Abstract
Sea cucumbers are marine organisms with uses in food, cosmetics, and medicine. This study aimed to identify Indonesian sea cucumbers with high antioxidant and antibacterial activities. Twenty-one sea cucumber species were used for this study. Antioxidant capacity was evaluated using the 2,2-diphenyl-β-picrylhydrazine assay. Antibacterial activity was assessed using the disk diffusion assay, whereas the resazurin-based assay was employed to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). Volatile compounds possibly related to the biological activity of sea cucumbers were analyzed via gas chromatography–mass spectrometry (GC–MS). Holothuria atra had the strongest antioxidant capacity (IC50 = 14.22 ± 0.87 µg µL−1). Stichopus vastus displayed the best antibacterial activity against Staphylococcus aureus, whereas Stichopus ocellatus extract was most potent against Vibrio cholerae. Holothuria albiventer, which controlled Bacillus subtilis most effectively while also being active against S. aureus and V. cholerae, was the optimal antimicrobial species. H. albiventer and Actinopyga echinites inhibited B. subtilis growth at 12.5 µg µL−1. The MBC tests indicated that the antibacterial activities of sea cucumbers at the MIC were bacteriostatic, rather than bactericidal, in nature. GC–MS analysis uncovered long-chain fatty acids that might be associated with the antibacterial activities of sea cucumbers.
Keywords: Antioxidant capacity, Disk diffusion, DPPH, Volatile compounds, Pathogenic bacteria, Long-chain fatty acids
Introduction
Indonesia, with its extensive coastlines based on its multiple islands, features a high diversity of marine species (Kasanah et al. 2015). Among the marine invertebrate species, sea cucumbers represent a potential source of new biologically active materials with uses in pharmaceutical applications, food supplements, and industrial applications. Sea cucumbers in Asian–Pacific waters display large biodiversity, with 1716 species identified in Indonesia alone. The region is host to as many as 350 species, including at least 26 species of known economic value (Pangestuti and Arifin 2017). The sea cucumber is important economically in various Asian countries such as China, in which it is used in both gourmet and traditional medical preparations with purported therapeutic benefits against disorders and diseases such as hypertension, eczema, arthritis, and tissue injury (Esmat et al. 2013; Dhinakaran and Lipton 2014). In particular, Holothuroidea species that sometimes reside in extreme environments produce various secondary metabolites that with beneficial effects (Pangestuti et al. 2016) such as antifouling, antipredation, and UV-protective activity (Murniasih et al. 2015).
Extracts of subtropical sea cucumbers are known antidotes against free radicals with positive effects on human health because they can protect against reactive species-induced damage at the cellular level (Pangestuti et al. 2016), and protective compounds been detected in sea cucumbers such as Holothuria leucospilota (Soltani and Baharara 2014), H. scabra (Nobsathian et al. 2017), and Stichopus chloronotus (Murniasih et al. 2015). In addition, sea cucumbers are also reported to possess antibacterial activity (Farjami et al. 2013), especially against pathogenic bacteria (Adibpour et al. 2014). Thus, they could be potential sources of novel antibiotics in view of several recent reports on the appearance of antibiotic-resistant pathogenic bacteria triggered by unregulated and excessive antibacterial agent use (Omar et al. 2012), in addition to naturally occurring resistance mechanisms (Spizek et al. 2010).
Reactive oxygen species (ROS) and free radicals have attracted increasing attention over the past decade. ROS, including superoxide anion radicals (O2•−), hydroxyl radicals (OH•), and non-free radical species such as H2O2 and singlet oxygen (1O2), comprise various forms of activated oxygen. These molecules exacerbate cellular injury and the aging process (Gulcin 2006). ROS are continuously produced during normal physiologic events, and they can easily initiate membrane lipid peroxidation, leading to the accumulation of lipid peroxides (Gulcin 2006). Moreover, ROS induce oxidative damage in biomolecules such as proteins, lipids, carbohydrates, and nucleic acids. This damage causes diseases including cancer and aging. ROS have been implicated in more than 100 diseases, including malaria, heart disease, acquired immunodeficiency syndrome, arteriosclerosis, stroke, cancer, and diabetes (Gulcin 2011). Thus, the development of safer antioxidants with natural origins has attracted increasing interest.
Although sea cucumbers represent a key non-fishing income stream for coastal peoples throughout the Indian and Pacific Oceans, information about biological activities such as antioxidant and antibacterial activities for some sea cucumber species is lacking. This study focused on the potential antioxidant and antibacterial activities of Indonesian sea cucumber species and characterization of their crude extracts regarding bioactive components using gas chromatography–mass spectrometry (GC–MS).
Materials and methods
Sample acquisition
In total, 21 sea cucumber species were collected in 2019 from Lampung Province and Lombok Island, Indonesia. Eight species of sea cucumbers (Actinopyga echinites LPG, H. atra, H. leucospilota LPG, H. scabra, S. hermanni, S. ocellatus, S. vastus, and Thelenota ananas) were collected from Lampung waters by fishermen, and thirteen species (Actinopyga echinites LBK, Bohadschia marmorata, H. albiventer, H. colluber, H. fuscogilva, H. hilla, H. impatiens, H. lesson, H. leucospilota LBK, H. pardalis, H. rigida, H. verrucosa, and Bohadschia sp.) were collected in tidal waters along Lombok Island via direct sampling. Species-level identification was based on the morphological characteristics of the specimens.
Extraction
Samples (5 g wet weight) of each species were cut into small pieces and extracted with methanol at room temperature for 24 h. The maceration method followed that of Jones and Kinghom (2006). The samples were filtered and re-extracted with methanol. The filtrate was then concentrated under vacuum at a maximum temperature of 40 °C to obtain the extract.
2,2-Diphenyl-β-picrylhydrazine (DPPH) radical scavenging assay
The antioxidant potential of each sample was assessed at concentrations of 0.1–50 µg µL−1. To 160 μL of each extract in the wells of a 96-well plate were added 40 μL of 1 mM DPPH solution (Sigma, D9132), and the mixture was incubated for 30 min at room temperature. The absorbance was then measured at a wavelength of 515 nm using a Tecan® Microplate Reader. Butylated hydroxytoluene (BHT, Sigma, W218405-1 KG-K) was used as a calibration standard at concentrations of 0.005–0.01 µg µL−1. The measurement was repeated three times. Free radical inhibitory activity was calculated using the following formula:
where A Sample is the absorbance of the sample and A control is the absorbance of the control (Ak and Gulcin 2008). A straight-line equation based on the calibration curve was used to obtain the 50% inhibitory concentration (IC50). Inhibitory activity was calculated using a previously reported formula (Sánchez‐Moreno et al. 1998). The percent inhibition was also obtained at 10 µg µL−1 using the regression equation y = ax + b, where y is the percent inhibition (%) and x is the targeted concentration (10 µg µL−1). The concentration (10 µg µL−1) was entered into the regression formula to obtain the percent inhibition (y = Inhibition [%]).
Determination of the antibacterial activity of sea cucumber extracts
Disk diffusion assay
Inhibition zone testing was conducted using three replications of disposable Petri dishes containing Mueller–Hinton agar (Radjasa et al. 2001) and Mueller–Hinton broth enriched medium. All sample extracts were tested at the same concentration (25 µg µL−1, 20 µL) on paper disks (6 mm). Each agar plate contained a bacterial strain spread using a sterile cotton swab. The plates were incubated for 18–24 h at 37 °C as described previously (Sukmiwati et al. 2019). Four species of human pathogenic bacteria, namely the gram-positive bacteria Staphylococcus aureus (ATCC 25923) and Bacillus subtilis (ATCC 6633) and the gram-negative bacteria Escherichia coli (ATCC 25922) and Vibrio cholerae (ATCC 14035), were used at OD600nm 0.3 in the antibacterial tests. The positive control wells contained 20 µL (10 µg µL−1) of erythromycin, ampicillin, or kanamycin, and sterile dimethyl sulfoxide (DMSO, 10%) served as the solvent control. By measuring the zone of inhibition against the test organisms, antimicrobial activity was evaluated (Gulcin et al. 2010). The diameters of the zones of inhibition were measured using calipers. The method followed a previous report (Telahigue et al. 2020) with modifications.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) measurements
The serial dilution technique was performed in a 96-well microplate to determine the MIC of sea cucumber samples. Yield extracts were dissolved in DMSO to obtain a 100 µg µL−1 stock solution. Overnight cultures of each bacterium were diluted in sterile Mueller–Hinton broth. Following 18–24 h of incubation, the inocula were prepared by adjusting the turbidity of the suspension to OD660nm 0.3. For each of the bacteria used (S. aureus, V. cholerae, E. coli, and B. subtilis), 50 µL of each extract and antibiotic controls (erythromycin, ampicillin, and kanamycin) were serially diluted with 50 µL of sterile distilled water. Twenty microliters of bacterial culture and 30 µL of medium were added to each well, with some wells reserved for the sterile control (Mueller–Hinton broth), positive controls (antibiotics), and negative control (DMSO). The plates were aerobically incubated at 35 ± 2 °C for 18–20 h, and the MIC was evaluated by observing the lowest concentration of the test extract that resulted in blue coloration following the addition of 20 µL of resazurin (0.1 µg µL−1), indicating an antibacterial reaction. Conversely, pink coloration was indicative of bacterial activity and hence a negative antibacterial reaction. This method followed that described by Sarker et al. (2007) with modifications.
Meanwhile, bacteriostatic activity was assessed using the MBC test as reported by Elshikh et al. (2016). To determine the bactericidal concentration, bacteria from the MIC wells were re-cultured on sterile Mueller–Hinton agar and incubated for 24 h at room temperature. The development of bacterial colonies indicated bacteriostatic activity, whereas the absence of bacterial colonies indicated bactericidal activity.
GC–MS analysis of sea cucumber extracts
Sea cucumber extracts were characterized by GC–MS at the Regional Health Laboratory (Labkesda), DKI Jakarta. Samples were injected into an Agilent Technologies 7890 gas chromatograph–mass spectrometer equipped with an autosampler and the 5975 Mass Selective Detector and linked to the Chemstation Data System. The HP Ultra 2L capillary column used in the analysis was 30 m × 0.25 mm i.d. with a film thickness of 0.25 μm. The instrument was set to the electron collision using ionization mode with an electron energy of 70 eV. After heating the oven to an initial temperature of 70 °C, the temperature was increased by 3 °C/min to 150 °C and held for 1 min. The temperature was then increased by 20 °C/min to 280 °C and held for 26 min. The settings of the system were as follows: gas carrier, helium; column flow rate, 1.0 µL; injection port temperature, 250 °C; interface temperature, 280 °C; ion source temperature, 230 °C; and quadrupole temperature, 140 °C. GC–MS analysis was performed using sea cucumber samples with bioactive components based on the highest antibacterial activity against S. aureus, V. cholerae, B. subtilis, and E. coli.
Statistical analysis
All data analyses were performed in triplicate, and data are expressed as the mean ± SD. Statistical significance was determined using one-way ANOVA. SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for the analysis. Differences were considered statistically significant at P < 0.05.
Results and discussion
DPPH radical scavenging assay
Sea cucumbers as natural products contain valuable ingredients including health-promoting substances (Qi et al. 2017). Knowing the differences in antioxidant capacity among various sea cucumber species is necessary to determine the source of the product with the best potential for utilization. In this study, antioxidant activity was tested to select the species of sea cucumbers with the best capacity using the DPPH method (Blois 1958; Abdille et al. 2005). Briefly, antioxidants react with stable free radicals such as 2,2-diphenyl-β-picrylhydrazyl, which is converted into DPPH, with a loss of color indicating antioxidant potential and scavenging of free radicals in the test sample (Hossain and Shah 2015). The use of DPPH in this study had advantages of minimal cost, simple procedures, and the ability to analyze large numbers of samples (Sveinsdottir et al. 2014).
The results of the revealed that H. atra has the strongest antioxidant activity (IC50 = 14.22 ± 0.87 µg µL−1), and the percent inhibition was 36.36 ± 1.58% at 10 µg µL−1 (Table 1). Meanwhile the IC50 of H. leucospilota LBK was 23.37 ± 0.37 µg µL−1, and the percent inhibition at 10 µg µL−1 was 23.39 ± 0.21%. BHT, as an indicator of antioxidant capacity, had stronger effects than all tested sea cucumber extract, and based on research conducted by Murniasih et al. (2015), the antioxidant capacity of fractionated compounds is much better than that of the crude extract. In this research, the ethyl acetate extract of H. leucospilota had the strongest antioxidant activity, with a percent inhibition of 11.12% at 1 µg µL−1. Meanwhile, the antioxidant activity of H. atra was verified using GC–MS. In other studies, antioxidant activity has reported for other sea cucumbers such as H. scabra (Nobsathian et al. 2017), H. leucospilota (Soltani and Baharara 2014), H. atra (Dhinakaran and Lipton 2014), and S. chloronotus (Althunibat et al. (2009). In the future, it is strongly recommended to perform phytochemical screening to clarify the relationships of the observed biological activity with the detected bioactive compounds.
Table 1.
Antioxidant activity (IC50 and percent inhibition) of the extracts of 21 sea cucumber species
| No | Species | Inhibition at 10 µg µL1 (%) | Regression | IC50 (µg µL−1) |
|---|---|---|---|---|
| 1 | Actinopyga echinites LPG | 14.68 ± 0.34 | y = 1.1021x + 3.6561 | 42.05 ± 2.40 |
| 2 | Holothuria atra | 36.36 ± 1.58 | y = 3.2314x + 4.0452 | 14.22 ± 0.87 |
| 3 | Holothuria leucospilota LPG | 17.17 ± 0.26 | y = 1.7687x − 0.5188 | 28.56 ± 0.53 |
| 4 | Holothuria scabra | 12.31 ± 0.76 | y = 1.1223x + 1.088 | 43.58 ± 4.25 |
| 5 | Stichopus hermanni | 8.34 ± 0.39 | y = 0.6969x + 1.3744 | 69.77 ± 0.40 |
| 6 | Stichopus ocellatus | 17.26 ± 0.17 | y = 1.1051x + 6.2097 | 39.63 ± 2.08 |
| 7 | Stichopus vastus | 13.95 ± 0.35 | y = 0.6323x + 7.6311 | 67.01 ± 0.74 |
| 8 | Thelenota ananas | 11.12 ± 0.20 | y = 0.7978x + 3.1443 | 58.73 ± 12.83 |
| 9 | Actinopyga echinites LBK | 19.33 ± 0.12 | y = 1.7171x + 2.1573 | 27.86 ± 0.31 |
| 10 | Bohadschia marmorata | 6.23 ± 0.22 | y = 0.4828x + 1.4017 | 100.66 ± 7.16 |
| 11 | Holothuria albiventer | 18.64 ± 0.58 | y = 1.9569x − 0.9313 | 26.03 ± 0.72 |
| 12 | Holothuria colluber | 7.01 ± 0.38 | y = 9.1797x + 4.6075 | 95.40 ± 5.22 |
| 13 | Holothuria fuscogilva | 9.37 ± 0.27 | y = 0.8854x + 0.5208 | 55.88 ± 4.87 |
| 14 | Holothuria hilla | 17.47 ± 1.34 | y = 1.6317x + 1.151 | 29.94 ± 3.06 |
| 15 | Holothuria impatiens | 9.29 ± 0.48 | y = 0.8039x + 1.2519 | 60.64 ± 3.13 |
| 16 | Holothuria lesson | 7.71 ± 0.21 | y = 0.8352x − 0.6412 | 60.63 ± 1.21 |
| 17 | Holothuria leucospilota LBK | 23.39 ± 0.21 | y = 0.8352x − 0.6412 | 23.37 ± 0.37 |
| 18 | Holothuria pardalis | 7.27 ± 0.15 | y = 0.6828x + 0.439 | 72.58 ± 1.77 |
| 19 | Holothuria rigida | 7.79 ± 0.26 | y = 0.7732x + 0.0592 | 64.59 ± 2.22 |
| 20 | Holothuria verrucosa | 11.48 ± 0.59 | y = 0.9305x + 2.173 | 51.40 ± 2.83 |
| 21 | Bohadschia sp. | 11.46 ± 0.43 | y = 1.2415x − 0.9575 | 41.05 ± 2.85 |
| 22 | Butylated hydroxytoluene | > 100 ± 105.88 | y = 1236x + 5.5851 | 0.04 ± 0.00 |
Collection location codes: LBK: Lombok, LPG: Lampung
IC50, 50% inhibitory concentration
Determination of the antibacterial activity of sea cucumber extracts
Disk diffusion assay
Because of its convenience, efficiency, and cost, the disk diffusion method, also termed the Kirby–Bauer test, is the most widely used method globally for assessing antibacterial activity, especially antimicrobial resistance. The advantages of the disc diffusion method include its simplicity and the absence of special equipment (Sandle 2016). In this research, the antibacterial properties of the methanol extracts of sea cucumber species were tested against four human pathogenic bacteria.
The results revealed that fourteen extracts had antibacterial activity against S. aureus and V. cholerae, four species were active against B. subtilis, and no extract was active against E. coli. Regarding S. aureus, S. vastus had the strongest growth-inhibitory effects (10.38 ± 0.09 mm), whereas S. ocellatus (14.60 ± 0.21 mm) displayed the strongest effects against V. cholerae Meanwhile, H. albiventer (10.60 ± 0.57 mm) most strongly inhibited the growth of B. subtilis. Four species (A. echinites LPG, H. albiventer, H. fuscogilva, and H. hilla) exhibited antimicrobial activity against S. aureus, V. cholerae, and B. subtilis. However, none of the crude extracts exhibited commensurate inhibitory activity as ampicillin, erythromycin, and kanamycin despite the use of lower concentrations for the antibiotics (1.25 µg µL−1). It is likely that the result would differ if the test subject were a single compound opposed to the entire extract.
In addition to its strong inhibitory effects on S. aureus, S. vastus also exhibited activity against Enterobacter and Klebsiella spp. as ethyl acetate and methanol extracts and against E. coli only as an ethyl acetate extract in prior research (Pringgenies et al. 2017). Moreover, the n-butanol, ethyl acetate, and n-hexane extracts of S. vastus exhibited activity against S. aureus, S. epidermidis, E. coli, and P. aeruginosa, and saponins, terpenoids, and steroid compounds were reportedly contained in the extracts (Sukmiwati et al. 2018). In other bacterial tests, S. ocellatus displayed antibacterial activity against V. eltor and E. coli (Rasyid et al. 2018), and saponins, terpenoids, steroids, and fatty acid compounds (Chasanah et al. 2016) were reportedly present in the extract of S. ocellatus. The antibacterial activity of H. albiventer has not been examined previously. Concerning its beneficial compounds, saponins have the ability to induce the leakage of proteins and certain enzymes in cells. Terpenoids can dissolve the cell wall of microorganisms by weakening the membranous tissue. Moreover, steroids are known for their effects on membrane lipids and ability to cause liposome leakage (Mujeeb et al. 2014).
Escherichia coli is gram-negative pathogenic bacteria that can cause diseases by its ability to invade tissues and induce toxigenesis (Manu et al. 2011). The bacterium is becoming highly resistant to conventionally used antibiotics (both newer and older medicines) as evidenced by many studies, perhaps because of the unregulated use of antibacterial agents (Omar et al. 2012). Antimicrobial resistance in E. coli in developing countries including Ethiopia is reported as a major cause of treatment failure in patients with infectious diseases. (Tuem et al. 2018). Thus, new therapeutic compounds are urgently needed (Manilal et al. 2009; Moran et al. 2011), prompting the inclusion of E. coli in the panel of bacteria treated with various sea cucumber extracts in the present study. Unfortunately, none of the sea cucumber species tested in the present study appeared to have any antibacterial effects on E. coli.
MIC and MBC assays
The MIC is a primary indicator of the antibacterial potential of agents (Elshikh et al. 2016). Based on their activity, antibiotics can be classified as bactericidal or bacteriostatic. Bactericidal antibiotics can kill bacteria, whereas bacteriostatic agents only inhibit their growth. Both definitions indicate that bactericidal antibiotics have stronger effects than bacteriostatic antibiotics (Nemeth et al. 2014). The MIC is considered a basic reference in determining the susceptibility of organisms to antimicrobials, supporting its wide use in vitro to identify novel therapies (Andrews 2001). Thus, the MIC was tested in this study identify sea cucumbers with the strongest antibacterial activity.
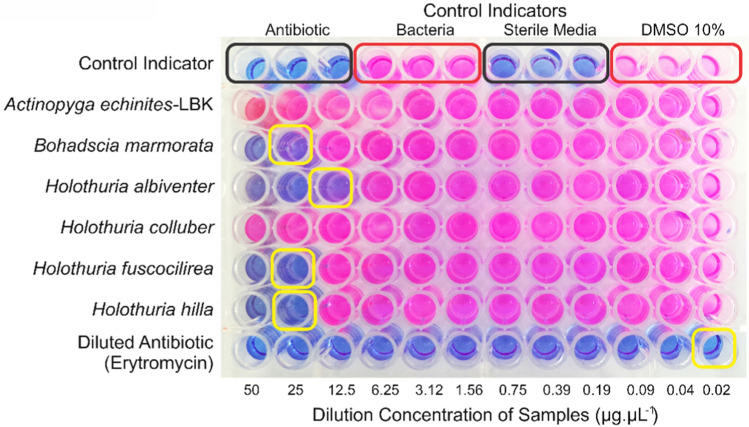
Despite the standard and easy use of the disk diffusion assay (Jorgensen and Ferraro 2009), minor technical problems can occur, such as the inaccurate diffusion of a test sample because of differences in polarity between the test compound and the agar medium (Sánchez and Kouznetsov 2010). For this reason, a second assay was employed in which the blue resazurin indicator would change in color to pink when reduced by oxidoreductase in bacterial cells (Sarker et al. 2007). The MIC results demonstrated that eleven species had antibacterial activity against only B. subtilis: A. echinites LPG (12.5 µg µL−1), T. ananas (50 µg µL−1), B. marmorata (25 µg µL−1), H. albiventer (12.5 µg µL−1), H. fuscogilva (25 µg µL−1), H. hilla (25 µg µL−1), H. impatiens (50 µg µL−1), H. lessoni (50 µg µL−1), H. leucospilota LBK (25 µg µL−1), H. verrucosa (50 µg µL−1), and Bohadschia sp. (25 µg µL−1). Of these, the strongest effects against B. subtilis were recorded for A. echinites LPG and H. albiventer (Table 3 and Fig. 1). The concentration was generally started at 50 µg µL−1, but the concentration was started at 25 µg µL−1 in some instances because of the limited yield.
Table 3.
Potential antibacterial activity tested using the resazurin-based minimum inhibitory concentration method
| No | Sea cucumber species | Bacteriostatic concentration (μg.μL−1) | |||
|---|---|---|---|---|---|
| S. aureus | V. cholerae | B. subtilis | E. coli | ||
| 1 | Actinopyga echinites LPG | > 50 | > 50 | 12.5 | > 50 |
| 2 | Holothuria atra | > 50 | > 50 | > 50 | > 50 |
| 3 | Holothuria leucospilota LPG | > 50 | > 50 | > 50 | > 50 |
| 4 | Holothuria scabra | > 25 | > 25 | > 25 | > 25 |
| 5 | Stichopus hermanni | > 25 | > 25 | > 25 | > 25 |
| 6 | Stichopus ocellatus | > 50 | > 50 | > 50 | > 50 |
| 7 | Stichopus vastus | > 50 | > 50 | > 50 | > 50 |
| 8 | Thelenota ananas | > 50 | > 50 | 50 | > 50 |
| 9 | Actinopyga echinites LBK | > 50 | > 50 | > 50 | > 50 |
| 10 | Bohadschia marmorata | > 50 | > 50 | 25 | > 50 |
| 11 | Holothuria albiventer | > 50 | > 50 | 12.5 | > 50 |
| 12 | Holothuria colluber | > 50 | > 50 | > 50 | > 50 |
| 13 | Holothuria fuscogilva | > 50 | > 50 | 25 | > 50 |
| 14 | Holothuria hilla | > 50 | > 50 | 25 | > 50 |
| 15 | Holothuria impatiens | > 50 | > 50 | 50 | > 50 |
| 16 | Holothuria lessoni | > 50 | > 50 | 50 | > 50 |
| 17 | Holothuria leucospilota LBK | > 50 | > 50 | 25 | > 50 |
| 18 | Holothuria pardalis | > 50 | > 50 | > 50 | > 50 |
| 19 | Holothuria rigida* | – | – | – | – |
| 20 | Holothuria verrucosa | > 50 | > 50 | 50 | > 50 |
| 21 | Bohadschia sp. | > 50 | > 50 | 25 | > 50 |
| Antibiotic (control) | |||||
| 22 | Ampicillin | 1.25 | 0.625 | < 0.0048 | < 0.0048 |
| 23 | Erythromycin | < 0.0048 | < 0.0048 | < 0.0048 | < 0.0048 |
| 24 | Kanamycin | < 0.0048 | < 0.0048 | < 0.0048 | < 0.0048 |
*Insufficient sample for tests
Collection location codes: LBK: Lombok, LPG: Lampung
Fig. 1.
Resazurin-based assay to determine minimum inhibitory concentration
Based on the results using the MIC and disk diffusion assays, seven sea cucumber species exhibited antibacterial activity against three pathogenic species: A. echinites LPG, T. ananas, H. albiventer, H. fuscogilva, H. hilla, H. lessoni, and H. leucospilota LBK. In line with our previous results, none of the sea cucumber samples displayed stronger antibacterial activity than the control antibiotics. Nonetheless, it should be noted that the active antibacterial factors were not purified and concentrated from the sea cucumber extracts to make an objective comparison. Overall, H. albiventer exhibited the greatest potential as an antibacterial agent in the two assays. This species was active against three bacterial species, producing inhibitory zones in the disk diffusion assay against S. aureus (8.30 ± 0.38 mm), V. cholerae (10.48 ± 0.14 mm), and B. subtilis (10.60 ± 0.57 mm) (Table 2), and this sea cucumber species was also bacteriostatic (MIC) against B. subtilis (12.5 µg µL−1) in the resazurin-based assay. A similar result also achieved for A. echinites LPG as the most potent antibacterial agent with activity against S. aureus (8.75 ± 0.18 mm), V. cholerae (9.77 ± 0.42 mm), and B. subtilis (8.48 ± 0.19 mm) in the disk diffusion assay (Table 2) and bacteriostatic activity (MIC) against B. subtilis at 12.5 µg µL−1 (Table 3). Another study conducted by Abraham et al. (2002) explained that A. echinites has antibacterial activity against S. aureus, and its bioactive compounds were triterpene glycosides (saponins) (Bordbar et al. 2011). In line with the previous discussion about H. albiventer, no related study of this species has been conducted, especially concerning antibacterial activity. This study could be the first evidence of the value of H. albiventer, especially regarding its antibacterial activity and antioxidant capacity.
Table 2.
Potential antibacterial activity of sea cucmbers against four bacteria tested using the disk diffusion method
| No | Sea cucumber species | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| S. aureus | V. cholerae | B. subtilis | E. coli | ||
| 1 | Actinopyga echinites LPG | 8.75 ± 0.18 | 9.77 ± 0.42 | 8.48 ± 0.19 | 0.00 ± 0.00 |
| 2 | Holothuria atra | 0.00 ± 0.00 | 13.72 ± 0.22 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 | Holothuria leucospilota LPG | 8.02 ± 0.37 | 10.55 ± 0.33 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 4 | Holothuria scabra | 0.00 ± 0.00 | 13.95 ± 0.28 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 5 | Stichopus hermanni | 0.00 ± 0.00 | 13.47 ± 0.22 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 6 | Stichopus ocellatus | 8.17 ± 0.26 | 14.60 ± 0.21 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 7 | Stichopus vastus | 10.38 ± 0.09 | 8.60 ± 0.31 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 8 | Thelenota ananas | 8.17 ± 0.26 | 12.55 ± 0.51 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 9 | Actinopyga echinites LBK | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 10 | Bohadschia marmorata | 7.50 ± 0.29 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 11 | Holothuria albiventer | 8.30 ± 0.38 | 10.48 ± 0.14 | 10.60 ± 0.57 | 0.00 ± 0.00 |
| 12 | Holothuria colluber | 7.97 ± 0.38 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 13 | Holothuria fuscogilva | 9.90 ± 0.21 | 8.63 ± 0.15 | 9.15 ± 0.32 | 0.00 ± 0.00 |
| 14 | Holothuria hilla | 7.30 ± 0.10 | 9.40 ± 0.15 | 9.17 ± 0.37 | 0.00 ± 0.00 |
| 15 | Holothuria impatiens | 8.05 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 16 | Holothuria lessoni | 8.08 ± 0.09 | 9.15 ± 0.62 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 17 | Holothuria leucospilota LBK | 7.98 ± 0.24 | 8.73 ± 0.44 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 18 | Holothuria pardalis | 0.00 ± 0.00 | 9.60 ± 0.10 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 19 | Holothuria rigida | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 20 | Holothuria verrucosa | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 21 | Bohadschia sp. | 8.35 ± 0.32 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Antibiotic (control, 1.25 μg μL−1) | |||||
| 22 | Ampicillin | 14.20 ± 0.40 | 23.70 ± 0.33 | 37.70 ± 0.06 | 19.67 ± 0.28 |
| 23 | Erythromycin | 20.26 ± 0.31 | 25.20 ± 0.41 | 23.90 ± 0.21 | 11.83 ± 0.72 |
| 24 | Kanamycin | 20.85 ± 0.15 | 19.43 ± 0.12 | 29.13 ± 0.33 | 20.60 ± 0.31 |
Collection location codes: LBK: Lombok, LPG: Lampung
The MBC test is performed to determine whether compounds are bactericidal or bacteriostatic. S. aureus, V. cholerae, and E. coli were not included in the MBC test because the findings were negative for these microbes in the MIC test. Seven species exhibited potential activity against B. subtilis in the MIC test. Of these species, all were revealed to have bacteriostatic effects opposed to bactericidal activity.
GC–MS analysis of sea cucumber extracts
In recent years, GC–MS studies have been increasingly applied to analyze medicinal plants, as this technique has proven valuable for analyzing non-polar components and volatile essential oils, fatty acids, lipids, and alkaloids. It has also been exclusively used for the analysis of esters, fatty acids, alcohols, aldehydes, terpenes, and other compounds. Based on the principle adsorption and partition of gas chromatography, this technique is the most widely used technique for this purpose (Al-Rubaye et al. 2017).
GC–MS analysis was performed using the three sea cucumber extracts with the strongest growth-inhibitory effects against each bacterial strain, revealing the diversity of the bioactive molecules. H. albiventer, which had the strongest effects against B. subtilis, featured eleven active compounds, and three compounds each were detected in the extracts of S. vastus and S. ocellatus (Table 2). The eleven compounds detected in H. albiventer were hexadecanoic acid, methyl ester (C17H34O2); 7-hexadecanoic acid, methyl ester (C17H32O2); hexadecanoic acid, 15-methyl-, methyl ester (C18H36O2); octadecanal (C18H36O); methyl stearate (C19H38O2); cis-13-octadecanoic acid, methyl ester (C19H36O2); 11-octadecanoic acid, methyl ester (C19H36O2); nonadecanoic acid, methyl ester (C20H40O2); methyl nonadecanoate (C20H40O2); methyl 9-eicosenoate (C21H40O2); and 5,8,11,14-eicosatetraenoic acid, methyl ester (C21H34O2). The bioactive compounds in S. ocellatus were hexadecanoic acid, methyl ester; methyl stearate; and hexadecanoic acid, bis (2-ethylhexyl) ester (C22H42O4). The active constituents of S. vastus were hexadecanoic acid, methyl ester; methyl stearate; and 9-octadecanoic acid, methyl ester (Table 4).
Table 4.
Gas chromatography–mass spectrometry analysis of antibacterial activity in sea cucumbers
| No | RT (min) | Compounds | Molecular formula | Molecular weight (g/mol) | Peak area (%) |
|---|---|---|---|---|---|
| Holothuria albiventer extract | |||||
| 1 | 29.093 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.50 | 4.06 |
| 2 | 29.265 | 7-Hexadecanoic acid, methyl ester | C17H32O2 | 268.4 | 1.47 |
| 3 | 29.727 | Hexadecanoic acid, 15-methyl-, methyl ester | C18H36O2 | 284.48 | 1.29 |
| 4 | 29.893 | Octadecanal | C18H36O | 268.47 | 1.34 |
| 5 | 30.279 | Methyl stearate | C19H38O2 | 298.50 | 7.66 |
| 6 | 30.389 | Cis-13-octadecanoic acid, methyl ester | C19H36O2 | 296.49 | 3.03 |
| 7 | 30.417 | 11-Octadecanoic acid, methyl ester | C19H36O2 | 296.49 | 4.18 |
| 8 | 30.761 | Nonadecanoic acid, methyl ester | C20H40O2 | 312.53 | 2.50 |
| 9 | 31.230 | Methyl nonadecanoate | C20H40O2 | 312.5 | 4.55 |
| 10 | 31.327 | Methyl 9-eicosenoate | C21H40O2 | 324.5 | 7.04 |
| 11 | 31.865 | 5,8,11,14-Eicosatetraenoic acid, methyl ester | C21H34O2 | 318.5 | 5.59 |
| Stichopus ocellatus extract | |||||
| 1 | 29.08 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.50 | 7.03 |
| 2 | 30.26 | Methyl stearate | C19H38O2 | 298.50 | 3.60 |
| 3 | 32.17 | Hexadecanoic acid, bis (2-ethylhexyl) ester | C22H42O4 | 370.56 | 28.57 |
| Stichopus vastus extract | |||||
| 1 | 29.093 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.50 | 10.18 |
| 2 | 30.272 | Methyl stearate | C19H38O2 | 298.50 | 4.82 |
| 3 | 30.382 | 9-Octadecanoic acid, methyl ester | C19H36O2 | 296.48 | 11.43 |
GC–MS analysis revealed the presence of long-chain fatty acids (LCFAs) without other secondary metabolites with potential antibacterial activity such as phenolic compounds or terpene compounds. LCFAs display interesting biological activities such as antibacterial activity, and they are the key ingredients of some antibacterial herbs and antimicrobial food additives. C16–C20 LCFAs are known to actively inhibit bacterial growth (Zheng et al. 2005). In other words, the detected compounds possess strong correlations with the inhibitory effects on bacterial growth. In a deep analysis, each compound has unique abilities to inhibit bacterial growth, but generally, the primary mechanisms include increased membrane permeability and cell lysis, disruption of the electron transport chain and uncoupling of oxidative phosphorylation, and inhibition of the activity of bacterial enzymes (Yoon et al. 2018). Other studies described LCFAs in other sea cucumber species such as S. horrens (Rasyid et al. 2018), H. atra (Murniasih et al. 2015), S. hermanni, Thelenota anax, H. fuscogilva, H. leucospilota, and H. scabra (Pangestuti and Arifin 2017).
Conclusion
The results in this study could represent an effective introduction to the antioxidant capacities and antibacterial activities of sea cucumbers, especially rare species. Antimicrobial and antioxidant activities were detected in extracts from various species of sea cucumbers collected from Lombok Island and Lampung, Indonesia. The most promising sea cucumber regarding antioxidant activity was H. atra, whereas the most promising antibacterial species was H. albiventer. Based on this research, those species could be further studied to obtain valuable information for researchers or industries with interest in developing functional foods or medicinal ingredients from sea cucumbers. Further studies are needed for other biological activities such as cytotoxic and antifungal activity as well as to characterize bioactive components such as phenolic, terpenoid, and saponin compounds.
Acknowledgements
The authors would like to thank EnagoTM English Editing Service for editing a draft of this manuscript.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AN, IAH, AB, ARA, AS, MYP. The first draft of the manuscript were written by AN, AB, TM and MYP and all authors reviewed and approved the final manuscript.
Funding
This research was supported by a research grant awarded to Masteria Yunovilsa Putra by the Coral Reef Rehabilitation and Management Program-Coral Triangle Initiative (COREMAP-CTI) 2019 and National Research Priority (PRN), Indonesian Institute of Sciences.
Data availability
All data generated or analysed during this study are included in this published article.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Informed consent
This article does not contain any individual person’s data in any form.
Footnotes
Aji Nugroho, Iskandar Azmy Harahap, and Ardi Ardiansyah are contributed equally.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdille MdH, Singh RP, Jayaprakasha GK, Jena BS. Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 2005;90:891–896. doi: 10.1016/j.foodchem.2004.09.002. [DOI] [Google Scholar]
- Abraham TJ, Nagarajan J, Shanmugam SA. Antimicrobial substance of potential biomedicinal importance from holothurian species. Indian J Mar Sci. 2002;31:161–164. [Google Scholar]
- Adibpour N, Nasr F, Nematpour F, Shakouri A, Ameri A. Antibacterial and antifungal activity of Holothuria leucospilota isolated from Persian gulf and Oman sea. Jundishapur J Microbiol. 2014;7:1–4. doi: 10.5812/jjm.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Al-Rubaye AF, Hameed IH, Kadhim MJ. A review: Uses of gas chromatography-mass spectrometry (GC-MS) technique for analysis of bioactive natural compounds of some plants. Int J Toxicol Pharmacol Res. 2017;9:81–85. doi: 10.25258/ijtpr.v9i01.9042. [DOI] [Google Scholar]
- Althunibat OY, Hashim RB, Taher M, Daud JM, Ikeda M, Zali BI. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur J Sci Res. 2009;37:376–387. [Google Scholar]
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs. 2011;9:1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasanah E, Fawzya YN, Tarman K, Januar HI, Nursid M. Fatty acid profile, carotenoid content, and in vitro anticancer activity of Karimunjawa and Lampung sea cucumber. Squalen Bull Mar Fish Postharvest Biotechnol. 2016;11:117–124. doi: 10.15578/squalen.v11i3.269. [DOI] [Google Scholar]
- Dhinakaran DI, Lipton AP. Bioactive compounds from Holothuria atra of indian ocean. Springer Plus. 2014;3:1–10. doi: 10.1186/2193-1801-3-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Marchant R, Banat IM. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett. 2016;38:1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmat AY, Said MM, Soliman AA, El-Masry KSH, Badiea EA. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition. 2013;29:258–267. doi: 10.1016/j.nut.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Farjami B, Nematollahi MA, Moradi Y, Irajian G, Nazemi M, Ardebili A, Pournajaf A. Antibacterial activity of the sea cucumber Holothuria leucospilota. Int J Mol Clin Microbiol. 2015;1:225–230. [Google Scholar]
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Gulcin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2011;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Kirecci E, Akkemik E, Topal F, Hisar O. Antioxidant, antibacterial, and anticandidal activities of an aquatic plant: Duckweed (Lemna minor L. Lemnaceae) Turk J Biol. 2010;34:175–188. doi: 10.3906/biy-0806-7. [DOI] [Google Scholar]
- Hossain MA, Shah MD. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab J Chem. 2015;8:66–71. doi: 10.1016/j.arabjc.2011.01.007. [DOI] [Google Scholar]
- Jones WP, Kinghom AD. Extraction of plant secondary metabolites. In: Sarker SJ, Latif Z, Gray AI, editors. Natural products isolation, 2nd edition. Totowa, New Jersey: Humana Press Inc.; 2006. pp. 323–351. [Google Scholar]
- Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 2009;49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- Kasanah N, Triyanto SDS, Amalia W, Isnansetyo A. Antibacterial compounds from red seaweeds (rhodophyta) Indones J Chem. 2015;15:201–209. doi: 10.22146/ijc.21215. [DOI] [Google Scholar]
- Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Gandhimathi R, Lipton AP. Antimicrobial potential and seasonality of red algae 23 collected from the southwest coast of india tested against shrimp, human and phytopathogens. Ann Microbiol. 2009;59:207–219. doi: 10.1007/BF03178319. [DOI] [Google Scholar]
- Manu D, Lupan I, Popescu O. Mechanisms of pathogenesis and antibiotics resistance in Escherichia coli. Ann Rom Soc Cell Biol. 2011;16:7–19. [Google Scholar]
- Moran LF, Aronsson Bo, Manz C, Gyssens IC, Sob AD, Monnet DL, Cars O. Critical shortage of new antibiotics in development against multidrug resistant bacteria - time to react is now. Drug Resist Update. 2011;14:118–124. doi: 10.1016/j.drup.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Mujeeb F, Bajpai P, Pathak N. phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Bio Med Res Int. 2014;497606:1–11. doi: 10.1155/2014/497606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murniasih T, Putra MY, Pangestuti R. Antioxidant capacities of Holothuria sea cucumbers. Ann Bogor. 2015;19:21–26. doi: 10.14203/ann.bogor.2015.v19.n2.21-26. [DOI] [Google Scholar]
- Nemeth J, Oesch G, Kuster SP. Bacteriostatic versus bactericidal antibiotics for patients with serious bacterial infections: systematic review and meta-analysis. J Antimivrobial Chemotheraphy Adv Access. 2014;10:1–14. doi: 10.1093/jac/dku379. [DOI] [PubMed] [Google Scholar]
- Nobsathian S, Tuchinda P, Sobhon P, Tinikul Y, Poljaroen J, Tinikul R, Sroyraya M, Poomton T, Chaichotranunt S. An antioxidant activity of the whole body of Holothuria scabra. Chem Biol Technol Agric. 2017;4:1–5. doi: 10.1186/s40538-017-0087-7. [DOI] [Google Scholar]
- Omar HH, Shiekh HM, Gumgumjee NM, El-Kazan MM, El-Gendy AM. Antibacterial activity of extracts of marine algae from the red sea of Jeddah, Saudi Arabia. Afr J Biotechnol. 2012;11:13576–13585. doi: 10.5897/AJB12.780. [DOI] [Google Scholar]
- Pangestuti R, Arifin Z. Medicinal and health benefit effects of functional sea cucumbers. J Trad Complement Med. 2017;8:341–351. doi: 10.1016/j.jtcme.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangestuti R, Murniasih T, Putra MY, Rasyid A, Wibowo JT, Ardiansyah A, Untari F. Free radical scavenging activity of selected sea cucumber species from Mataram-Lombok, Indonesia. J Teknol Sci Eng. 2016;78:179–185. doi: 10.11113/jt.v78.8202. [DOI] [Google Scholar]
- Pringgenies D, Ridlo A, Sembiring N. Antibacterial activity for multi drug resistance (MDR) bacteria bysea cucumber stichopus vastus extract from Karimunjawa islands—Indonesia. J Ilmu dan Teknologi Kelautan Tropis. 2017;9:695–707. doi: 10.29244/jitkt.v9i2.19302. [DOI] [Google Scholar]
- Qi H, Ji X, Liu S, Feng D, Dong X, He B, Srinivas J, Yu C. Antioxidant and anti-dyslipidemic effects of polysaccharidic extract from sea cucumber processing liquor. Electron J Biotechnol. 2017;28:1–6. doi: 10.1016/j.ejbt.2017.04.001. [DOI] [Google Scholar]
- Radjasa OK, Urakawa H, Kita-Tsukamoto K, Ohwada K. Characterization of psychotropic bacteria in the surface and deep-sea waters from Northwestern Pacific Ocean based on 16S ribosomal DNA approach. Mar Biotechnol. 2001;3:454–462. doi: 10.1007/s10126-001-0050-1. [DOI] [PubMed] [Google Scholar]
- Rasyid A, Wahyuningsih T, Ardiansyah A. Profil metabolit sekunder, aktivitas antibakteri dan komposisi senyawa yang terkandung dalam ekstrak methanol teripang Stichopus horrens. J Ilmu dan Teknologi Kelautan Tropis. 2018;10:333–340. doi: 10.29244/jitkt.v10i2.19480. [DOI] [Google Scholar]
- Sanchez JGB, Kouznetsov VV. Antimycobacterial susceptibility testing methods for natural products research. Brazil J Microbiol. 2010;41:270–277. doi: 10.1590/S1517-83822010000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric. 1998;76:270–276. doi: 10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9. [DOI] [Google Scholar]
- Sandle T. Antibiotics and preservatives. Pharm Microbiol. 2016 doi: 10.1016/b978-0-08-100022-9.00014-1. [DOI] [Google Scholar]
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in IHE in vitro antibacterial screening of phytochemicals. Methods J. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani M, Baharara J. Antioxidant and antiproliferative capacity of dichloromethane extract of Holoturia leucospilota sea cucumber. Int J Cell Mol Biotechnol. 2014;204:1–9. doi: 10.5899/2014/ijcmb-00013. [DOI] [Google Scholar]
- Spızek J, Novotna J, Ezanka TR, Demain AL. Do we need new antibiotics? The search for new targets and new compounds. J Ind Microbiol Biotechnol. 2010;37:1241–1248. doi: 10.1007/s10295-010-0849-8. [DOI] [PubMed] [Google Scholar]
- Sukmiwati M, Diharmi A, Mora E, Susanti E. Aktivitas antimikroba teripang kasur (Stichopus vastus Sluiter) dari Perairan Natuna Kepulauan Riau. J Pengolahan Hasil Perikanan Indonesia. 2018;21:328–335. doi: 10.17844/jphpi.v21i2.23088. [DOI] [Google Scholar]
- Sukmiwati M, Ilza M, Putri AE, Sidauruk SW. Antibacterial activity of sea cucumber (Holothuria atra) against Pseudomonas aeruginosa. IOP Conf Ser Earth Environ Sci. 2019;012047:1–6. doi: 10.1088/1755-1315/404/1/012047. [DOI] [Google Scholar]
- Sveinsdottir H, Hamaguchi PY, Bakken HE, Kristinsson HG. Method for assessing the antioxidative activity of aquatic food compounds. In: Hordur GK, editor. Antioxidant and functional component in aquatic foods. New York: Wiley; 2014. pp. 151–174. [Google Scholar]
- Telahigue K, Ghali R, Nouiri E, Labidi A, Hajji T. Antibacterial activities and bioactive compounds of the ethyl acetate extract of the sea cucumber Holothuria forskali from Tunisian coasts. J Mar Biol Assoc UK. 2020 doi: 10.1017/s0025315420000016. [DOI] [Google Scholar]
- Tuem KB, Gebre AK, Atey TM, Bitew H, Yimer EM, Berhe DF. Drug resistance patterns of Escherichia coli in ethiopia: a meta-analysis. BioMed Res Int. 2018 doi: 10.1155/2018/4536905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BK, Valle-González JJA, ER, Cho NJ, Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int J Mol Sci. 2018;19:1–40. doi: 10.3390/ijms19041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579:5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.