Abstract
In this paper, the components of Zanthoxylum bungeanum Maxim. essential oil (ZBMEO) were analyzed. The efficacy of different concentrations of ZBMEO on the change in physical and chemical indicators of the rabbit meat patty was evaluated. Furthermore, kinetics models were employed to calculate the lipid oxidation induction period and microbial growth lag time. GC–MS analysis revealed that the major chemical components in ZBMEO included linalool, limonene, and sabinene. Results of the storage experiment indicated that ZBMEO had a good inhibition effect on lipid and protein oxidation, microbial growth, and formation of TVB-N, as well as slowed down the rate of change in color and pH during the 12 days storage time of rabbit meat. The models showed that adding ZBMEO could delay the lipid oxidation induction period, and extend the microbial growth lag time. Overall data showed that ZBMEO is a promising natural additive to maintain the quality of rabbit meat patty.
Keywords: Rabbit meat, Lipid and protein oxidation, Zanthoxylum bungeanum maxim. essential oil, Quality index, Kinetic models
Introduction
Lipid and protein oxidation in meat and meat products can cause rancid odor, off-flavor, discoloration, and toxic compounds, which can affect the consumers’ acceptance of products and reduce shelf life (Eckl and Bresgen 2017). Rabbit meat is favored by consumers due to its high contents of polyunsaturated fatty acids, proteins, and essential amino acids (Dalle Zotte and Szendrő 2011); it meets the demand for a healthy diet, and its global production has steadily increased according to Food and Agriculture Organization of the United Nations, Statistics Division (2018). However, rabbit meat is prone to spoilage during chilled storage, resulting in loss of nutrition and even leading to poisoning when eaten.
In meat products, synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole, and tert-butylhydroquinone have been used successfully for the shelf-life extension. These chemicals have residual toxicity, raising the demand for natural antioxidants in the processed food industry (Lee et al. 2019). Thus, safe and novel natural antioxidants to prolong the shelf life of rabbit meat are urgently needed. Recently, essential oils have attracted scientific interest due to their potential as natural antioxidants consisting of biologically active compounds.
Zanthoxylum bungeanum Maxim. (ZBM), the Zanthoxylum genus of the family Rutaceae, is one of the most widely used pungent spices in Chinese cuisine (Li et al. 2015). Z. bungeanum Maxim. essential oil (ZBMEO) has been extracted from ZBM by hydrodistillation or supercritical fluid CO2 extraction (Fu et al. 2018). Given its rich amount of active compounds such as eucalyptol, olefins, alcohols, ketones, and esters (Zhang et al. 2016), ZBMEO is used as an antifungal, antioxidant, and popular flavoring agent in the food processing industry (Li et al. 2015; Zeng et al. 2018).
To the best of our knowledge, no research has been conducted on the inhibition of microbial reproduction and lipid and protein oxidation in rabbit meat using ZBMEO. Thus, this study aimed to evaluate the inhibitory effect of different concentrations (0.1%, 0.3%, and 0.5%) of ZBMEO on microbial spoilage, quality index change, lipid and protein oxidation in rabbit meat. Models were used to calculate the lipid oxidation induction period (IP) and microbial growth lag time during 12 days chilled storage.
Materials and methods
Materials
ZBMEO was purchased from Guangzhou TIANXU Food Additive Co. Ltd. (Guangzhou, China) and stored at 4 °C before analysis. The fresh carcass (seventy male Ira rabbits, 2.3–2.5 kg/live rabbit) were purchased from A Xing Ji Food Co. Ltd. (Chongqing, China). The company was slaughtered according to the method prescribed by Agricultural Industry Standard of the People's Republic of China (NY/T 3470-2019). The carcasses were immediately transported to the laboratory in a chilled box (approximately 6 °C) within 4 h. Then removed the skin, head, and viscera Aging at 4 °C for 24 h was performed to dissipate rigor mortis. All chemicals were analytical grade or higher.
GC–MS analysis of ZBMEO
ZBMEO samples were analyzed by GC–MS Shimadzu corporation 2010 equipped with a flame ionization detector and HP-5MS (30 m × 0.25 mm × 0.25 μm) capillary column according to the method by Ahmed and Tavaszi-Sarosi (2019) with little modification. The temperature was programmed for isothermal setting at 50 °C for 2 min, increased from 50 to 150 °C at 2 °C/min, held at 150 °C for 2 min, increased from 150 to 250 °C at 10 °C/min, and held at 250 °C for 5 min. The injector temperature was 250 °C, and the flow rate of helium (the carrier gas) was 1.0 mL/min. Samples were diluted (1% solution, v/v, diluted in hexane) and then manually injected in the split mode.
Manufacture
All samples of Longissimus thoracis et lumborums (LTL) were dissected, and visible fat and connective tissue were removed. Subsequently, 14 kg of rabbit meat (LTL) was ground using a professional meat grinder. After mincing, the ground meat was equally divided into five treatments with three replicates and five patties in each replicate. Each treatment was performed in accordance with the following formulation: (1) control (add nothing); (2) BHT (add 0.02% BHT, w/w); (3) 0.1% ZBMEO (add 0.1% ZBMEO, w/w); (4) 0.3% ZBMEO (add 0.3% ZBMEO, w/w); and (5) 0.5% ZBMEO (add 0.5% ZBMEO, w/w). Each treatment group was blended for 2 min and placed inside molds to form round patties (diameter 3 cm, height 2 cm). A total of 525 meat patties (30 g each) were formed and placed in a tray (polypropylene, 182 cm × 136 cm × 30 cm) with a polyethylene bag (Topu daily-use chemicals China Co. Ltd, China). The samples were stored at 4 °C until analysis at 0, 2, 4, 6, 8, 10, and 12 days.
Microbial analysis
The determination of total bacterial counts followed the method of Imazaki et al. (2019) using plate count agar medium. In brief, 10 g of rabbit meat sample was mixed with 90 mL of sterilized saline (0.85%), and further dilution was performed via transferring 1 mL of the homogenate to 9 mL of sterile saline. Serial decimal dilutions (10−1–10−6) were then prepared. About 1 mL of each dilution was poured into a Petri dish, and the sterilized medium was added. The solution was incubated at 37 °C ± 1 °C for 48 h, and the results are presented in log CFU/g sample.
Determination of total volatile basic nitrogen
Total volatile basic nitrogen (TVB-N) was measured by semi microsteam distillation, as described by Sujiwo et al. (2019) with minor modifications. About 10 g of ground rabbit meat was dispersed in 100 mL of distilled water and stirred for 60 min. The mixture was then filtered. The TVB-N value was determined based on the consumption of hydrochloric acid.
pH values
pH was measured according to Chang et al. (2019) with slight modification. Rabbit meat was ground, and 5.0 g of ground rabbit meat was homogenized with 45 mL of 0.1 M potassium chloride (pH 7.0). The pH was determined with a digital pH meter.
Protein oxidation
Carbonyl contents
Carbonyl content was determined following the method by Wang et al. (2019) with minor modification. Carbonyls were reacted with 2,4-dinitrophenylhydrazine, and products were detected by measuring the absorbance at 370 nm. Protein concentrations were calculated by measuring the absorbance at 280 nm with bovine serum protein as standard. The content of carbonyls was calculated using an extinction coefficient of 22,000 M−1 cm−1.
Sulfhydryl contents
Sulfhydryl content was determined following the method of Xu et al. (2019). The protein concentration was determined using the biuret method and bovine serum protein as standard. Sulfhydryl contents were calculated using an extinction coefficient of 13,600 M−1 cm−1.
Lipid oxidation
Determination of peroxide value
The peroxide values (PVs) of rabbit meat were determined by using the ferric thiocyanate method of Firuzi et al. (2019) with slight modifications. Approximately 5.0 g of ground rabbit meat was homogenized in 20 mL of 1:1 chloroform: methanol (v/v). The solution was mixed with 6 mL of 0.5% NaCl, shaken well, and centrifuged at 1800 g (6 min, 4 °C). About 5 mL of chloroform layer solution was transferred into a glass tube; 3 mL of chloroform: methanol (1:1), 100 μL of ammonium thiocyanate (3.94 M), and 100 μL of FeCl2 (18 mM) were added successively into the tube. The resulting solution was incubated for 20 min at room temperature, and absorbance was measured at 500 nm.
Determination of TBARS (thiobarbituric acid reactive substances) values
TBARS was measured according to the procedure developed by Souza et al. (2019) with slight modifications. In brief, 10.0 g of ground rabbit meat was homogenized (3720×g, 1 min) with 20 mL of 20% trichloroacetic acid. The supernatant was filtered after centrifugation, and 5 mL of filtrate was transferred into a test tube, then 5 mL of 2-thiobarbituric acid solutions (0.02 mol/L) was added. The mixed solution was reacted for 20 min in a boiling water bath, and the resulting solution was cooled with running water to room temperature. The absorbance of the solution was measured at 532 nm.
Determination of hexanal content
The hexanal content in rabbit meat was monitored by gas chromatography (GC-2010 plus Shimadzu, Japan) coupled to headspace solid-phase microextraction (automatic headspace injector HSS 86.50 Plus DANI Instruments S.P.A., Italy). The methods were based on the procedure described as follows: 3 g of sample was weighed and placed in a crimp headspace vial; 3 mL of saturated saline water was added, and the vial was sealed with a crimp seal. The sample was equilibrated at 80 °C for 30 min. The flow rate of helium on the HP-5MS capillary column was 1 mL/min. The temperature program for the procedure was as follows: initial 40 °C for 1 min, raised to 90 °C at 8 °C/min, held for 1 min at 90 °C, increased to 250 °C at 6 °C/min, and held for 5 min at 250 °C. The qualitative analysis of hexanal was based on its retention time, and the quantification of hexanal in the sample was made using a standard curve of hexanal solution.
Surface color evaluation
The color of rabbit meat sample was measured following the method described by Xu et al. (2019). The change in color was represented by , which was calculated by Eq. (1).
| 1 |
where = − , = − , and = − . , represents lightless, and (the positive value) represent redness and yellowness, respectively. While and (the negative value) represent greenness and blueness, respectively. The subscript ‘‘0′’ indicates the initial color at day 0. The color change during storage was expressed as the total color difference .
Sensory evaluation
Patties were analyzed by descriptive analysis according to Cullere et al. (2018) with slight modification. The rabbit meat patties (every patty about 30 g) were cooked (steamed) in a pan (about 1 L water) until the core temperature reached 80 °C and maintained 30 min, then cut into small pieces (2 cm × 2 cm × 1 cm, 10 g approx.) (Özünlü et al. 2018). Each sample (without addition) was served at 20 ± 1 °C on a separate plate. The laboratory environment for sensory evaluation is set according to the international standard (ISO 2007). In brief, the temperature of the laboratory is set at 20 °C, the relative humidity is set at 55%, and the illumination intensity is set at 1000 Lx. Private booths were prepared for each panelist. The sensory assessment panel consisted of 10 trained and experienced members (5 males, 5 females, age range 20–30 years old) from the College of Food Science of Southwest University. Before the sensory assessment, characteristics were discussed and explained. The cooked rabbit meat patties were evaluated based on the characteristics of odor, color, flavor, texture, and overall acceptance by the sensory assessment panel. All samples were randomly coded and presented to the panelists. using a 9‐score hedonic scale with the following descriptors: 1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8 = like very much, and 9 = like extremely.
Modeling
Lipid oxidation IP measurement
IP represents a critical time when lipid oxidation shifts from initiation to propagation phase. In this paper, the IP was the point with a sudden rise in PV of rabbit meat during chilled storage. Tangent method and combinational kinetic model were used for IP determination. Thus, a combination of the pseudo-first- and -second-order reaction kinetics was employed to fit the lipid peroxidation curve and predict the IP. The pseudo-first- and -second-order reactions were expressed as Eqs. (2) and (3) (Okpala et al. 2016; Farhoosh 2018), respectively. The turning point was the maximum growth rate of PV, and the turning point time was calculated and expressed as Eq. (4). The first straight line was obtained from the turning point and growth rate.
| 2 |
| 3 |
| 4 |
The second straight line was obtained from the integration of the basic rate expression for the pseudo-zero-order reaction kinetics, as shown in Eq. (5). On the basis of Eqs. (3) and (5), the value of IP could be calculated and expressed as Eq. (6).
| 5 |
| 6 |
where (1/d), (g/mmol·d), and (mmol/g d) were the first-, second- and zero-order rate constants, respectively. After integration, Eq. (2) was converted to Eq. (3), and C (mmol/g) was the integration constant. was the PV value storage at day 0. was the turning point time, which was mathematically expressed as the turning point, and its second derivative was 0 (). (d) was the induction period time.
Mathematical model of microbial growth
In this paper, the full Huang model was used to analyze. The growth curve, as shown in Eqs. (7) and (8) (Sommers et al. 2018). The kinetic parameter of lag time of bacterial growth was described by an empirical relationship [Eq. (9)].
| 7 |
| 8 |
| 9 |
where and are the initial and real-time bacterial concentrations, respectively; t is the storage time(d); is the maximum cell concentration in the sample; is the specific growth rate (ln CFU/g d−1); is the lag time (d); and α and β are regression coefficients.
Statistical analysis
Statistical analysis was performed using MATLAB (R2016b, USA). The data were assessed using one-way ANOVA to examine the effect of time and differences between treatments on characteristics of rabbit meat patties. The results are expressed as the mean ± standard error of three repetitions, and the significances of the means were evaluated using Duncan’s multiple range test (P < 0.05).
Results and discussion
Chemical analysis of ZBMEO
The chemical composition of ZBMEO was determined by GC–MS, as shown in Table 1, and 17 components were detected. The most dominant component was linalool (51.91%), followed by limonene (18.17%) and sabinene (13.27%). Three components, namely, α-pinene (1.65%), β-myrcene (4.9%), and linalyl acetate (3.78%), were in the range of 1–10% of the peak area. The remaining component peak areas (%) were lower than 1%.
Table 1.
Chemical composition of the ZBMEO by GC–MS analysis
| Compounds | Retention time (min) | Peak area (%) |
|---|---|---|
| Methyl heptane | 4.832 | 0.3 |
| phenylethylene | 7.492 | 0.38 |
| α-Pinene | 9.107 | 1.65 |
| Sabinene | 11.008 | 13.27 |
| (1S)-(1)-beta-pinene | 11.183 | 0.98 |
| β-Myrcene | 11.885 | 4.9 |
| Limonene | 14.064 | 18.17 |
| Eucalyptol | 14.208 | 0.08 |
| β-Pinene | 14.513 | 0.1 |
| (Z)-β-Ocimene | 15.125 | 0.39 |
| 5-Ethyl-2-methyl-octane | 15.589 | 0.46 |
| Cis-4-thujanol | 16.601 | 0.57 |
| Linalool | 18.814 | 51.91 |
| α-Thujone | 18.990 | 0.71 |
| Linalyl acetate | 28.581 | 3.78 |
| β-Caryophyllene | 39.018 | 0.47 |
| Germacrene D | 42.855 | 0.57 |
The raw materials came from different areas, and different extraction methods had a major influence on the composition of EO; however, the main components were monoterpenes and sesquiterpenes. Concentrations of 12.36% limonene were reported by Cairo et al. (2017) in Brazilian red pepper EO. Uliana et al. (2016) reported that Brazilian rose pepper leaves contain myrcene and α-pinene. Brazilian rose and red pepper belong to the Zanthoxylum genus of the family rutaceae, and the main components were similar to ZBM. EOs exhibited antimicrobial properties because they contained monoterpenes or sesquiterpenes or their oxygenated derivatives (Diao et al. 2014).
Microbial, TVB-N, and pH analysis
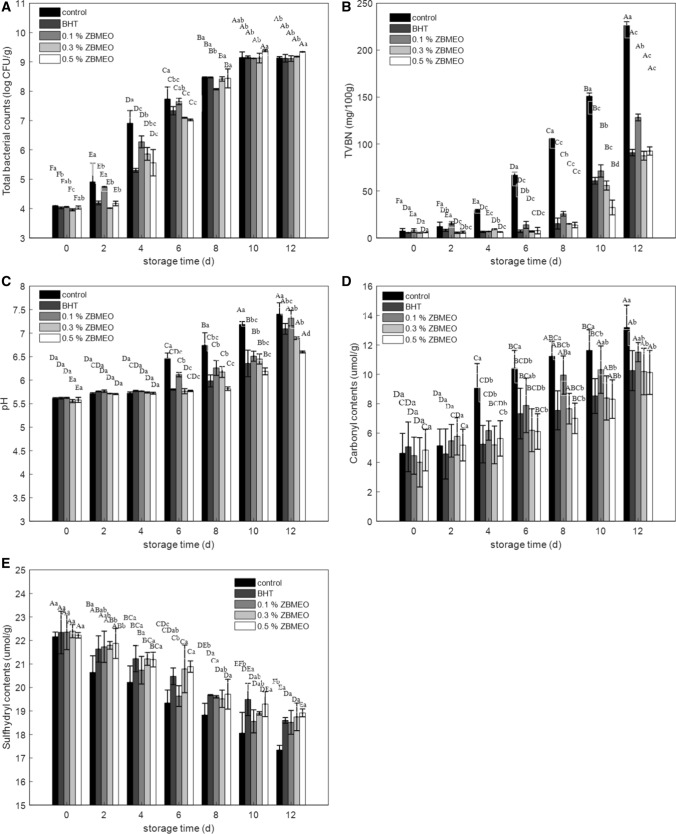
The microbiological analysis of rabbit meat patties during chilled storage is presented in Fig. 1a. The microbial counts of all groups increased significantly (P < 0.05) in the first 10 days of storage but did not further increase beyond 10 storage days, which indicated that the microbial growth entered a stable stage. The initial bacterial counts of the control, BHT, 0.1% ZBMEO, 0.3% ZBMEO, and 0.5% ZBMEO groups were 4.089, 4.024, 4.055, 3.957, and 4.033 log CFU/g, respectively. Figure 1a shows that the addition of BHT, 0.3% ZBMEO, and 0.5% ZBMEO led to a significant (P < 0.05) decrease in the microbial growth rate during the storage time of the initial 6 days. On day 6, the lowest total viable count (7.024 log CFU/g) was observed for 0.5% ZBMEO compared with the control’s 7.735 log CFU/g. These results were due to the anti-bacterial activity of BHT and ZBMEO, whereas 0.5% ZBMEO had a better effect than BHT. During 8–12 storage days, no significance was observed among all groups in the total viable counts (P > 0.05).
Fig. 1.
Effect of ZBMEO and BHT on the change of total microbial counts (a), TVB-N values (b), pH values (c), carbonyl contents (d), and sulfhydryl contents (E) for all groups of rabbit meat patties during chilled storage. BHT, butylated hydroxyl toluene. Control, no additive; 0.1% ZBMEO, 0.3% ZBMEO and 0.5% ZBMEO were represent add 0.1%, 0.3% and 0.5% ZBMEO, respectively. Different capital letters (A–F) indicate the significant differences of the same treatment at different storage time (P < 0.05). Different lowercase letters (a–d) indicate significant differences between different treatments within the same storage time (P < 0.05)
TVB-N is a quality index used to assess the deterioration and shelf life of meat products. The TVB-N increased for all groups with storage time (Fig. 1b). In the first 2 days of storage, no significance was observed among all groups. However, after 4 days of storage, the TVB-N in the ZBMEO treatment groups was significantly lower than that in the control group (P < 0.05). If a TVB-N value of 15 mg/100 g [According to National Standard of the People’s Republic of China (GB 5009.228-2016)] in rabbit meat was proposed as an acceptable limit (Lan et al. 2016), then the acceptable shelf life was less than 4 days for the control group; 6 days for the 0.1% ZBMEO group; and longer than 8 days for the 0.3% ZBMEO, 0.5% ZBMEO, and BHT groups. The results showed that ZBMEO could extend the shelf life of rabbit meat during chilled storage.
On the first day of storage, the pH values of the control, BHT, 0.1% ZBMEO, 0.3% ZBMEO, and 0.5% ZBMEO groups were 5.61, 5.62, 5.62, 5.56, and 5.58, respectively. The pH values of all groups were not significant at the beginning of storage. Figure 1c shows that the pH values of all groups increased continuously with increased storage time. Until the 4th day, the pH value of the control group was significantly higher than that of the other groups. At the end of the chilled storage time, the lowest pH of 6.6 was observed in 0.5% ZBMEO, followed by those in 0.3% ZBMEO (6.89) and BHT (7.09). The pH values of the 0.1% ZBMEO and control groups increased to 7.31 and 7.40, respectively. The results showed that ZBMEO delayed the increase in pH during chilled storage. The pH, TVB-N, and microbial counts had a strong correlation due to the accumulation of biogenic amines produced by the microbiological degradation of the protein (Fan et al. 2019).
Protein oxidation analysis
Protein oxidation can cause meat deterioration, especially in color and texture, in meat or meat products (Wang et al. 2018). Protein oxidation in meat or meat products can be reflected by changes in the carbonyl and sulfhydryl contents (Davies 2016).
The oxidative degradation of the protein resulted in carbonyl compounds. The changes in the concentrations of carbonyl contents during chilled storage of rabbit meat patties are illustrated in Fig. 1d. The carbonyl contents increased in all groups during chilled storage. This result was consistent with the observations reported by Wang et al. (2018) on refrigerated and super chilled rabbit meat. The carbonyl content of the control group was significantly higher than that of the other groups. Compared with the control, adding BHT, 0.3% ZBMEO, and 0.5% ZBMEO significantly inhibited (P < 0.05) the formation of protein carbonyl rabbit meat patties in the first 8 days of storage. At the 12 days of storage, the lowest carbonyl content among all groups was 10.12 nmol/mg protein in 0.5% ZBMEO, followed by that of BHT (10.25 nmol/mg protein). The carbonyl content of control group increased to 13.18 nmol/mg protein. Protein side-chain amino acids were oxidized under free radical attack to form carbonyl groups (Davies 2016). Studies have shown that BHT and ZBMEO could scavenge free radicals (Aminzare et al. 2018; Pateiro et al. 2018). Thus, BHT and ZBMEO inhibited protein oxidation maybe by scavenging free radicals.
The effect of chilled storage on the sulfhydryl contents of rabbit meat patties is illustrated in Fig. 1e. The number of total sulfhydryl contents decreased with increasing storage time for both groups. Similarly, a decreased trend in sulfhydryl group was previously reported on pork during super chilled storage (Pomponio and Ruiz-Carrascal 2017). The sulfhydryl contents of the control group were lower than that of the other groups. Moreover, the decreased sulfhydryl was consistent with carbonyl formation for both groups, supporting that BHT and ZBMEO could inhibit the degradation of sulfhydryl groups and formation of carbonyls in rabbit meat.
The side-chain amino acids of protein are vulnerable to free radical attacks and are oxidized, especially sulfur-containing amino acids such as cysteine, and previous studies indicated side-chain amino acids protein oxidation were oxidized under free radical attack to form carbonyl group, at the same time, sulfhydryl group damage by free radicals (Davies 2016). ZBMEO could inhibit carbonyl formation and sulfhydryl destruction, that means ZBMEO maybe inhibited protein oxidation by scavenging free radicals or other ways.
Lipid oxidation analysis
The main primary product measured as PV, secondary products measured as TBARS values, and hexanal were obtained to assess lipid oxidation in rabbit meat.
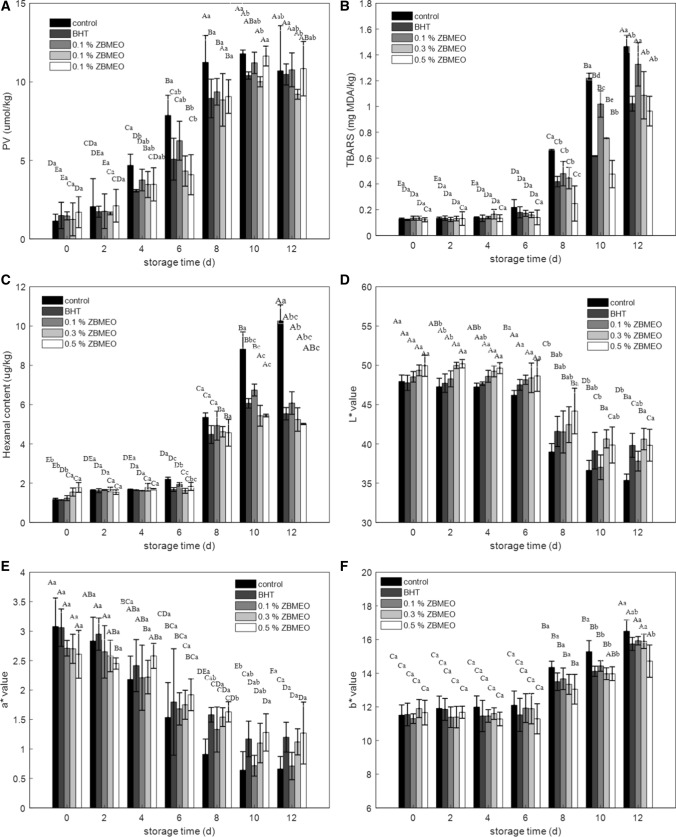
The change in PV during chilled storage is presented in Fig. 2a. PV increased in the first 10 days but decreased beyond 10 days for all groups due to the continued accumulation of hydroperoxides during the early days of storage; during the late days of storage, the lipid oxidation reactions lessened, and hydroperoxides degraded to other small molecules (Li et al. 2019). This phenomenon strongly supported the concept that hydroperoxides decomposition can yield secondary lipid oxidation products. The PV of the control group increased rapidly from 0 to 8 days, reaching 11.24 μmol/kg, which was significantly higher than that of other groups. the results indicated that BHT and ZBMEO could inhibit lipid oxidation. To further understand the effect of ZBMEO on lipid oxidation, the lipid hydroperoxide model was exploited.
Fig. 2.
Effect of ZBMEO and BHT on the change of PV (a), TBARS values (b), hexanal values (c), L* value (d), a* value (e), and b* value (f) of rabbit meat patties during chilled storage. BHT, butylated hydroxyl toluene. Control, no additive; 0.1% ZBMEO, 0.3% ZBMEO and 0.5% ZBMEO were represent add 0.1%, 0.3% and 0.5% ZBMEO, respectively. Different capital letters (A–F) indicate the significant differences of the same treatment at different storage time (P < 0.05). Different lowercase letters (a–c) indicate significant differences between different treatments within the same storage time (P < 0.05)
Malondialdehyde as the main secondary product of lipid oxidation was measured as TBARS. The initial TBARS values of all groups ranged from 0.121 mg/kg to 0.135 mg/kg, as shown in Fig. 2B, TBARS increased slowly in the first 6 days but increased significantly (P < 0 0.05) beyond 6 days for all the groups; the result was similar to those of Li et al. (2019). The tendency could be explained as follows. In the early days of storage, the primary oxidation products mainly accumulated. With increased concentration of primary oxidation products, the decomposition rate of primary oxidation products was accelerated, and the concentration of secondary oxidation products increased. The results corresponded to the change in PV conclusion. TBARS of the control group increased more rapidly than that of other groups, followed by 0.1% ZBMEO, 0.3% ZBMEO, BHT, and 0.5% ZBMEO. After 6 days, TBARS of the control group was significantly higher than that of the other groups. At the end of the storage time, the lowest TBARS among all groups was 0.96 mg/kg in 0.5% ZBMEO, followed by that of BHT (1.02 mg/kg) and 0.3% ZBMEO (1.08 mg/kg). The TBARS of the 0.1% ZBMEO and control groups increased to 1.46 and 1.32 mg/kg, respectively.
Hexanal was another secondary product of lipid oxidation. The change in the hexanal contents for all groups is shown in Fig. 2c, where the changing trend of hexanal was similar to that of TBARS. The initial hexanal contents of all groups had no significant difference, ranging from 1.15 μg/kg to 1.79 μg/kg, after which it increased slightly but not significantly (P > 0.05) in the first 6 days. The hexanal content of the control group increased in the rest storage time and was noticeably higher than that of other groups from 10 to 12 days. These results indicated high correlations between TBARS and hexanal formation, which agreed with a previous study (Mi et al. 2016).
Lipid oxidation is a chain reaction induced by free radicals, and the decomposition of hydroperoxides is the homolytic cleavage between two oxygen atoms, yielding one each of alkoxyl and hydroxyl radicals. At the same time, the alkoxyl radical can further yield lipid secondary oxidation products such as MDA and hexanal. ZBMEO is rich in monoterpenes and sesquiterpenes, and these components exhibited antioxidation properties (Diao et al. 2014). Thus, ZBMEO maybe could scavenge free radicals and inhibit the propagation of free radical reactions (Aminzare et al. 2018; Pateiro et al. 2018), and ZBMEO has good effect on inhibiting lipid oxidation.
Color evaluation
The acceptability of meat is first dependent on the change in sensory attributes, where color is the first criterion. Changes in the color of all groups from beginning to the end of the storage are presented in Fig. 2. The b* values increased (Fig. 2f), whereas the a* values (Fig. 2e) and L* values (Fig. 2d) decreased during the storage period; this trend was more noticeable in the control. The changes in the L*, a*, and b* values in this study were similar to those reported by Fan et al. (2019) for pork meat containing Portulaca oleracea L. extract during refrigerated storage. In the first 6 days, the L* and b* values for all groups slightly changed, whereas the a* values decreased thereafter. After 8 days, the L* and a* values of the BHT group were higher than those of the other groups. These results indicated that BHT was more effective in keeping meat color than others, followed by 0.3% ZBMEO. Between the beginning and the end of storage, ΔE of the control, BHT, 0.1% ZBMEO, 0.3% ZBMEO, and 0.5% ZBMEO groups was 13.76, 9.20, 11.86, 9.74, and 10.66, respectively. Thus, groups with natural and synthetic antioxidants exhibited lower ΔE compared with the control group. These results indicated that the addition of BHT and ZBMEO inhibited the discoloration of rabbit meat. Lipid and oxidation could produce reactive oxygen species and various aldehydes with powerful biological activities, these components could promote protein and heme iron oxidation. Thus, the L* and a* values decreased while b* value increased. The above results indicated that BHT and ZBMEO inhibited the protein and lipid oxidation, which means the groups treated ZBMEO lower ΔE compared with the control group. Several studies indicated that the discoloration of meat or meat products during storage is caused by oxidative reactions (Lin et al. 2019; Wibowo et al. 2015).
Sensory evaluation
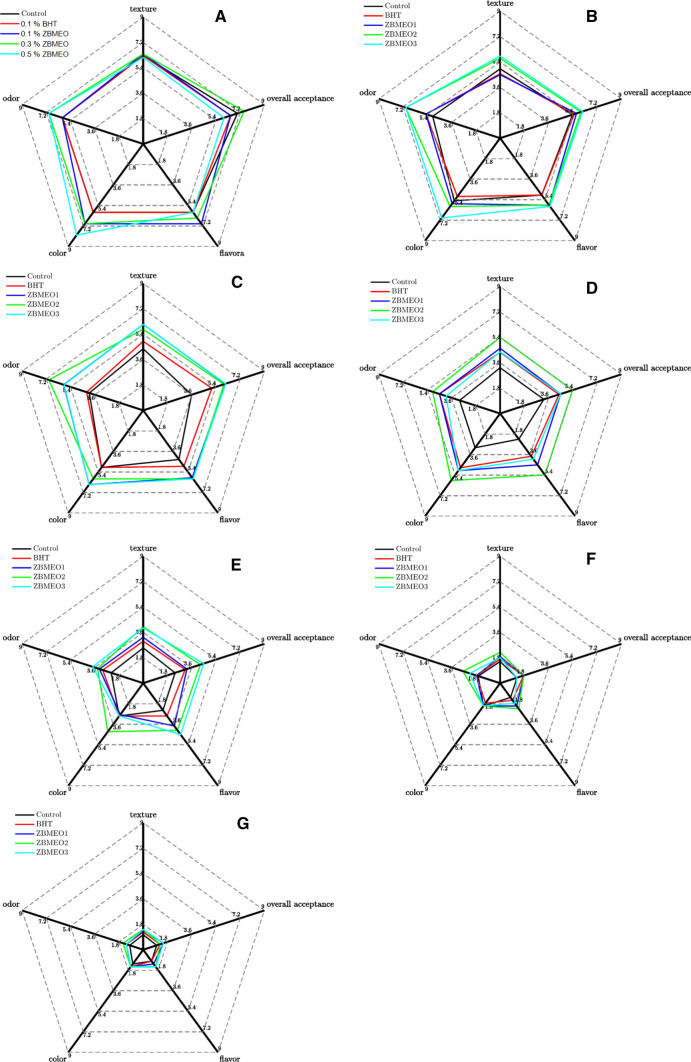
Figure 3 shows the loss of all sensory attributes of rabbit meat patties with the storage time for all groups. The addition of ZBMEO and BHT considerably reduced the storage effect on odor, color, flavor, texture, and overall acceptance, resulting in a milder quality decrease. The addition of 0.3% and 0.5% ZBMEO significantly improved the quality of rabbit meat. Given that a score of 5 indicated neither a like or a dislike; we defined it as an approximated threshold value for acceptance. The control group was considered nonacceptable, except for color since day 4. The BHT, 0.1% ZBMEO, and 0.5% ZBMEO groups were considered below this acceptance limit since day 6 regarding all attributes, whereas the 0.3% ZBMEO group was considered below this acceptance limit since day 8. All groups were unacceptable after 8 days, and no significant difference between groups was noted. The results of sensory evaluation had high correlations with microbial growth. Thus, BHT and ZBMEO could only extend but not completely stop the degenerative processes, and a proper amount of ZBMEO could improve the flavor of rabbit meat.
Fig. 3.
Effect of ZBMEO and BHT on Sensory evaluation of rabbit meat patties during chilled storage. BHT, butylated hydroxyl toluene. Control, no additive; 0.1% ZBMEO, 0.3% ZBMEO and 0.5% ZBMEO were represent add 0.1%, 0.3% and 0.5% ZBMEO, respectively. a Sensory evaluation on the initial day of storage for all groups; b sensory evaluation on the 2 days of storage for all groups; c sensory evaluation on the 4 days of storage for all groups; d Sensory evaluation on the 6 days of storage for all groups; e Sensory evaluation on the 8 days of storage for all groups; f Sensory evaluation on the 10 days of storage for all groups; g sensory evaluation on the 12 days of storage for all groups
Model
Microbial growth model
The fitting procedure was performed in MATLAB R2016b (MathWorks, USA) for a growth curve. The model parameters and microbial growth parameters were estimated, as illustrated in Table 2. In Table 2, the values of the growth parameters were estimated by the fitting of the full Huang model to the experimental data obtained in rabbit meat for all groups. The full Huang model fitted very well (, log CFU/g) with the changes in the microbial counts during storage time.
Table 2.
The parameters of the full Huang model fitted on the microbial growth curve of the total microbial counts of all groups during the chilled storage
| Groups | Model parameters | Microbial growth parameters | |||||
|---|---|---|---|---|---|---|---|
|
(log CFU/g) |
(log CFU/g) |
(log CFU/g d−1) |
(d) |
||||
| Control | 0.982 | 0.233 | 0.279 | 4.088 | 9.139 | 0.760 | 0.634 |
| 0.02% BHT | 0.997 | 0.048 | 0.127 | 4.099 | 9.165 | 1.011 | 2.747 |
| 0.1% ZBMEO | 0.983 | 0.210 | 0.265 | 4.054 | 9.198 | 0.697 | 0.8572 |
| 0.3% ZBMEO | 0.995 | 0.075 | 0.158 | 3.915 | 9.281 | 0.819 | 1.821 |
| 0.5% ZBMEO | 0.997 | 0.055 | 0.135 | 4.090 | 9.359 | 1.110 | 2.624 |
Analysis of the results of the full Huang model showed that the addition remarkably influenced several growth parameters (, λ) in rabbit meat but had minimal influence on and . However, the lag time () for the control, BHT, 0.1% ZBMEO, 0.3% ZBMEO, and 0.5% ZBMEO groups ranged from 0.634 to 2.747. Compared with the control group, the values of the BHT and 0.5% ZBMEO groups increased more than four times, and the values of in 0.3% ZBMEO and 0.1% ZBMEO increased to 0.857 and 1.821 days, respectively. BHT and ZBMEO had high antimicrobial activity. The value of increased with the amount of ZBMEO added. These results indicated that ZBMEO had good antibacterial effects due to the antimicrobial activity reported for components such as terpenes, terpenoids, and molecules with an aromatic ring in ZBMEO against Gram-positive bacteria (Myszka et al. 2019).
Lipid oxidation IP model
The kinetic parameters of the combinational kinetic model of the accumulation of lipid hydroperoxides and the parameters of lipid oxidation including the IP and the turning point time of all groups during chilled storage are shown in Table 3. The combinational kinetic model fitted well (, µmol/kg) with the changes in the PVs during storage time (Table 3) and provided a set of highly precise kinetic parameters.
Table 3.
The parameters of the combinational kinetic model fitted on the kinetic curve of the accumulation of lipid hydroperoxides during peroxidation of the all groups during the chilled storage
| Groups | Model parameters | Lipid oxidation parameters | |||||
|---|---|---|---|---|---|---|---|
|
(d−1) |
(g mmol−1 d−1) |
(d) |
(d) |
||||
| Control | 0.974 | 2.068 | 0.719 | 0.6277 | 0.05379 | 4.564 | 2.667 |
| 0.02% BHT | 0.967 | 2.011 | 0.709 | 0.4650 | 0.04002 | 6.026 | 3.112 |
| 0.1% ZBMEO | 0.980 | 1.376 | 0.587 | 0.4857 | 0.04116 | 5.493 | 2.754 |
| 0.3% ZBMEO | 0.925 | 4.191 | 1.024 | 0.4904 | 0.04685 | 5.704 | 3.040 |
| 0.5% ZBMEO | 0.911 | 6.411 | 1.266 | 0.4176 | 0.03214 | 6.585 | 3.592 |
The parameters of lipid oxidation were calculated. Table 2 shows that the first-order rate constants of the control, BHT, 0.1% ZBMEO, 0.3% ZBMEO, and 0.5% ZBMEO groups were 0.6277, 0.4650, 0.4857, 0.4904, and 0.4176 day−1, respectively. The group with the minimum second-order rate constant was 0.5% ZBMEO, followed by BHT, 0.3% ZBMEO, 0.1% ZBMEO, and control groups. The lower the rate constant was, the slower the oxidation of lipid. Therefore, BHT and ZBMEO could delay lipid oxidation to some extent. The longest IP among all groups was 3.592 days observed in 0.5% ZBMEO, followed by those in BHT (3.112 days) and 0.3% ZBMEO (3.040 days). The IP of the 0.1% ZBMEO and control groups were 2.754 and 2.667 days, respectively. BHT and ZBMEO in rabbit meat during chilled storage. The results were similar to the findings of a previous report (Amiri et al. 2019).
Conclusion
In this study, GC–MS analysis results showed that the most dominant component was linalool (51.91%), followed by limonene (18.17%) and sabinene (13.27%) in ZBMEO. The addition of ZBMEO to rabbit meat patty effectively reduced lipid oxidation compared with the control. The addition of 0.3% ZBMEO and 0.5% ZBMEO retarded protein oxidation during the chilled storage time. Treatment with ZBMEO decreased discoloration and pH compared with the control. The antimicrobial activity of ZBMEO was also observed. The models indicated that ZBMEO could prolong the IP of lipid oxidation and the microbial growth lag time. The antioxidant and antimicrobial activities of ZBMEO could be related to monoterpenes and sesquiterpenes and their oxygenated derivatives. The results of the storage experiment indicated that ZBMEO has a good protective effect on the quality of rabbit meat, and was recommended as a natural and effective additive to rabbit meat. Further studies are necessary to elucidate the antioxidant and antimicrobial mechanisms of ZBMEO in meat systems.
Acknowledgements
Hongjun Li would like to thank the financial support from the Earmarked Fund for China Agriculture Research System (Grant No. CARS-43-E-1) and Chongqing Herbivorous livestock Industry Technology System (Y201706).
Abbreviations
- ZBMEO
Zanthoxylum bungeanum Maxim. Essential oil
- IP
Induction period
- TVB-N
Total volatile basic nitrogen
- BHT
Butylated hydroxytoluene
- ZBM
Zanthoxylum bungeanum Maxim.
- LTL
Longissimus thoracis et lumborums
- TBARS
Thiobarbituric acid reactive substances
- PV
Peroxide value
Authors’ contributions
Zefu Wang: conceived and designed the investigation, performed most of experiments, and wrote the paper. Zhifei He: performed statistical analysis about the models. Dong Zhang: collected data and do some experiments about color evaluation for the paper. Xiaosi Chen: helped revise the paper. Hongjun Li: funding acquisition, and conceived the investigated together with the first author.
Data availability
Research data are not shared.
Code availability
Not applicable.
Compliance with ethical standards
Conflicts of interest
Authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zefu Wang, Email: 1137224683@qq.com.
Zhifei He, Email: 2628576386@qq.com.
Dong Zhang, Email: 1277136118@qq.com.
Xiaosi Chen, Email: 2939333903@qq.com.
Hongjun Li, Email: 983362225@qq.com.
References
- Ahmed HM, Tavaszi-Sarosi S. Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. 2019;275:730–738. doi: 10.1016/j.foodchem.2018.09.155. [DOI] [PubMed] [Google Scholar]
- Aminzare M, Tajik H, Aliakbarlu J, Hashemi M, Raeisi M. Effect of cinnamon essential oil and grape seed extract as functional-natural additives in the production of cooked sausage-impact on microbiological, physicochemical, lipid oxidation and sensory aspects, and fate of inoculated Clostridium perfringens. J Food Saf. 2018;38(4):e12459. doi: 10.1111/jfs.12459. [DOI] [Google Scholar]
- Amiri E, Aminzare M, Azar HH, Mehrasbi MR. Combined antioxidant and sensory effects of corn starch films with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on fresh ground beef patties. Meat Sci. 2019;153:66–74. doi: 10.1016/j.meatsci.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Cairo PLG, Gois FD, Sbardella M, Silveira H, De Oliveira RM, Allaman IB, Cantarelli VS, Costa LB. Effects of dietary supplementation of red pepper (Schinus terebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J Sci Food Agric. 2017;98(2):541–548. doi: 10.1002/jsfa.8494. [DOI] [PubMed] [Google Scholar]
- Chang W, Liu F, Sharif HR, Huang Z, Goff HD, Zhong F. Preparation of chitosan films by neutralization for improving their preservation effects on chilled meat. Food Hydrocoll. 2019;90:50–61. doi: 10.1016/j.foodhyd.2018.09.026. [DOI] [Google Scholar]
- Cullere M, Dalle Zotte A, Tasoniero G, Giaccone V, Szendrő Z, Szín M, Odermatt M, Gerencsér Z, Dal Bosco A, Matics Z. Effect of diet and packaging system on the microbial status, pH, color and sensory traits of rabbit meat evaluated during chilled storage. Meat Sci. 2018;141:36–43. doi: 10.1016/j.meatsci.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Dalle Zotte A, Szendrő Z. The role of rabbit meat as functional food. Meat Sci. 2011;88(3):319–331. doi: 10.1016/j.meatsci.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Davies MJ. Protein oxidation and peroxidation. Biochem J. 2016;473(Pt 7):805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao W, Hu Q, Zhang H, Xu J. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35(1):109–116. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- Eckl PM, Bresgen N. Genotoxicity of lipid oxidation compounds. Free Rad Biod Med. 2017;111:244–252. doi: 10.1016/j.freeradbiomed.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Fan X, Liu S, Li H, He J, Feng J, Zhang X, Yan H. Effects of Portulaca oleracea L. extract on lipid oxidation and color of pork meat during refrigerated storage. Meat Sci. 2019;147:82–90. doi: 10.1016/j.meatsci.2018.08.022. [DOI] [PubMed] [Google Scholar]
- FAOSTAT (2018) https://www.fao.org/faostat/en/#data/QL
- Farhoosh R. Reliable determination of the induction period and critical reverse micelle concentration of lipid hydroperoxides exploiting a model composed of pseudo-first and -second order reaction kinetics. LWT. 2018;98:406–410. doi: 10.1016/j.lwt.2018.09.003. [DOI] [Google Scholar]
- Firuzi MR, Niakousari M, Eskandari MH, Keramat M, Gahruie HH, Mousavi Khaneghah A. Incorporation of pomegranate juice concentrate and pomegranate rind powder extract to improve the oxidative stability of frankfurter during refrigerated storage. LWT. 2019;102:237–245. doi: 10.1016/j.lwt.2018.12.048. [DOI] [Google Scholar]
- Fu L, Xie H, Shi S. Multielement analysis of Zanthoxylum bungeanum Maxim. essential oil using ICP-MS/MS. Anal Bioanal Chem. 2018;410(16):3769–3778. doi: 10.1007/s00216-018-1040-8. [DOI] [PubMed] [Google Scholar]
- Imazaki PH, Elansary M, Scippo M, Daube G, Clinquart A. Effect of sex and sub-zero storage temperature on the microbial and oxidative stability of beef packed in a high-oxygen atmosphere after different vacuum ageing times. Meat Sci. 2019;148:198–205. doi: 10.1016/j.meatsci.2018.09.005. [DOI] [PubMed] [Google Scholar]
- ISO (2007) Sensory analysis—general guidance for the design of test rooms. International Organization for standardization ISO 8589
- Lan Y, Shang Y, Song Y, Dong Q. Changes in the quality of superchilled rabbit meat stored at different temperatures. Meat Sci. 2016;117:173–181. doi: 10.1016/j.meatsci.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Lee MA, Kim TK, Hwang KE, Choi YJ, Park SH, Kim CJ, Choi YS. Kimchi extracts as inhibitors of colour deterioration and lipid oxidation in raw ground pork meat during refrigerated storage. J Sci Food Agric. 2019;99(6):2735–2742. doi: 10.1002/jsfa.9441. [DOI] [PubMed] [Google Scholar]
- Li B, Xu Y, Li J, Niu S, Wang C, Zhang N, Yang M, Zhou K, Chen S, He L, Liu S, Yin S, Yang Y. Effect of oxidized lipids stored under different temperatures on muscle protein oxidation in Sichuan-style sausages during ripening. Meat Sci. 2019;147:144–154. doi: 10.1016/j.meatsci.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Li J, Hui T, Wang F, Li S, Cui B, Cui Y, Peng Z. Chinese red pepper (Zanthoxylum bungeanum Maxim.) leaf extract as natural antioxidants in salted silver carp (Hypophthalmichthys molitrix) in dorsal and ventral muscles during processing. Food Control. 2015;56:9–17. doi: 10.1016/j.foodcont.2015.03.001. [DOI] [Google Scholar]
- Lin L, Mao X, Sun Y, Rajivgandhi G, Cui H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int J Food Microbiol. 2019;292:21–30. doi: 10.1016/j.ijfoodmicro.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Mi H, Guo X, Li J. Effect of 6-gingerol as natural antioxidant on the lipid oxidation in red drum fillets during refrigerated storage. LWT. 2016;74:70–76. doi: 10.1016/j.lwt.2016.07.029. [DOI] [Google Scholar]
- Myszka K, Leja K, Majcher M. A current opinion on the antimicrobial importance of popular pepper essential oil and its application in food industry. J Essent Oil Res. 2019;31(1):1–18. doi: 10.1080/10412905.2018.1511482. [DOI] [Google Scholar]
- Okpala COR, Bono G, Falsone F, Cani MV, Scannella D, Di Maio F. Aerobic Microbial Inactivation Kinetics of Shrimp Using a Fixed Minimal Ozone Discharge: A Fact or Fib During Iced Storage. Procedia Food Science. 2016;7:47–52. doi: 10.1016/j.profoo.2016.02.084. [DOI] [Google Scholar]
- Özünlü O, Ergezer H, Gökçe R. Improving physicochemical, antioxidative and sensory quality of raw chicken meat by using acorn extracts. LWT. 2018;98:477–484. doi: 10.1016/j.lwt.2018.09.007. [DOI] [Google Scholar]
- Pateiro M, Barba FJ, Domínguez R, Sant'Ana AS, Mousavi Khaneghah A, Gavahian M, Gómez B, Lorenzo JM. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: a review. Food Res Int. 2018;113:156–166. doi: 10.1016/j.foodres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Pomponio L, Ruiz-Carrascal J. Oxidative deterioration of pork during superchilling storage. J Sci Food Agric. 2017;97(15):5211–5215. doi: 10.1002/jsfa.8403. [DOI] [PubMed] [Google Scholar]
- Sommers C, Huang C, Sheen L, Sheen S, Huang L. Growth modeling of Uropathogenic Escherichia coli in ground chicken meat. Food Control. 2018;86:397–402. doi: 10.1016/j.foodcont.2017.12.007. [DOI] [Google Scholar]
- Souza VGL, Pires JRA, Vieira ÉT, Coelhoso IM, Duarte MP, Fernando AL. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: from in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019;89:241–252. doi: 10.1016/j.foodhyd.2018.10.049. [DOI] [Google Scholar]
- Sujiwo J, Kim H, Song S, Jang A. Relationship between quality and freshness traits and torrymeter value of beef loin during cold storage. Meat Sci. 2019;149:120–125. doi: 10.1016/j.meatsci.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Uliana MP, Fronza M, Silva AGD, Vargas TS, Andrade TUD, Scherer R. Composition and biological activity of Brazilian rose pepper (Schinus terebinthifolius Raddi) leaves. Ind Crops Prod. 2016;83:235–240. doi: 10.1016/j.indcrop.2015.11.077. [DOI] [Google Scholar]
- Wang Z, He Z, Emara AM, Gan X, Li H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019;288:405–412. doi: 10.1016/j.foodchem.2019.02.126. [DOI] [PubMed] [Google Scholar]
- Wang Z, He Z, Gan X, Li H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018;146:131–139. doi: 10.1016/j.meatsci.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Wibowo S, Vervoort L, Tomic J, Santiago JS, Lemmens L, Panozzo A, Grauwet T, Hendrickx M, Van Loey A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015;171:330–340. doi: 10.1016/j.foodchem.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Xu L, Cheng J, Liu X, Zhu M. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. LWT. 2019;100:62–68. doi: 10.1016/j.lwt.2018.10.025. [DOI] [Google Scholar]
- Zeng M, Wang J, Zhang M, Chen J, He Z, Qin F, Xu Z, Cao D, Chen J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018;239:111–118. doi: 10.1016/j.foodchem.2017.06.097. [DOI] [PubMed] [Google Scholar]
- Zhang W, Guo S, You C, Geng Z, Liang J, Deng Z, Wang C, Du S, Wang Y. Chemical composition of essential oils from Zanthoxylum bungeanum Maxim. and their bioactivities against Lasioderma serricorne. J Oleo Sci. 2016;65(10):871–879. doi: 10.5650/jos.ess16038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.
Not applicable.