Abstract
Tannin acyl hydrolase referred commonly as tannase catalyzes the hydrolysis of the galloyl ester bond of tannin to release gallic acid. The tannase TanBLp which cloned from Lactobacillus plantarum ATCC14917T has high activity in the pH range (7.0–9.0) at 40 °C, it would be detrimental to the utilization at acidic environment. The catalytic sites and stability of TanBLp were analyzed using bioinformatics and site-specific mutagenesis. The results reiterated that the amino acid residues Ala164, Lys343, Glu357, Asp421 and His451 had played an important role in maintaining the activity. The optimum pH of mutants V75A, G77A, N94A, A164S and F243A were shifted from 8.0 to 6.0, and mutant V75A has the highest pH stability and activity at acidic conditions than other mutants, which was more suitable for industrial application to manufacture gallic acid. This study was of great significance to promote the industrialization and efficient utilization of tannase TanBLp.
Keywords: Tannase, TanBLp, Acid resistance, Catalytic activity, Site-specific mutagenesis
Introduction
Gallnut (Rhus chinensis Mill.) is one of the important insect resource products in China. Its main components include various natural medicine ingredients such as Chinese gallotannin and gallic acid, which are widely used in the fields of biology, medicine, chemical and food industry [1, 2]. The plant extraction and deep processing of gallnut as a raw material has become a characteristic industry with resource advantages in the western area of Hunan province. At present, the industrial production of gallic acid from gallnut mainly adopted chemical hydrolysis [3], which are not only complicated and highly corrosive, but also produces a large amount of refractory industrial waste water. Therefore, creating an efficient and stable tannase and establishing an enzymatic-based green process for gallic acid production from Chinese gallotannin is the best way to exploit the resources of gallnut [4–6].
Tannases (Tannin acyl hydrolase E.C.3.1.1.20) are a family of serine esterase A which have the activities of depsidase and esterase, and can hydrolyze the depside bond and ester bond [7]. Tannases can be produced by plants, animals and microorganisms [8–10]. Tannases derived from microorganisms were widely used in industry because of the advantages of biochemical diversity and simplification of gene editing [11, 12]. The gene sequences of bacteria, yeast and fungi tannases were quite different from each other, while the amino acid sequence analysis showed that an active-site motif of Gly-X-Ser-X-Gly was pervasive among them[13, 14]. However, the fungal tannases have a polymer composition and high degree of glycosylation, the clone of tannase genes from fungi were difficult and inefficient, which are limiting the industrial application of tannases from eukaryotes [15, 16]. The recombinant tannases, which are easy to be purified from bacteria, have gradually become the focus of tannase research, and the cloning and expression of tannases from Lactobacillus plantarum (Generally recognized as safe, GRAS/FDA) have been extensively studied for the sake of biological safety [17–20].
Tannase TanBLp (AB379685.1, formerly TanLpl) forming a distinct cluster from TanA, TanASg, and TanAAp [21, 22], was amplified from Lactobacillus plantarum ATCC14917T. Molecular weight of TanBLp was 50.7 KDa, TanBLp displayed an α/β structure with eighteen α-helices and thirteen β-strands, and formed a large cap domain inserted into the classical serine hydrolase fold. TanBLp can function as a monomer, a catalytic triad was composed of Ser163, Asp419 and His451. In the structure of TanBLp, the location of the middle of a surface groove formed at the junction of the hydrolase fold and the inserted cap domain was the binding site of the catalytic triad and substrate. During the binding of gallic acid, the carboxyl group makes interaction with the catalytic triad of TanBLp to form a usual hydrogen bonding structure, while the other three hydroxyl groups make contact with Lys343, Glu357, and Asp421 to form another hydrogen-bonding network. TanBLp was most active in the pH range 7.0–9.0 at 40 °C, while with the production of tannic acid, the fermentation broth gradually becomes acidic, which will be detrimental to the utilization of TanBLp [17, 23, 24]. Besides, the active sites affecting pH stability of TanBLp were still unclear. In order to obtain higher pH stability mutants of TanBLp in acidic environment and promote the industrial application of tannase. Computer-aided design methods including homology modeling and molecular docking were used for catalytic sites prediction of TanBLp, combined with site-specific mutagenesis and enzymatic properties analysis were used for analyzing the relationship between active sites and acid resistance of tannase TanBLp in this study.
Materials and Methods
Acquisition of the Tannase Gene TanBLp
The tannase gene TanBLp (AB379685.1) was synthesized and cloned into pET30a vector, and then transformed into E. coli BL21 by Changsha Youbao Biotechnology Co., Ltd.
Prediction of the Active Sites in Tannase TanBLp
The docking of substrate propyl gallate (PG) with tannase TanBLp 3D Model (PDB ID: 4J0K) were using induced fit docking in Schrodinger and displayed by PyMOL [25].
Extraction, Purification and Enzyme Activity Assay of Tannase
Escherichia coli BL21 strains carrying the recombinant plasmid were inoculated in LB broth containing 100 μg/mL kanamycins and 0.05 mmol/L IPTG with 2% amount of inoculation, incubated at 15 °C for 10 h, then the crude enzyme was extracted by ultrasonic. The crude enzyme solution was filtered by 0.22 μm membrane and purified using His-labeled protein purification kit (Beijing Kangwei Century Biotechnology Co., Ltd) [26, 27]. The enzyme activity was calculated from the change in absorbance of gallic acid at 520 nm, i.e. ΔA520 = (Atest − Ack) − (Acontrol − Ack). One unit of activity was defined as the amount of enzyme which liberated 1 mmol of gallic acid from methyl gallate formed per min [28].
Site-Directed Mutagenesis of Tannase TanBLp
Based on the bioinformatics prediction results of TanBLp, site-directed mutagenesis was performed in the catalytic domain of tannase TanBLp. The PCR site-directed mutagenesis of plasmid DNA was carried out according to the method established by Miyazaki and Takenouchi [29]. The PCR primers, which were shown in Table 1, were designed using DNAMAN software and synthesized by GENEWIZ.lnc. The mutants’ plasmid library established by site-directed mutagenesis methods was transformed into E. Coli BL21 for future study.
Table 1.
PCR primer sequence for the site-specific mutagenesis of tannase TanBLp
| Primer name | Primer sequence |
|---|---|
| V75A-R/F |
5′-CTGGCAAATAACCGCCGGCCGTATTCGGCATCAG-3′ 5′-CTGATGCCGAATACGGCCGGCGGTTATTTGCCAG-3′ |
| G77A-R/F |
5′-CGGTCCTGGCAAATAAGCGCCGACCGTATTCGG-3′ 5′-CCGAATACGGTCGGCGCTTATTTGCCAGGACCG-3′ |
| N94A-R/F |
5′-TTGAATCGTCCCTGCAGCCGTCGGCCAAGTGACAC-3′ 5′-GTGTCACTTGGCCGACGGCTGCAGGGACGATTCAA-3′ |
| A164X-R/F |
5′-GCCGAAGTGGCACCCCCNNNACTCGTTCCATTCGTG-3′ 5′-CACGAATGGAACGAGTNNNGGGGGTGCCACTTCGGC-3′ |
| F243A-R/F |
5′-GACCACTAACCGGTTCAGCTTTTGGTCGCCCATT-3′ 5′-AATGGGCGACCAAAAGCTGAACCGGTTAGTGGTC-3′ |
| K343A-R/F |
5′-CAAACGCCGGGACGGCTGCCATGCGAGTTAACGA-3′ 5′-TCGTTAACTCGCATGGCAGCCGTCCCGGCGTTTG-3′ |
| E357A-R/F |
5′-CGCCAAATAAATTATTCGCTGGACTCGTCAAATC-3′ 5′-GATTTGACGAGTCCAGCGAATAATTTATTTGGCG-3′ |
| D421A-R/F |
5′-GATTGCAAAACTCGTAGCTCGGTCGGCCGCACC-3′ 5′-GGTGCGGCCGACCGAGCTACGAGTTTTGCAATC-3′ |
| H451A-R/F |
5′-ATCATAGTCACCACTGGCGGGAATATCCCACGG-3′ 5′-CCGTGGGATATTCCCGCCAGTGGTGACTATGAT-3′ |
The Acid Resistance of the Recombinant Mutants of Tannase TanBLp
The optimum pH was investigated according to the spectrophotometric method described above [28] in 20 mL citric acid-sodium citrate buffer (pH 4.0, 5.0, and 6.0), 20 mL Tris–HCl buffer (pH 7.0, 8.0, and 9.0), and 20 mL sodium bicarbonate-sodium hydroxide buffer (pH 10.0 and 11.0) at 40 °C for 10 min with 10 μL propyl gallate (10 mmol/L) and 10 μL diluted crude enzyme solution. For acid resistance analysis, samples in buffer solution (pH 4.0–11.0) were incubated at 40 °C for 24 h, and then immediately cooled on ice for 30 min. The treated samples were further diluted tenfold with citric acid-sodium citrate buffer (pH 6.0) and the residual activities were measured. On calculation of the Michaelis–Menten constant (Km), the mutants’ activity was measured under their own optimum pH conditions using methyl gallate as a substrate at different concentrations.
Results and Discussion
The Prediction Results of Catalytic Sites in Tannase TanBLp
The tannase TanBLp (PDB ID:4J0K) and propyl gallate (PG) were selected as acceptor and ligand, respectively. The optimal conformation between the ligand and acceptor were obtained using Schrodinger, and the three-dimensional structure model was displayed by PyMOL (Fig. 1a). The results showed that the docking score of 4J0K and PG was − 5.868. The binding site of substrate (propyl gallate) to tannase TanBLp (PDB ID: 4J0K) was a pocket structure, which was existed in the hollow tunnel of tannase, and the propyl gallate could fully incorporated into the binding pocket of TanBLp, the space complementarity of propyl gallate and TanBLp was high. The relative forces between the amino acid residues N73, V75, N94, R116, T162, R228, Q230, P231, F243, D421, H451, S452 and the propyl gallate were larger than others. Amino acid residues K343, E357 and D421 act on three phenolic hydroxyl groups of propyl gallate and amino acid residues G77 and A164 act on the carbonyl group of propyl gallate, which were consistent with Matoba et al. [17] (Fig. 1b). These analyses of the catalytic sites of tannase TanBLp had great guiding significance for the selection of mutation sites.
Fig. 1.
Binding model of tannase TanBLp and substate PG. a Docking model of tannase TanBLp and substate PG; b Schematic diagram of the binding site for tannase TanBLp and substrate PG
Construction and Screening of the Mutants of Tannase TanBLp
The mutation of TanBLp was carried out using site-specific mutagenesis technology basing on the results of previous bioinformatics predictions, the results showed that the sequences of the mutants were consistent with the predicted mutation sites, and no other mutations were occurred. The enzymatic activities of mutants V75A, G77A, N94A, F243A and A164S were 97%, 84%, 96.6%, 85.3% and 15.3% of that of the original tannase TanBLp, respectively (Fig. 2). The mutants K343A, E357A, D421A, H451A, A164P, A164T, A164G, A164R, A164D, A164V and A164E were all completely lost the tannase activity. Previous study has shown that the mutants S163A, K343A, E357A, D419A, D421A and H451A almostly lead to inactivation of tannase TanBLp [24], Ala164 that form the oxyanion hole was correlated with the stability of a tetrahedral intermediate [17], the results of our study reiterated that amino acid residues Ala164, Lys343, Glu357, Asp421 and His451 had played an important role in maintain the activity of tannase TanBLp.
Fig. 2.
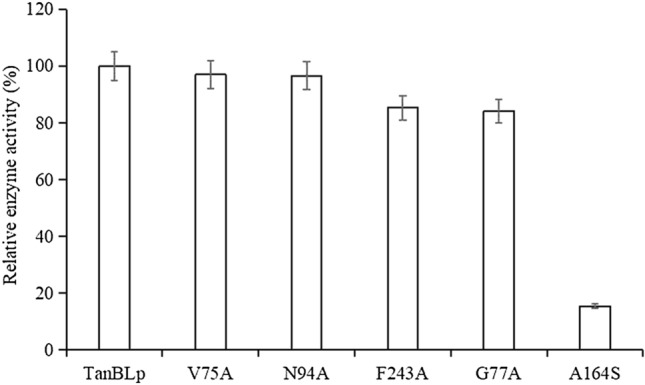
The relative enzyme activity of tannase TanBLp and its mutants
Effect of pH on the Activity and Stability of TanBLp and its Mutants
The effect of pH on enzyme activity showed that the pH ranges of all mutants were enlarged, the highly enzymatic activities of all the mutants were at pH6.0-pH10.0. The optimum pH of all mutants shifted from 8.0 to 6.0 by comparison with the TanBLp, as showed in Fig. 3. Mutant V75A has the highest activity than other mutants at pH 6.0. At the same time, mutant V75A could keep more than 50% activity at pH 5.0, which will expand the application of mutant V75A in an acidic environment.
Fig. 3.
The effect of pH on the activity of TanBLp and its mutants
After 24 h of different pH treatments, the relative enzyme activity of tannase TanBLp was above 80% at pH 8–10, the relative enzyme activities of mutants V75A, G77A, and N94A were more than 80% at pH 4–11 and the relative enzyme activity of mutant F243A was above 80% at pH 4–9. The pH stabilities of mutants V75A, G77A, N94A and F243A were slightly higher than that of tannase TanBLp (Fig. 4), The stability of mutant V75A at acidic conditions was higher than that of tannase TanBLp.
Fig. 4.
The pH stability of TanBLp and its mutants
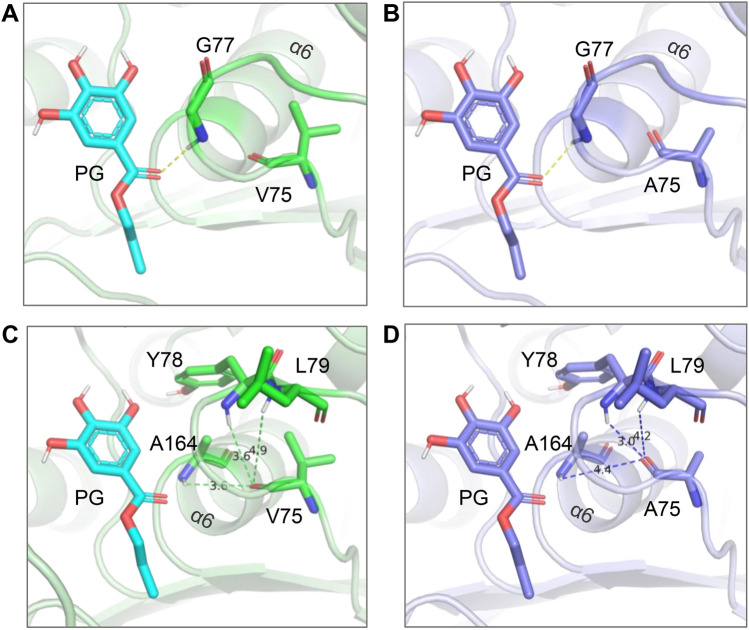
During the fermentation, the pH of fermentation liquor were decreased gradually along with the growth of microorganisms [30], the mutations of amino acids Val75, Gly77, Asn94 and Phe243 will benefit the fermentation process in Tannin. The pH stability of mutant V75A was significantly higher than that of the original tannase TanBLp and other mutant enzymes after 24 h of treatment at pH 4, 5 and 6, the relative enzyme activities of the mutant V75A were 97.2%, 96.1% and 94.4%, respectively. There was no significant change in the hydrogen bonds interaction between PG and Gly77 in catalytic triad, which may be the reason of no significant change in tannase activity before and after the mutation (Fig. 5a, b). When the residue Val79 mutated to Ala, the distance between this residue and Tyr78, Leu79 were decreased, while the distance between it and A164 was increased (Fig. 5c, d), which indicated that the binding pocket of PG becomes larger after the mutation and may be the important reason of the increase of pH stability of tannase TanBLp, the mutant V75A was more suitable for hydrolyzing gallnut to produce gallic acid and has wide industrial application prospect.
Fig. 5.
Changes in spatial structure of TanBLp before and after Val75 mutation. a and b were the changes in the distance from Gly77 before and after Val75 mutation; c and d were the changes in the distance from Tyr78, Leu79 and Ala164 before and after the Val75 mutation
Determination of Kinetic Constants of the Mutants
The results of enzyme-catalyzed reaction of tannase TanBLp and its mutants based on Lineweaver–Burk method showed that the Km values of mutants V75A, G77A, N94A, A164S and F243A were higher than that of tannase TanBLp, while the Vmax values of all mutants were lower than that of tannase TanBLp. The results of specific activity showed that mutants V75A, G77A, N94A and F243A were similar to that of tannase TanBLp, while the specific activity of TanBLp was 6.5 times than that of mutant A164S (Table 2), the decrease of enzyme activity of mutant A164S was related to the decrease of affinity between enzyme and substrate, which was indicated that this mutant site was related to the substrate binding of tannase TanBLp [17, 24].
Table 2.
The specific activity of TanBLp and its mutants
| Tannase | Michaelis–Menten equation | Correlation (R2) | Km (mmol/L) | Vmax (μmol/mg min) | Specific activity (U/mg) |
|---|---|---|---|---|---|
| TanBLp | y = 0.038x + 0.011 | 0.996 | 3.455 | 90.909 | 30.278 |
| V75A | y = 0.065x + 0.012 | 0.996 | 5.417 | 83.333 | 29.372 |
| G77A | y = 0.145x + 0.012 | 0.996 | 12.083 | 83.333 | 25.447 |
| N94A | y = 0.080x + 0.012 | 0.993 | 6.667 | 83.333 | 29.256 |
| F243A | y = 0.129x + 0.016 | 0.993 | 8.063 | 62.500 | 25.837 |
| A164S | y = 0.413x + 0.018 | 0.993 | 22.944 | 55.556 | 4.640 |
Conclusion
The amino acid residues Ala164, Lys343, Glu357, Asp421 and His451 had played an important role in maintaining the activity of tannase TanBLp, which was consistent with previous researches. The mutants V75A, G77A, N94A, and F243A had higher pH stability and mutant V75A has the highest stability and activity at acidic conditions than other mutants, which was more suitable for industrial application to manufacture gallic acid in acidic environment.
Author's Contribution
HP, JZ and HY conducted most of the experiments and contributed equally to this work. YT and HZ conceived and designed the study. CW and HL designed the primer by site-directed mutagenesis. HZ, XS and XL predicted the catalytic sites of tannase TanBLp using bioinformatics. HP, JZ and HY wrote the paper. All authors discussed the data and commented on the manuscript.
Funding
National Key R&D Program of China (2019YFC1604903, 2019YFC1604905), National Natural Science Foundation of China (21808052, 31971042), Science and Technology Department of Hunan Province (2020NK4194, 2018RS3086, 2019GK4018), Open Research Project of State Key Laboratory Breeding Base of Crop Germplasm Innovation and Resource Utilization (18KFXM11), Training Program for Excellent Young Innovators of Changsha (kq2009019) and “Double First-Class” construction project of Hunan Agricultural University (SYL201802002) funded this work.
Data Availability
The data underlying this article are available in the article.
Declarations
Conflict of interest
All authors declare that they have conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hu Pan, Jingjing Zhan and Hui Yang contributed equally to this article.
References
- 1.Zhu X, Hou X, Ma B, Xu H, Yang Y. Chitosan/gallnut tannins composite fiber with improved tensile, antibacterial and fluorescence properties. Carbohydr Polym. 2019;226:115311. doi: 10.1016/j.carbpol.2019.115311. [DOI] [PubMed] [Google Scholar]
- 2.Watrelot AA, Guernevé CL, Halle H, Meudec E, Cheynier V. Multimethod approach for extensive characterization of gallnut tannin extracts. J Agric Food Chem. 2017;68:13426–13438. doi: 10.1021/acs.jafc.9b08221. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Xia X, Dong S, Zhou K. Industrial scale extraction and stripping devices for continuous recovery of gallic acid from Chinese nutgall processing wastewater. Environ Eng Res. 2017;22:288–293. doi: 10.4491/eer.2016.143. [DOI] [Google Scholar]
- 4.Chávez-González M, Rodríguez-Durán LV, Balagurusamy N, Prado-Barragán A, Rodríguez R, Contreras JC, Aguilar CN. Biotechnological advances and challenges of tannase: an overview. Food Bioprocess Technol. 2012;5:445–459. doi: 10.1007/s11947-011-0608-5. [DOI] [Google Scholar]
- 5.Jana A, Halder SK, Banerjee A, Paul T, Pati BR, Mondal KC, Mohapatra PKD. Biosynthesis, structural architecture and biotechnological potential of bacterial tannase: a molecular advancement. Bioresour Technol. 2014;157:327–340. doi: 10.1016/j.biortech.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Naby MA, El-Tanash AB, Sherief ADA. Structural characterization, catalytic, kinetic and thermodynamic properties of Aspergillus oryzae tannase. Int J Biol Macromol. 2016;92:803–811. doi: 10.1016/j.ijbiomac.2016.06.098. [DOI] [PubMed] [Google Scholar]
- 7.Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan LA, Ramírez-Coronel A, Contreras-Esquivel JC. Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol. 2007;76:47–59. doi: 10.1007/s00253-007-1000-2. [DOI] [PubMed] [Google Scholar]
- 8.Begovic S, Duzic E. Purifying of tannase from the mucous membrane of the rumen and small intestines of bovines and a comparison of activity. Vetenaria (Yugoslavia) 1977;26:227–233. [Google Scholar]
- 9.Govindarajan RK, Revathi S, Rameshkumar N, Krishnan M, Kayalvizhi N. Microbial tannase: current perspectives and biotechnological advances. Biocatal Agric Biotechnol. 2016;6:168–175. doi: 10.1016/j.bcab.2016.03.011. [DOI] [Google Scholar]
- 10.Dai XL, Liu YJ, Zhuang JH, Yao SB, Liu L, Jiang XL, Zhou K, Wang YS, Xie DY, Bennetzen JL, Gao LP, Xia T. Discovery and characterization of tannase genes in plants: roles in hydrolysis of tannins. New Phytol. 2020;226:1104–1116. doi: 10.1111/nph.16425. [DOI] [PubMed] [Google Scholar]
- 11.Chhokar V, Sharma J, Kumar A, Beniwal V. Recent advances in industrial application of tannases: a review. Recent Patents Biotechnol. 2013;7:228–233. doi: 10.2174/18722083113076660013. [DOI] [PubMed] [Google Scholar]
- 12.Sharma KP, John PJ, Goswami P, Soni M. Enzymatic synthesis of gallic acid from tannic acid with an inducible hydrolase of Enterobacter spp. Biocatal Biotransform. 2017;35:177–184. doi: 10.1080/10242422.2017.1306740. [DOI] [Google Scholar]
- 13.Yao J, Guo GS, Ren GH, Liu YH. Production, characterization and applications of tannase. J Mol Catal B Enzym. 2014;101:137–147. doi: 10.1016/j.molcatb.2013.11.018. [DOI] [Google Scholar]
- 14.Govindarajan RK, Mathivanan K, Rameshkumar N, Shyu DJH, Krishnan M, Kayalvizhi N. Purification, structural characterization and biotechnological potential of tannase enzyme produced by Enterobacter cloacae strain 41. Process Biochem. 2019;77:37–47. doi: 10.1016/j.procbio.2018.10.013. [DOI] [Google Scholar]
- 15.Fuentes-Garibay JA, Aguilar CN, Rodríguez-Herrera R, Guerrero-Olazarán M, Viader-Salvadó JM. Tannase sequence from a xerophilic Aspergillus niger strain and production of the enzyme in Pichia pastoris. Mol Biotechnol. 2015;57:439–447. doi: 10.1007/s12033-014-9836-z. [DOI] [PubMed] [Google Scholar]
- 16.Venu Gopal KS, Cherita C, Anu Appaiah KA. Yeast as a potential candidate for tannase production: a review. Curr Biochem Eng. 2016;3:82–88. doi: 10.2174/2212711902666150519233022. [DOI] [Google Scholar]
- 17.Matoba Y, Tanaka N, Noda M, Higashikawa F, Sugiyama M. Crystallographic and mutational analyses of tannase from Lactobacillus plantarum. Proteins. 2013;81:2052–2058. doi: 10.1002/prot.24355. [DOI] [PubMed] [Google Scholar]
- 18.Aguilar-Zarate P, Cruz-Hernandez MA, Montanez JC, Belmares-Cerda RE, Aguilar CN. Enhancement of tannase production by Lactobacillus plantarum CIR1: validation in gas-lift bioreactor. Bioprocess Biosyst Eng. 2014;37:2305–2316. doi: 10.1007/s00449-014-1208-3. [DOI] [PubMed] [Google Scholar]
- 19.Aishwarya SS, Selvarajan E, Iyappan S, Rajnish KN. Recombinant l -asparaginase ii from Lactobacillus casei subsp. casei ATCC 393 and its anticancer activity. Indian J Microbiol. 2019;59:313–320. doi: 10.1007/s12088-019-00806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Zhao B, Liu CJ, Yang E. Optimization of biosynthesis conditions for the production of exopolysaccharides by lactobacillus plantarum sp8 and the exopolysaccharides antioxidant activity test. Indian J Microbiol. 2020;60:334–345. doi: 10.1007/s12088-020-00865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivas BDL, Rodríguez H, Anguita J, Muoz R. Bacterial tannases: classification and biochemical properties. Appl Microbiol Biotechnol. 2019;103:603–623. doi: 10.1007/s00253-018-9519-y. [DOI] [PubMed] [Google Scholar]
- 22.Ueda S, Nomoto R, Yoshida K, Osawa R. Comparison of three tannases cloned from closely related Lactobacillus species: L. plantarum, L. paraplantarum, and L. pentosus. BMC Microbiol. 2014;14:87. doi: 10.1186/1471-2180-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwamoto K, Tsuruta H, Nishitaini Y, Osawa R. Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum ATCC 14917T. Syst Appl Microbiol. 2008;31:269–277. doi: 10.1016/j.syapm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Ren B, Wu M, Qin W, Peng X, Chen Q. Crystal structure of tannase from Lactobacillus plantarum. J Mol Biol. 2013;425:2737–2751. doi: 10.1016/j.jmb.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Seeliger D, Groot BLD. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des. 2010;24:417–422. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmoud AE, Fathy SA, Rashad MM. Purification and characterization of a novel tannase produced by Kluyveromyces marxianus using olive pomace as solid support, and its promising role in gallic acid production. Int J Biol Macromol. 2018;107:2342–2350. doi: 10.1016/j.ijbiomac.2017.10.117. [DOI] [PubMed] [Google Scholar]
- 27.Tseng YH, Hsieh CC, Kuo TY, Liu JR, Hsu TY, Hsieh SC. Construction of a lactobacillus plantarum strain expressing the capsid protein of porcine circovirus type 2d (pcv2d) as an oral vaccine. Indian J Microbiol. 2019;59:490–499. doi: 10.1007/s12088-019-00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaitanyakumar A, Anbalagan M. Expression, purification and immobilization of tannase from Staphylococcus lugdunensis MTCC 3614. AMB Express. 2016;6:89. doi: 10.1186/s13568-016-0261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazaki K, Takenouchi M. Creating random mutagenesis libraries using megaprimer PCR of whole plasmid. Biotechniques. 2002;33:1036–1038. doi: 10.2144/02335st03. [DOI] [PubMed] [Google Scholar]
- 30.Pulido RP, Omar NB, Abriouel H, López RL, Gálvez A. Characterization of lactobacilli isolated from caper berry fermentations. J Appl Microbiol. 2007;102:583–590. doi: 10.1111/j.1365-2672.2006.03067.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.