, & Zhou Y (2016). Effects of α-Thalassemia on HbA1c Measurement. Journal of Clinical Laboratory Analysis, 30(6), 1078–1080. doi: 10.1002/jcla.21983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Yu Z, Li H, Franco Oscar H, Liu Y, & Lin X (2007). Distributions of C-Reactive Protein and its Association With Metabolic Syndrome in Middle-Aged and Older Chinese People. Journal of the American College of Cardiology, 49(17), 1798–1805. doi: 10.1016/j.jacc.2007.01.065 [DOI] [PubMed] [Google Scholar]
- Yudkin John S, Stehouwer CDA, Emeis JJ, & Coppack SW (1999). C-Reactive Protein in Healthy Subjects: Associations With Obesity, Insulin Resistance, and Endothelial Dysfunction. Arteriosclerosis, thrombosis, and vascular biology, 19(4), 972–978. doi: 10.1161/01.ATV.19.4.972 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study can be made available from the corresponding author and the PIs of the studies which funded this work, upon reasonable request.


