Abstract
The effect of high-intensity ultrasound (HIU), antibrowning agents (C6H806 & CaCl2), and hot water bath (65 °C) treatments on the prevention of enzymatic browning and 2 weeks shelf-life study of fresh-cut mango fruit slices were evaluated. Results showed that HIU treated mangoes have the lowest PPO activity ranging from 76.17 U/mL to 134.12 U/mL, while improved sensorial properties including decay and off-odor, and enhanced bioactive compounds (ascorbic acid, antioxidant capacity and total phenolics) during storage (4 °C). It is stated that HIU treatment as a promising alternative to replace chemical and/or physical methods to prolong the shelf-life and pursue the quality properties in mango fruit during cold storage.
Keywords: Enzymatic browning, Bioactive compounds, High-intensity ultrasound, Mango fruit, Shelf life
Introduction
Nutritional studies suggest the regular consumption of fresh fruit & vegetables for a healthier diet. The importance of fresh-cut products based on the freshness, convenience, improved nutrient and sensory properties with prolonged shelf life. Fresh-cut processing makes the product highly perishable and undesirable by the consumer. Therefore, suitable processing techniques to keep the wholesomeness and consumer desirability of fresh-cut produce are needed to satisfy today’s increasing consumer demands (Salinas-Roca et al. 2016). Mango (Mangifera indica) is a well-supplied source of ascorbic acid, but unfortunately the content decreases at the ripening period. Furthermore, mangoes are a rich source of carotenoids including p-carotene, P-cryptoxantin, zeaxathin, luteoxathin isomers, violxanthin and neoxantin, pro-vitamin A (Wang et al. 2020). However, several changes take place at the time of harvesting, production and storage of the fruit. Those developments cause loss of the microbiological and antioxidant properties, and the main oxidative reaction observed in the fruits is enzymatic browning (Wang et al. 2020). Enzymatic browning is a development which happens in various fruits and vegetables and has a significant role in the loss of food product. Due to enzymatic browning, around 50% of the fruits are discarded as a consequence of quality loss and the browning which is catalysed by the polyphenol oxidase (PPO) enzyme (Moon et al. 2020). When the product structure is injured by physical forces including cutting, PPO is activated by releasing into the cytosol. In the case of O2 and PPO, monophenol is hydroxylated to o-diphenol, and diphenol could be oxidized to o-quinones, which goes through polymerization process for the production of black-brownish pigments (Yildiz et al. 2020). Several approaches are explored such as thermal, chemical and physical treatments in order to limit this occurrence. Thermal process is very efficient to reduce the enzymatic browning, but it depends upon the basis of differences in the appearance, texture, colour & flavour of food crops (Izli et al. 2018). Hence, conducted experiments aimed to study and compare the effect of several pretreatments including non-thermal, physical and chemical treatments on the visual appearance, enzymatic activity, colour, bioactive constituents, microbial activity and sensory properties of fresh-cut mango fruit slices during fourteen days of storage under refrigeration conditions.
Materials and methods
Mango slices and treatments
Mango fruits (Mangifera indiica) cv. Kent was obtained from a local market in Iğdır, Turkey. Mangoes after washing with tap water for 2 min that was subjected to 7 treatments in total following the slicing of mango pieces with a sharp stainless-steel knife (10 mm thickness). The sample without any pretreatments was stated as a control (untreated). In chemical treatments, mango pieces were cleaned uo by tap water for sixty-seconds and immediately submerged into 3.0% (w/w) antibrowning preservatives for 5 min. All antibrowning agents (ascorbic acid (AA) & calcium chloride (CC)) were organized at 3% (w/v) with distilled water along with the combined AA and CC in different percentages including 1% AA-2% CC; 1.5% AA-1.5% CC, and 2% AA-1% CC. In hot water treatment, fresh-cut mango pieces were immersed in a water bath (Thermoscientfic. MA, US) at 65 °C for 5 min. Mangoes were kept in an ultrasonic water bath (Wiseclean, WU-C-A10H, Germany) for 15 min. After the application of all treatments including antibrowning agents, hot water and high-intensity ultrasound (HIU), fresh-cut mangoes were drained with the help of a paper towel for a minute & instantly packaged in polyethylene bags, labelled and stored at cold storage (4 °C) for 14 days for the shelf life study and the experiments on storage were conducted at 0th, 7th, and 14th days.
Colour measurement
Colour readings of the fresh-cut mangoes were determined by a Minotla Chromo Meter CR-3000 (Minolata Camera Co. Ltd., Osoka, Japan) from the cut surface of the mango fruit slices exposed to antibrowning solutions, hot water and the HIU (Yildiz 2018). The white standard plate was utilized in order to calibrate the color meter. The colour results were shown by the lightnesss (L*), redness (a*) and yelowness (b*). These three values were obtained from 10 separate locations from the cut surface of mango fruit slices at 25 °C and the averaged L*, a*, and b* values were recorded.
Polyphenol oxidase activity
Based on the process stated by Montgomey and Sgarberi (1975) was performed for the PPO analyses in the fresh-cut mango fruit pieces by a spectrophotmeter (Cary 60UV Vis, Ageint Technologie, USA). About 30 g of mango tissues were blended in a Osterizoer 12 sped blender in 0.2 g polyvinylpolypyrolidone and 70 mL of 0.5 M phosphate buffer for an extraction step during 60 s. The clear supernatant right after the use of a centrifuge (1200 g, 20 min, room temperature) was obtained as an enzyme extract. 2 mL of buffer solution at refrigeration condition and 0.5 mL mango fruit extract were combined. Later, fresh 0.5 mL catechol was added to the mixture. The mixture was placed into a cuvette, and the absorbance was recorded during three minutes every fifteen seconds at 420 nm. The enzyme activity of PPO analysis was conducted at the first, seventh & fourteenth day. The absorbance of the assay emulsion versus the reaction (rxn) time was ploted for the demostration of the enzyme kinetics. The ratio of the absorbance vs. time curve was determined, and the values were expressed in Unite/g of fresh weight. The result was divided by 0.01 & enzyme activity was described as “U/mL” in the deal with Cemerogu (2007).
| 1 |
where E: the proportion of the absorboance vs. rxn time, 0.001: a constant value, He: the volume of the PPO extract, Hrk: the total volume of the mixture (mL), and Sf: a dilution determinant.
Bioactive metabolites
Ascorbic acid (AA, Vitamin C)
Vitamin C composition of mango fruit slices was analyzed using titration following the procedure proposed by Ranganna (1986) based on the reduction of 2,6-dichloropenol indohenol dye (SigmaAldrich, StLouis, MO, USA) by Vitamin C and it was expressed in mg/100 g of fresh weight.
Extraction of sample
The extraction stage was achieved based on the procedure reported by Yildiz et al. ( 2020). Homogenized untreated and processed mango fruit pieces (1 g) were blended with 4.5 mL of CH3OH and H20 combination (80/20 v/v, respectively) at room tempeture and shaken at 140 rpm with an orbital shaker (Biosan OS-20, Latvia) for two hours. The supernatant was obtained right after the centrifugation process (10000 g, 15 min) (Sigma 3 K-30, UK). The supernatant was filtered by using a 0.45 μm PTEE filter for the detection of total phenolics as well as DPPH antioxidant activity of the mango fruit pieces.
Total phenolics content (TPC)
The procedure stated by Igual et al. (2012) along with small changes was applied to measure total phenolics of mango fruit slices by using gallic acid as a standard. The basis of the procedure is the degradation of Folin–Ciocalteua reagent with phenolic substance. Concisely, the mango fruit extract (0.25 mL) was mixed with 1.25 mL of Folin–Ciocalteau indicator and 15 mL of deionized water on a vortex blender (WisMisx VM-10, Dahan, Korean). Directly after 10 min in the dark place, 3.75 ml of 7.5% Na2CO3 was added into the mix and the volume was rounded up to 25 mL by distiled water. Following that, the absorbance was measured at 765 nm using a spectrophotometer right after 2 h incubation at room temperature. Galic acid was used as a standard and the outcome were pointed out as mg of galic acid per 100 g of dry weight.
Antioxidant capacity (AOC)
DPPH analysis was utilized to analyze the antioxidant activity of mango fruit samples by the approach defined previously by Alothman and Karim (2009). Mango fruit extract (around 0.1 ml) was mixed with a 3.9 mL of 1.1-diphenly-2-picrylhydrazyl solution (25 mM) and vortexed from fifteen to thirty seconds and incubated at 25 °C throughout thirty-minutes. At the end of the time, the absorbance of the blend was read at 515 nm with a spectrophotometer and AOC of the mango fruit specimens was expressed as µmol TE/g.
Microbial identification
The microbial detection was carried out as per the procedure mentioned by Zhang et al. (2005). About 25 g of control and processed mango specimens were added into 250 mL of aseptica physilogical saline with 0.2% Tween 80 surfactant. The blend was shaken for 5 min and diluted in series (1:10). The mango fruit specimens were plated in triplicate on plate count agar, incubated for a day at 37 °C for aerobic bacteria and for 3 days at 28 °C for mold and yeast.
Visual appearance
The photographs of fresh-cut mango fruit slices exposed to antibrowning agents, hot water and high-intensity ultrasound (HIU) were taken by the camera (Canon Powershot SX72-HSS Digital Stil Camera). The colour differences on cut surfaces of mango fruit slices were recorded for 14 days, and the photos were taken at 0, seventh and fourteenth days.
Sensory Evaluation
Panelists consist of 22 people were chosen for the evaluation of the symptom of decay visually and off-odor of the mango fruit slices. A 4-grade scale was supplied to the people for their scores as below:
no decay observation (0),
slight (1): decay comprises up to 25% of the mango fruit sample surface,
moderate (2): decay comprising > 25% but < 50% of the mango fruit sample surface,
severe (3): decay comprising > 50% of the mango fruit sample surface (Cao et al. 2010).
More than that, for off-odor, no off-odor (0) and very strong off-odor (7) were provided to the panelists. Decay and off-odor analysis were performed at days seven and fourteen of the storage.
Shelf life
Storage analysis of all mango fruit samples (untreated and/or processed) kept at refrigeration conditions was conducted at the intervals of first, seven and fourteen days.
Statistical data analysis
A randomized plots factorial experimental design was employed. The results were determined using the JMP (Ver. 7 0, SAS Insttute Inc., NC, US). Differences among the mean value were obtained by Fisher’s least signifcant differences (LSD) test at alpha: 0.05.
Results and discussion
Effect of storage on the colour of fresh cut mangoes
In fruits and vegetables, colour is a crucial parameter which has a big potential on consumer preference and enables people to have an idea about various other properties connected with final food product (Yildiz et al. 2019). The colour measurements of control and treated mango fruits (antibrowning, hot water, and HIU) are shown in Table 1. The lightness indexes of HIU-treated mango fruit samples were significantly higher than antibrowning and hot water treatment after 14 days storage interval (Table 1). This might be explained as the ultrasound treatment creates physical destruction and membrane deteriorration of the cells, which causes easy elution of pigment component from mango fruit tissues (Wanng et al. 2011). As the storage time was increased, the lightness value was significantly decreased in all the mango pieces (untreated & treated mango fruit slices). The redness value of the mango fruits displayed the identical tendency with the lightness value and decreased all along the storage time (Table 1). While the lowest redness values were detected on the last day (all mango fruit slices), the highest redness values were found on the first-day mango slices. On the other hand, yellowness values of the mango fruit slices were increased all along with the storage in all processed mangoes. While the highest yellowness values were found out on the last day (all mango fruit slices), the lowest yellowness values were found on the first-day mango fruit samples. Browning phenomena is not very hard to occur if the product is cut, because the cut surfaces let O2 to proceed with enzymes and other chemical substances (Yıldlız 2019). It is pointed out a decrease in lightness index and an increase in yellowness index is the reason of browning activities (Putnik et al. 2020). In the current work, the lightness values of all mango slices were decreased, while yellowness values was increased after 2 weeks cold storage, which displays the development of browning activity as a function of time. Yiliz (2018) reported comparable findings where the colour indexes of fresh-cut banana fruit pieces exhibited a decrease in lightness and an increase in yellowness at the time of storage. Furthermore, colour parameters of fresh-cut apple fruit pieces were pointed out by a decline in lightness & an increase in yellownness all along with the storage in the research of Yildiz et al. (2019). On a large scale, ultrasonic treatment is more favourable to preserve the colour of mangoes, thus showing the high quality and prolonged-shelf life of fruit samples (Putnik et al. 2020).
Table 1.
Change of color in control and treated mango samples over storage at 4 °C
| Treatment | Storage (day) | L* | a* | b* |
|---|---|---|---|---|
| Control | 0 | 67.13 ± 1.13b (x) | 4.14 ± 0.32a (x) | 12.17 ± 0.15c (x) |
| 7 | 54.98 ± 0.71e (y) | 3.53 ± 0.15b (y) | 13.98 ± 0.22c (y) | |
| 14 | 42.91 ± 0.45e (z) | 2.56 ± 0.87c (z) | 15.12 ± 0.13c (z) | |
| Ascorbic acid (3%) | 0 | 67.32 ± 1.33b (x) | 4.11 ± 0.13a (x) | 21.38 ± 0.19b (y) |
| 7 | 60.14 ± 0.98c (y) | 3.58 ± 0.78b (y) | 21.17 ± 0.13b (y) | |
| 14 | 53.21 ± 0.17c (z) | 2.95 ± 0.65a (y) | 22.17 ± 0.32b (x) | |
| Calcium chloride (3%) | 0 | 65.88 ± 1.12c (x) | 4.32 ± 0.29a (x) | 21.33 ± 0.81b (z) |
| 7 | 56.43 ± 0.88d (y) | 3.55 ± 0.43b (y) | 22.16 ± 0.17b (y) | |
| 14 | 53.23 ± 0.56c (z) | 2.54 ± 0.09c (z) | 23.28 ± 0.15b (x) | |
| AA (1%)—CC(2%) | 0 | 65.15 ± 1.22c (x) | 4.35 ± 0.22a (x) | 21.12 ± 0.88b (y) |
| 7 | 60.56 ± 1.13c (y) | 3.53 ± 0.24b (y) | 22.93 ± 0.01b (x) | |
| 14 | 54.28 ± 0.12c (z) | 2.74 ± 0.04b (y) | 22.42 ± 0.33b (x) | |
| AA (1.5%)—CC | 0 | 67.68 ± 0.12b (x) | 3.06 ± 0.13d (x) | 22.13 ± 0.68b (y) |
| (1.5%) | 7 | 59.74 ± 0.88c (y) | 2.61 ± 0.54c (y) | 22.94 ± 0.14b (y) |
| 14 | 54.56 ± 0.09c (z) | 2.13 ± 0.66d (y) | 23.34 ± 0.77b (x) | |
| AA (2%) – CC (1%) | 0 | 65.19 ± 0.94c (x) | 3.49 ± 0.72ab (x) | 22.54 ± 0.45b (z) |
| 7 | 60.67 ± 0.07c (y) | 3.55 ± 0.41b (x) | 23.67 ± 0.13b (y) | |
| 14 | 59.12 ± 0.11b (z) | 2.19 ± 0.18d (y) | 22.53 ± 0.19b (x) | |
| Hot water (65 °C) | 0 | 67.18 ± 0.35b (x) | 4.20 ± 0.17a (x) | 21.12 ± 0.09b (y) |
| 7 | 63.09 ± 1.14b (y) | 4.11 ± 0.75a (x) | 22.66 ± 0.21b (x) | |
| 14 | 48.47 ± 0.14d (z) | 2.18 ± 0.18d (y) | 22.21 ± 0.31b (x) | |
| Ultrasound (15 min) | 0 | 69.34 ± 0.16a (x) | 4.33 ± 0.83a (x) | 27.13 ± 0.15a (y) |
| 7 | 67.36 ± 1.32a (y) | 3.57 ± 0.55b (y) | 27.56 ± 0.18a (y) | |
| 14 | 65.15 ± 0.44a (z) | 2.16 ± 0.29d (z) | 28.13 ± 0.16a (x) |
a−e Treatment means showed the effect of different treatments for the same day are not significantly differet (p < 0.05)
x–z Tretment means showed the effect of storage times for the same treatment are not signifiantly different (p < 0.05)
Effect of storage on polyphenol oxidase activity
Different chemical preservatives (AA & CC), hot water and HIU were applied for browning reduction on fresh-cut mango fruit pieces. The PPO activity of mango fruit slices during fourteen days under refrigeration conditions is shown in Table 2. The PPO activities of mangoes exposed to HIU were changing from 76 to 134 U/mL during 14 days, and that was from 311 to 595 U/mL for untreated mango fruit slices. The PPO activities of all treatments were remarkably increased over the storage, specifically in untreated mango fruit samples. While PPO activity of mango fruit slices was low on the first day, these were higher on days seven and fourteen. The highest PPO activities were detected on the last day of stored mango fruit slices. Chemical preservatives and submerging mango fruit samples in water at 65 °C was significantly deceased (p < 0.05) the PPO activities of mangoes with the comparison of untreated mango fruit samples. Noticeably, mango fruit samples exposed to an ultrasonic treatment displayed the lowest PPO activity while compared with the other mango samples, which is the sign of the least browning. This is promoted by the colour findings (Table1). Mango fruit samples exposed to HIU showed less browning activity by showing a higher lightness and lower yellowness values as compared to untreated and other processed mango fruit slices. Diffrent application on food products such as cutting causes the removal of fruit skins and this is the reason of water loss & deterioration. It is the reason of rising in the respiration ratio and ethlyene production as well (Tapiai et al. 2015). Furthermore, the reaction between phenol and polyphenol oxidize causes browning discoloration (Yadav and Sinhgh 2014).
Table 2.
Changes of PPO activity in fresh-cut mango slices at 4 °C
| Treatments | Storage (days) | PPO activity (U/mL) at 4 °C |
|---|---|---|
| Control | 0 | 311.13 ± 1.12a (z) |
| 7 | 412.11 ± 1.15a (y) | |
| 14 | 595.12 ± 3.12a (x) | |
| Ascorbic acid (3%) | 0 | 216.12 ± 2.08c (z) |
| 7 | 311.44 ± 1.13c (y) | |
| 14 | 439.15 ± 1.25c (x) | |
| Calcium chloride (3%) | 0 | 283.36 ± 2.13b (z) |
| 7 | 373.26 ± 4.11b (y) | |
| 14 | 489.27 ± 1.13b (x) | |
| AA (1%)—CC (2%) | 0 | 201.84 ± 3.12c (z) |
| 7 | 302.72 ± 2.15c (y) | |
| 14 | 427.44 ± 1.91c (x) | |
| AA (1.5%)—CC (1.5%) | 0 | 205.77 ± 2.13c (z) |
| 7 | 288.31 ± 0.99d (y) | |
| 14 | 378.18 ± 1.15d (x) | |
| AA (2%)—CC (1%) | 0 | 156.43 ± 1.57d (z) |
| 7 | 219.25 ± 1.43e (y) | |
| 14 | 364.73 ± 1.23d (x) | |
| Hot water (65 °C) | 0 | 222.19 ± 0.54bc (z) |
| 7 | 307.66 ± 1.19c (y) | |
| 14 | 442.33 ± 2.05c (x) | |
| Ultrasound (15 min) | 0 | 76.17 ± 0.95e (z) |
| 7 | 101.35 ± 1.15f (y) | |
| 14 | 134.12 ± 1.99e (x) |
a−f Treatment means showed the effects of different treatment for the same day are not significantly different (p < 0.05)
x–z Treatment means showed the effect of storage times for the same treatment are not significantly different (p < 0.05)
Non-thermal techniques are a relevant challenge in the enzymatic browning of fruits. The most used methods are high hdyrostatic pressure processing, HIU, iradiation, and pulsed electric field (PEF) (Roobab et al. 2018). If we look at the effect of non-thermal techniques on browning, we can say that these are effective against enzyme inactivation. For example, Yamaguch et al. (2010) found that high-hydrostatic pressure (HHP) lowers the PPO activity (37%) in ginger plant. In another study, Schilling et al. (2008) achieved the total inhibition of browning enzymes in apple fruit samples by using pulsed electric field treatment at 60 °C. In present work, a relatively less studied method of HIU was examined in comprehensively rather than other non-thermal methods. The cavitation developed by ultrasonic waves is an important cause for the progress of microscopic channels in the fruit samples that promotes moisture removal (Yildiz et al. 2019). In addition, it might be helpful to get rid of the moisture that is firmly attached to the solid component which is exposed to ultrasonic waves. Deformation of porous solid components created by ultrasonic waves also decreases the diffusion boundary layer and increase the convective mass transfer in the food samples because of the development of microscopic chanels (Yao 2016).
Effect of storage on bioactive components of fresh cut mangoes
The ascorbic acid (vitamin C) changes of mango fruit slices for fourteen days of storage under refrigeration conditions are displayed in Table 3. A significant decrease for vitamin C was determined in all processed mango slices during storage. After 2 weeks of storage, the vitamin C content of mango fruit slices exposed to HIU was found significantly higher (p < 0.05) than untreated mangoes samples. A similar reduction in vitamin C content of mango fruit slices at the time of storage was reported by Kumar et al. (2019). During storage, this decline related to ascorbic acid content is prevalent in whole and/or minimally treated fruits & vegetables, and this is basically as a consequence of their engagement in oxidative reactions (De Sousa et al. 2017). Moreover, removal of O2 during cavitation might be the primary reason of vitamin C destruction (Yildiz et al. 2020). Vitamin C restricts the enzymatic browning of fruits with the help of inhibition of catalytic site of the PPO. Additionally, vitamin C may reduce the quinones produced by polyphenol oxidize to their initial structure by limiting the darkening (Reucuk et al. 2009). Accordingly, a decline in the inhibition stage was sustained via vitamin C in the site of the PPO (higher PPO activitiy, Table2) and the ability to make diphenols from mango fruit samples were likely blocked by a decrease in vitamin C of the treatments, which cause darkening of the mango fruit slices (lowest lightness value, Table1) over the storage time. The results were supported by the Pearson corelation coeeficient test where the adverse linkage between vitamin C & polyphenol oxidize (Table2) and positive correlation between vitamin C and the lightness values (Table1).
Table 3.
The effect of AA, AOC, and TPC in fresh-cut mango slices at 4 °C
| Treatment | Storage (day) | AA (mg/100 g) | AOC (µmol TE/g) | TPC (mg GA/100 g) |
|---|---|---|---|---|
| Control | 0 | 28.45 ± 0.16d (z) | 2.15 ± 0.15a (z) | 17.14 ± 0.88c (z) |
| 7 | 22.51 ± 0.13d (y) | 3.18 ± 0.42d (y) | 18.25 ± 0.23d (y) | |
| 14 | 16.21 ± 1.09e (x) | 5.43 ± 0.31b (x) | 19.19 ± 0.13d (x) | |
| AA (3%) | 0 | 30.15 ± 0.44c(z) | 2.25 ± 0.19b (z) | 19.34 ± 0.25bc(z) |
| 7 | 25.18 ± 0.76c (y) | 3.33 ± 0.59d (y) | 20.15 ± 0.18c (y) | |
| 14 | 19.33 ± 0.35d (x) | 4.17 ± 0.64c (x) | 21.93 ± 0.16c (x) | |
| CC (3%) | 0 | 31.18 ± 1.12bc (z) | 2.13 ± 0.32b (z) | 19.15 ± 0.26bc(z) |
| 7 | 24.19 ± 0.88c (y) | 3.45 ± 0.77d (y) | 20.37 ± 0.22c (y) | |
| 14 | 19.35 ± 0.13d (x) | 4.36 ± 0.83c (x) | 21.85 ± 0.35c (x) | |
| AA (1%) + CC (2%) | 0 | 32.47 ± 1.07b (z) | 3.37 ± 0.25b (y) | 19.33 ± 0.20bc(z) |
| 7 | 24.56 ± 0.07c (y) | 3.22 ± 0.20d (y) | 20.72 ± 0.54c (y) | |
| 14 | 21.71 ± 0.83c (x) | 4.13 ± 0.49c (x) | 21.15 ± 0.67c (x) | |
| AA (1.5%) + CC (1.5%) | 0 | 31.65 ± 0.44bc (z) | 2.45 ± 0.76b (y) | 20.13 ± 0.72b (y) |
| 7 | 25.19 ± 0.45c (y) | 4.18 ± 0.63c (x) | 20.62 ± 0.86c (y) | |
| 14 | 21.58 ± 0.87c (x) | 4.53 ± 0.16c (x) | 23.19 ± 0.14b (x) | |
| AA (2%) + CC (1%) | 0 | 32.14 ± 0.98b (z) | 3.33 ± 0.54c (y) | 19.14 ± 0.23bc(z) |
| 7 | 28.28 ± 0.27b (y) | 5.35 ± 0.13b (x) | 22.33 ± 0.11b (y) | |
| 14 | 24.88 ± 0.12b (x) | 5.16 ± 0.77b (x) | 23.45 ± 0.25b (x) | |
| Hot water (65 °C) | 0 | 30.19 ± 0.55c (z) | 2.79 ± 0.14b (z) | 20.64 ± 0.35b (y) |
| 7 | 25.29 ± 1.15c (y) | 4.37 ± 0.23c (y) | 20.29 ± 0.48c (y) | |
| 14 | 19.25 ± 0.43d (x) | 5.35 ± 0.44b (x) | 23.73 ± 0.73b (x) | |
| Ultrasound (15 min) | 0 | 36.42 ± 0.12a (z) | 4.44 ± 0.13d (y) | 24.76 ± 0.68a (z) |
| 7 | 32.18 ± 0.65a (y) | 6.42 ± 0.85a (x) | 27.27 ± 0.63a (y) | |
| 14 | 27.44 ± 0.77a (x) | 6.15 ± 0.93a (x) | 29.13 ± 0.42a (x) |
a−e Treatment means showed the effect of different treatments for the same day are not signifcantly diferent (p < 0.05)
x–z Treatment means showed the effect of storage times for the same treatment are not signifiIcantly diffrent (p < 0.05)
The impact of TPC on untreated and treated mango fruit samples is shown in Table 3. Among all treatments, HIU treatment shown the highest phenolic compounds all along with the storage. Similar findings were stated in the study of Bhat et al. (2011) and an increase in TPC in pear fruit pieces right after HIU treatment was pointed out (Cruz-Cansion et al. 2015). This difference could be related to the release of bound form of phenolic ingredient by cause of the deformation of cell wall via ultrasonic cavitation (Yıldız 2019). AOC of untreated and processed mango fruit slices are shown in Table 3. AOC was significantly increased (p < 0.05) with the storage time in mango fruit samples for all treatments. Mango fruit slices exposed to HIU had the highest AOC among all treatments (Table3). Zafra-Roja et al. (2013) investigated that HIU treated fruit slices led to higher AOC. So, this increment in AOC may be attributed to the enhanced extraction of antioxidant substances such as vitamin C and phenolic metabolites that could be as a result of the mechanical impact of cavitation and bubble implosions at the time of HIU treatment (Abid et al. 2015). This could approved the results that there is a positive correlation between AOC and total phenolic compounds of mango fruit samples (Table 3). The association of phenolic compound in the AOC of food products has been focused in other researches with the inclusion of processed mango fruit samples (Gonzalez-Aguilar et al. 2007).
Microbial activity
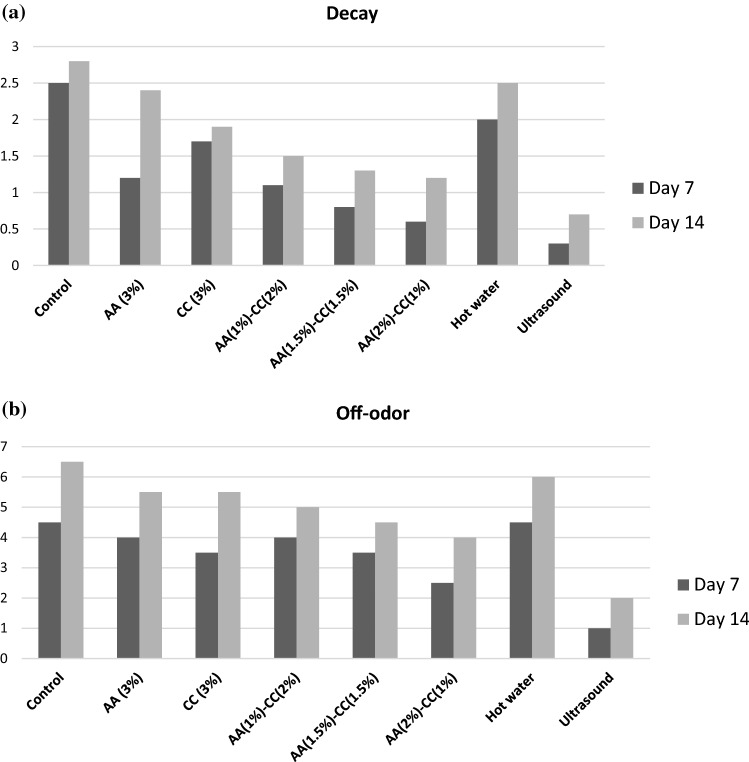
An increase in the numbers of total bacteria, mold and yeast in fresh-cut mango fruit samples were observed during the storage in Table4. In cold storage, the numbers of microorganism in mango fruit pieces were significantly decreased (p < 0.05) by HIU when compared with the other treated and non-treated mango fruit samples (Table 4). Moreover, HIU application efficiently reduced the spoilage arise from microorganisms on fresh-cut mango fruit samples. It is well- known that HIU treatment develops cytolytic effects which is very efficient for the microorganism inactivation, however the cell breakdown degree depended on the experimental circumstances and the type of microorganism (Roobab et al. 2018). The findings indicated that the lower decay value (Fig. 2) in mango fruit samples exposed to HIU might be linked to inhibited microbial populations.
Table 4.
The numbers of aerobic microorganisms in fresh-cut mango slices at 4 °C
| Treatments | Storage (days) | Bacteria (Log10 CFU g−1) | Mold and yeast (Log10 CFU g−1) |
|---|---|---|---|
| Control | 0 | 2.94 ± 0.04a (z) | 2.48 ± 0.06a (z) |
| 7 | 3.40 ± 0.08a (y) | 3.22 ± 0.04a (y) | |
| 14 | 3.85 ± 0.05a (x) | 3.89 ± 0.03a (x) | |
| AA (3%) | 0 | 2.76 ± 0.02b (z) | 2.42 ± 0.04a (z) |
| 7 | 3.34 ± 0.05a (y) | 3.15 ± 0.02a (y) | |
| 14 | 3.72 ± 0.01b (x) | 3.80 ± 0.06b (x) | |
| CC (3%) | 0 | 2.77 ± 0.01b (z) | 2.43 ± 0.03a(z) |
| 7 | 3.28 ± 0.03ab (y) | 3.18 ± 0.04a (y) | |
| 14 | 3.75 ± 0.04b (x) | 3.81 ± 0.02b (x) | |
| AA (1%) + CC (2%) | 0 | 2.64 ± 0.04c (z) | 2.28 ± 0.05b (z) |
| 7 | 3.28 ± 0.06ab (y) | 3.07 ± 0.08b (y) | |
| 14 | 3.65 ± 0.02bc (x) | 3.60 ± 0.01c (x) | |
| AA (1.5%) + CC (1.5%) | 0 | 2.48 ± 0.04d (z) | 2.02 ± 0.05cb (z) |
| 7 | 3.13 ± 0.07c (y) | 2.93 ± 0.05c (y) | |
| 14 | 3.62 ± 0.04c (x) | 3.47 ± 0.03d (x) | |
| AA (2%) + CC (1%) | 0 | 2.33 ± 0.03e (z) | 2.01 ± 0.04c (z) |
| 7 | 2.97 ± 0.05d (y) | 2.80 ± 0.02d (y) | |
| 14 | 3.42 ± 0.02d (x) | 3.40 ± 0.06e (x) | |
| Hot water (65 °C) | 0 | 2.85 ± 0.04b (z) | 2.45 ± 0.04a (z) |
| 7 | 3.40 ± 0.02a (y) | 3.10 ± 0.05b (y) | |
| 14 | 3.75 ± 0.07b (x) | 3.87 ± 0.07a (x) | |
| Ultrasound (15 min) | 0 | 1.25 ± 0.06f (z) | 1.27 ± 0.02d (z) |
| 7 | 2.21 ± 0.08e (y) | 2.20 ± 0.04e (y) | |
| 14 | 2.65 ± 0.03e (x) | 2.72 ± 0.04f (x) |
a−f Treatment means showed the effect of different treatments for the same day are not significantly different (p < 0.05)
x–z Treatment means showed the effect of storage times for the same treatment are not significantly different (p < 0.05)
Fig. 2.
a Decay of fresh-cut mango slices with different treatments, after being stored for 7 and 14 days at 4 °C. b Off-odor of fresh-cut mango slices with different treatments, after being stored for 7 and 14 days at 4 °C
Visual Appearance
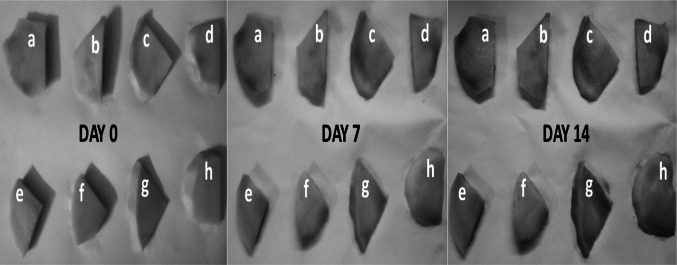
The visional display of fresh-cut mango specimens with various methods during fourteen days of storage at refrigeration conditions are shown in Fig. 1. All mango fruit slices exposed to antibrowning agents and hot water as well as control samples exhibited a darker colour as compared to HIU treatment (Fig. 1). The image displays a fine correlation with the Hunter lightness readings where fresh-cut mango fruit pieces exposed to HIU treatment exhibited the highest lightness readings between the applications (Table1). All mango slices displayed colour degradation starting from first day tol ast day. In particular, mango fruit pieces on day 14 displayed a significant colour degradation, specifically in the untreated mango fruit pieces (Fig. 1a). Appearance has a considerable function for the development of consumers’ preferences and subsequent approval of a food product (Wu et al. 2015). It can be anticipated that people would be in favor of shopping for fresh-cut mango fruits exposed to high intensity ultrasound by considering that they have desirable colour and less browning.
Fig. 1.
Appearance of mango slices with different treatments for a 14-day period at 4 °C (a no treatment; b CC (3%); c AA (3%); d AA (1%)—CC (2%); e AA (1.5%)—CC (1.5%); f AA (2%)—CC (1%); g hot water at 65 °C; h ultrasound)
Sensory evaluation
Figure 2 exhibits scores of decay degrees on the fresh-cut mango fruit slices employed with antibrowning agents, hot water and HIU treatments. The HIU treatment shown the most effective treatment in reducing browning and decay of fresh-cut mango slices (Fig. 2). Alternative applications such as hot water and antibrowning compounds were slightly effective fort he reduction of browning and decay symptoms with the comporison of untreated mango slices. At the end of 2 weeks storage, mango fruit slices exposed to HIU exhibited less decay symptoms. On the other hand, medium to severe decay problems were inspected in other treatments and untreated mango fruit specimens. At day 1, no decay was noticed for all applications (untreated, antibrowning agents, hot water, and HIU treatments). The decay evaluation of all 3 treatments on the day 14 was higher as compared to day 7. Khademi, Ashtari, & Razavi (2019) stated that the banana fruit pieces exposed to HIU displayed a less chilling injury severity along with improved firmness, total phenolic content and antioxidant activity.
On the other hand, Fig. 2b displays scores of off-odor assessment on the fresh-cut mango slices employed with antibrowning agents, hot water and HIU treatments. At day 1, no off-odor was observed in all mango samples. On the other hand, an off-odor was identified on all mango samples on day 14 with the exception of HIU treated mangoes. While, the lowest scores for decay, browning and off-odor were stated in mango fruit slices exposed to HIU treatments.
Conclusions
Even though HIU was proved to be more effective to inactivate microorganisms rather than enzyme inactivation, this work demonstrated that HIU treatment is a promising alternative of heat treatments for the inactivation of browning enzymes. Overall, compared to the chemical (ascorbic acid and calcium chloride agents) and physical (submerging in hot water) applications, HIU was reported to be the most effective technique for the prevention of browning in fresh-cut mangoes. Aside from the inactivation of the enzyme, HIU exhibited improved performance on the prevention of colour degradation of mango fruit pieces. Fresh-cut mango fruit samples exposed to HIU application showed improved bioactive content and sensory properties. HIU treatment might be utilized to prevent browning, decay, and deterioration of fresh-cut mango fruit samples. This study will be beneficial for the formation of processed mango fruit with a prolonged shelf life and higher customer acceptability supported by preserving the colour and inhibiting the enzymatic browning.
Author's contribution
GY: Conceptualization, Methodology, Data curation, Writing; RMA: Writing—review & editing.
Compliance with ethical standards
Conflicts of interest
There is no conflict of interest to report for the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abid M, Jabbar S, Wu T, Hashim MM, Hu B, Saeeduddin M, Zeng X. Qualitative Assessment of Sonicated Apple Juice during Storage. J Food Process Pres. 2015;39:1299–1308. doi: 10.1111/jfpp.12348. [DOI] [Google Scholar]
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Bhat R, Kamaruddin NS, Min-Tze L, Karim AA. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason Sonochem. 2011;18:1295–1300. doi: 10.1016/j.ultsonch.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Cao SF, Hu ZC, Pang B, Wang HO, Xie HX, Wu F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control. 2010;21(4):529–532. doi: 10.1016/j.foodcont.2009.08.002. [DOI] [Google Scholar]
- Cemeroglu B (2007) Gıda Analizleri. Gıda Teknolojisi Dernegi Yayınları, No: 34, Bizim Büro Basımevi. Ankara, 535
- Cruz-Cansino NS, Ramírez-Moreno E, León-Rivera J, Delgado- Olivares L, Alanís-García E, Ariza-Ortega JA et al (2015) Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason Sonochem 27: 277–286. [DOI] [PubMed]
- De De Sousa AED, Fonseca KS, da Silva Gomes WK, da Silva APM, de Oliveira Silva E, Puschmann R (2017) Control of browning of minimally processed mangoes subjected to ultraviolet radiation pulses. J Food Sci Technol 54:253–259.10.1007/s13197-016-2457-8 [DOI] [PMC free article] [PubMed]
- Gonzalez-Aguilar GA, Villegas-Ochoa MA, Martı´nez-Te´llez MA, Gardea AA, Ayala-Zavala JF, Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J Food Sci. 2007;72:197–202. doi: 10.1111/j.1750-3841.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- Igual M, García-Martínez E, Martín-Esparza ME, Martínez-Navarrete N. Effect of processing on the drying kinetics and functional value of dried apricot. Food Res Int. 2012;47:284–290. doi: 10.1016/j.foodres.2011.07.019. [DOI] [Google Scholar]
- Izli G, Izli N, Taskin O, Yildiz G. Convective drying of kumquat slices: Comparison of different drying temperatures on drying kinetics, colour, total phenolic content and antioxidant capacity. Lat Am Appl Res. 2018;48:37–42. [Google Scholar]
- Khademi O, Ashtari M, Razavi F (2019) Effects of salicylic acid and ultrasound treatments on chilling injury control and quality preservation in banana fruit during cold storage. Sci Hortic 249: 334–339.
- Kumar R, Vijayalakshmi S, Rajeshwara R, Sunny K, Nadanasaapathi S. Effect of storage on thermal, pulsed electric field and combination processed mango nectar. J Food Meas Charact. 2019;13:131–143. doi: 10.1007/s11694-018-9926-x. [DOI] [Google Scholar]
- Montgomery MW, Sgarbieri VC. Isoenzymes of Banana Polyphenol Oxidase. Phytochem. 1975;14:1245–1249. doi: 10.1016/S0031-9422(00)98602-3. [DOI] [Google Scholar]
- Moon KM, Kwon EB, Lee B, Kim CY. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules. 2020;25(12):2754. doi: 10.3390/molecules25122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnik P, Pavlić B, Šojić B, Zavadlav S, Žuntar I, Kao L, Kitonić D, Kovačević DB. Innovative Hurdle Technologies for the Preservation of Functional Fruit Juices. Foods. 2020;9:699. doi: 10.3390/foods9060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. 2. New Delhi, India: Tata Mc Graw Hill Publishing Co.; 1986. [Google Scholar]
- Reuck K, Sivakumar D, Korsten L. Integrated application of 1-methylcyclopropene and modified atmosphere packaging to improve quality retention of litchi cultivars during storage. Postharvest Biol Tec. 2009;52:71–77. doi: 10.1016/j.postharvbio.2008.09.013. [DOI] [Google Scholar]
- Roobab U, Aadil RM, Madni GM, Bekhit AED. The impact of nonthermal technologies on the microbiological quality of juices: a review. Compr Rev Food Sci. 2018;17(2):437–457. doi: 10.1111/1541-4337.12336. [DOI] [PubMed] [Google Scholar]
- Salinas-Roca B, Soliva-Fortuny R, Welti-Chanes J, Martín-Belloso O. Combined effect of pulsed light, edible coating and malic acid dipping to improve fresh-cut mango safety and quality. Food Control. 2016;66:190–197. doi: 10.1016/j.foodcont.2016.02.005. [DOI] [Google Scholar]
- Schilling S, Schmid S, Jaeger H, Ludwig M, Dietrich H, Toepfl S, Knorr D, Neidhart S, Schieber A, Carlet R. Comparative study of pulsed electric field and thermal processing of apple juice with particular consideration of juice quality and enzyme deactivation. J Agric Food Chem. 2008;56:4545–4554. doi: 10.1021/jf0732713. [DOI] [PubMed] [Google Scholar]
- Tapia MR, Gutierrez-Pacheco MM, Vazquez-Armenta FJ, Gonzlez-Aguilar GA, Ayala Zavala JF, Rahman MSH, Siddiqui MW, (2015) Washing, peeling and cutting of fresh-cut fruits and vegetables. In: Siddiqui MW, Rahman MS (eds) Minimally processed foods. Springer, Switzerland, pp 57–58
- Wang Y, Zhang M, Mujumdar AS. Trends in processing technologies for dried aquatic products. Dry Technol. 2011;29:382–394. doi: 10.1080/07373937.2011.551624. [DOI] [Google Scholar]
- Wang J, Liu Q, Xie B, Sun Z. Effect of ultrasound combined with ultraviolet treatment on microbial inactivation and quality properties of mango juice. Ultrason Sonochem. 2020;64:105000. doi: 10.1016/j.ultsonch.2020.105000. [DOI] [PubMed] [Google Scholar]
- Wu TY, Yen H, Lee GA. The effect of product appearances on consumer emotions and behaviors: a perspective of involvement. J Ind Prod Eng. 2015;32:486–499. doi: 10.1080/21681015.2015.1077352. [DOI] [Google Scholar]
- Yadav AK, Singh SV. Osmotic dehydration of fruits and vegetables: a review. J Food Sci Technol. 2014;51:1654–1673. doi: 10.1007/s13197-012-0659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Kato T, Noma S, Igura N, Shimoda M. The Effects of High Hydrostatic Pressure Treatment on the Flavor and Color of Grated Ginger. Biosci Biotech Bioch. 2010;74:1981–1986. doi: 10.1271/bbb.90712. [DOI] [PubMed] [Google Scholar]
- Yao Y. Enhancement of mass transfer by ultrasound: Application to adsorbent regeneration and food drying/dehydration. Ultrason Sonochem. 2016;31:512–531. doi: 10.1016/j.ultsonch.2016.01.039. [DOI] [PubMed] [Google Scholar]
- Yildiz G (2018) The Effect of Different Chemical Agents on the Prevention of Enzymatic Browning in Banana. J Food Sci Eng 7: 91–96. 10.17265/2159-5828/2018.02.005
- Yıldız G. Control of enzymatic browning in potato with calcium chloride and ascorbic acid coatings. Food and Health. 2019;5:121–127. doi: 10.3390/foods9060699. [DOI] [Google Scholar]
- Yildiz G, Palma S, Feng H. Ultrasonic cutting as a new method to produce fresh-cut red delicious and golden delicious apples. J Food Sci. 2019;84(12):3391–3398. doi: 10.1111/1750-3841.14798. [DOI] [PubMed] [Google Scholar]
- Yildiz G, Izli G, Aadil RM (2020) Comparison of chemical, physical, and ultrasound treatments on the shelf life of fresh‐cut quince fruit (Cydonia oblonga Mill.). J Food Process Preserv 44(3):e14366
- Zafra-Rojas QY, Cruz-Cansino N, Ramírez-Moreno E, Delgado- Olivares L, Villanueva-Sánchez J, Alanís-García E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason Sonochem. 2013;20:1283–1288. doi: 10.1016/j.ultsonch.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Zhang LK, Lu ZX, Yu ZF, Gao X. Preservation of fresh-cut celery by treatment of ozonated water. Food Control. 2005;16:279–283. doi: 10.1016/j.foodcont.2004.03.007. [DOI] [Google Scholar]