Abstract
Xanthosoma sagittifolium and Colocasia esculenta contain high levels of nutrients; but have naturally toxic compounds, oxalates and hydrocyanic acid (HCN). The objective of this work was to evaluate the effect of heat treatment on the concentration of antinutrients in malanga corms and its effect on mice. Malanga samples were heated to a boil for 0 to 120 min; oxalates and HCN were determined by spectrophotometry, at 710 and 510 nm, respectively. Pellets were prepared from raw malanga flour (15 and 50%), cooked malanga (15 and 50%) and wheat flour (control) and fed for nine weeks to five groups of six mice each. Cooking of X. sagittifolium corms for 80 min reduced oxalates present by 75% (143 to 35.6 mg/100 g sample), while oxalates in C. esculenta were reduced by 83% (345 to 57.8 mg/100 g sample). HCN levels became negligible after 20 min of cooking. During the nine weeks of feeding the different mice groups showed no significant difference (p > 0.05) between initial and final weight, with respect of the control; mice did not lose their appetite. The results indicate that the consumption of cooked malanga does not pose an evident risk to health, assessed by the reduced level of antinutrients, being an excellent alternative for feeding people in communities with prevalence of food insecurity.
Keywords: Xanthosma sagittifolium, Colocasia esculenta, Oxalate, HCN, Mice
Introduction
Malanga corms of Colocasia esculenta, are an underground stem that is widely grown as a staple food in many parts of Africa, the Americas, the Pacific Islands and Asia; they rank fifth and sixth in world production and planting, after cassava, potato, sweet potato, yam and taro (Pérez et al. 2007; Morales and Santacruz 2017); and corms of X. sagittifolium, known as taioba, tannia or cocoyam, belongs to the Araceae family and is native to tropical America (Almeida et al. 2013). Its corms and cormels, which contain mainly starch, are used as a subsistence food in many parts of the tropics and subtropics (Falade and Okafor 2015). There are several reports indicating that malanga corms are suitable for the production of many food products, especially for infants with allergic problems and people with gastrointestinal disorders. Antidiabetic, hypolipidemic, and antioxidant effects have been observed in a related species of X. violaceum (Faisal et al. 2014). Apigenin derivatives, vitexin and isovitexin have been reported in X. violaceum. The same authors have reported that apigenin can regulate diabetes mellitus, as well as diabetes-induced thyroid dysfunction and lipid peroxidation in aloxane-induced diabetic mice (Faisal et al. 2014).
One of the disadvantages in the use of raw malanga (either C. esculenta or X. Sagittifolium), is the presence of anti-physiological (anti-nutritional) factors, as well as the presence of oxalates in all parts of the plant. Oxalates originate in many plant species in a soluble or insoluble form as a final part of their metabolism; their content may vary according to the type of food, soil, or age, among other factors (Akhtar et al. 2011). The level of oxalate in Araceae can be reduced by peeling, grating, soaking, fermenting and cooking before consumption; improper cooking can cause irritable sensations (itching) in the throat and interfere with the biodistribution of other nutrients leading to health problems when ingested above threshold levels (Pérez et al. 2007; Boakye et al. 2018). However, a substance cannot be classified as an anti-nutrient based on the intrinsic characteristics of the compound. An anti-nutrient exerts its adverse effects generally at the digestive process in humans (Udousoro et al. 2013).
Cyanogenic glycosides (CNG) are derivatives of amino acids, a group of secondary metabolites present in more than 2500 species of plants. Chemically, CNG are described as the glycosides of α-hydroxynitriles (Natesh et al. 2017); these compounds, upon hydrolysis produce toxic hydrogen cyanide (HCN). Cyanide ions inhibit several enzyme systems and stop growth through interference with certain essential amino acids and the utilization of associated nutrients (Udousoro et al. 2013). High levels of HCN have been implicated in brain damage and lethargy in men and animals (Agbaire 2011); other studies indicate that HCN has inhibitory effects on the respiratory, nervous, endocrine and cardiovascular systems (Tuncel et al. 2017). In plants, HCN is metabolized to β+-cyanoalanine, which in turn is converted into asparagine or γ-glutamyl-cyanoalanine, as a detoxification pathway used to prevent accumulation of HCN; this occurs in plants even with high rates of ethylene biosynthesis (De la Cruz et al. 2010).
Several processing methods have been carried out to reduce the HCN content by inactivation of the enzyme β-glucosidase that include autoclaving, microwave heating, extrusion cooking, roasting and solvent treatment (Tuncel et al. 2017; Natesh et al. 2017). In recent years, malanga consumption has increased in some regions of Mexico, Central and South America. Despite this, the development of products from the different malanga plants has been limited. In contrast to the enormous potential it represents as a food product, especially in places where access to food is limited, and can be used as a strategy to improve food security conditions; therefore, it is necessary to carry out tests intended to evaluate the content of oxalates and HCN, and their effect on a biological system. The aim of the present study was to determine the content of oxalates and HCN in fresh and heat-treated malanga corms, as well as to assess the effect of these two anti-nutrients in a murine model through the addition of raw and heat-treated malanga corms flour in their feed.
Materials and methods
Proximate chemical composition and oxalate content was determined at the Research and Functional Products Development Laboratory (LIDPF), biological evaluation was performed at the Multidisciplinary Experimental Laboratory and Animal Facility, both at the University of Sciences and Arts of Chiapas. The determination of HCN was carried out at the Post-Harvest Laboratory of the Research Unit and Food Development (UNIDA) of the Veracruz Institute of Technology.
Biological material
Malanga corms of X. sagittifolium were used, obtained from the region of San Fernando, Chiapas, Mexico; as well as C. esculenta donated by the Colegio de Posgraduados Campus Veracruz, Mexico. The murine model was formed by adult CD-1® IGS mice (14 weeks) from the Institute of Biomedical Research of the National Autonomous University of Mexico. The description of the strain of mice used can be found at https://www.criver.com/sites/default/files/resources/CD-1IGSMouseModelInformationSheet.pdf.
Proximate chemical analysis
Moisture, ash, ethereal extract (fat), protein, and crude fiber were determined by methods published by the Official Methods of the Association of official Analytical Chemists (AOAC) (1995); the nitrogen-free extract (total carbohydrates) was calculated by difference; all analyses were performed in triplicate.
pH
Ten grams of finely ground malanga flesh and 5 g of flour were mixed separately in 50 mL of deionized distilled water at 25 °C; both solutions were filtered for pH measurement, using a pH-meter (Hanna Instruments®) previously calibrated with buffer solutions (Golden Bell) of pH 4.01 and 7.01.
Oxalate content
The cube-shaped samples (80 g) were placed in beakers with 100 mL of deionized distilled water and heated to a temperature above 95 °C. The solution was sampled at 0, 20, 40, 60, 80, 100 and 120 min, for analysis of calcium oxalate concentration. Quantification of these compounds was performed by titration of the extracts with a 0.05 N KMnO4 solution, using the method published by Olajide et al. (2011), considering the conversion factor:
| 1 |
HCN (hydrocyanic acid)
The hydrocyanic acid content in the samples of heat-treated malanga corms and cassava (used as reference) was determined by spectrophotometry using the method published by Olajide et al. (2011), with a UV–Vis diode array spectrophotometer (Agilent®, Model 8453) at 510 nm. The hydrocyanic acid content (ppm or mg HCN/kg sample) was calculated by multiplying the absorbance obtained by 396.
Biological test
Pellet production
First, two types of flour were made, one with fresh malanga (HF) corms and the other with boiled corms (HH). For the mice food preparation (pellet) were mixed, malanga flour (HF or HH) in two different proportions (15 and 50%) and wheat flour (Table 1), according to group of mice to feed. They were mixed to homogeneity, adding tap water until a dough with the appropriate consistency was obtained; pellets were formed with a size of approximately 3 cm long and a radius of approximately 1 cm, dried at 60 °C for 3 h in a convection oven (TERLAB, TEH35D, Mexico), these were stored in air-tight plastic containers at room temperature.
Table 1.
Flour mixture used for the production of pellets
| Treatment | Malanga flour (%) | Wheat flour (%) | |
|---|---|---|---|
| Raw | Boiled | ||
| Group 1 | 0 | 15 | 85 |
| Group 2 | 15 | 0 | 85 |
| Group 3 | 50 | 0 | 50 |
| Group 4 | 0 | 50 | 50 |
| Control group | 0 | 0 | 100 |
Mouse feeding period
For the biological evaluation, five groups of adult CD-1 mice (14 weeks) were formed, each group containing six mice (n = 6, m = 30). The animals were kept in the Multidisciplinary Experimental Laboratory and Animal Facility for a period of 9 weeks, under the following conditions: temperature 27 ± 2ºC, relative humidity of 65 ± 2%, and constant darkness. The experiment was approved by the Ethics and Biosecurity Committee of the Faculty of Nutrition and Food Sciences of the University of Sciences and Arts of Chiapas. The feeding period was from March 3 to May 28, 2020 (9 weeks) according to Table 1. During this period the average food consumption and weight of the mice per group were quantified; also, some aspects of grooming were observed and recorded.
Statistical analysis
The results of pH, oxalates content, HCN and weight were analyzed by ANOVA using MINITAB® statistical software version 16.0 for Windows.
Results and discussion
Appearance and proximate chemical analysis of malanga corms
The internal appearance in the corms of the two genera of malanga used, is shown in Fig. 1. The genus X. sagittifolium (Fig. 1a) from the Zoque region (San Fernando, Chiapas), displayed a white flesh with purple streaks, an attribute that makes it sensorially very appealing to the consumer, even after processing. The genus of C. esculenta (Fig. 1b) from Veracruz, had a yellowish flesh with brown streaks; the color of both products could be caused by the presence of carotenoids, and these in turn are related to the content of antioxidants.
Fig.1.
Malanga corms. a Xanthosoma sagittifolium from Chiapas, b Colocasia esculenta from Veracruz
Table 2 shows the results of the proximate chemical analysis of malanga corms from the states of Chiapas and Veracruz, as well as their flour. All components of fresh corms and their flours from the two evaluated species showed significant differences (p < 0.05), except for the carbohydrate content of the flours that showed no significant differences (p > 0.05). As it can be seen, the most abundant nutrients are carbohydrates, and within this group starch is the most important. Microscopic inspection indicates that malanga starch granules are smaller than potato granules, which suggests better nutritional characteristics. On the other hand, the protein content in the flour is greater than 4.0%, an amount higher than reported for some vegetables, such is the case of chard and spinach, which contain both about 2.9% (Cruz-Ordóñez et al. 2017). Pérez et al. (2007) reported a protein content of 6.3 and 6.5% in flours of C. esculenta and X. sagittifolium, respectively. The moisture content of both flours was less than 11%, which guarantees their stability during storage, since food products with high carbohydrate content and moisture less than 12% do not allow microbial growth.
Table 2.
Proximate chemical composition of fresh corms and malanga flour of the genera Xanthosoma sagittifolium (from Chiapas) and Colocasia esculenta (from Veracruz)
| Parameter | Fresh corms | Flour | ||
|---|---|---|---|---|
| X. sagittifolium | C. esculenta | X. sagittifolium | C. esculenta | |
| Moisture | 82.67 ± 0.28a | 64.90 ± 0.57b | 10.56 ± 0.22a | 7.29 ± 0.09b |
| Crude protein (N × 6.25) | 1.22 ± 0.07a | 2.89 ± 0.13b | 4.08 ± 0.13a | 5.56 ± 0.06b |
| Fat | 1.07 ± 0.10a | 0.82 ± 0.04b | 3.95 ± 0.09a | 2.45 ± 0.07b |
| Crude fiber | 0.88 ± 0.08a | 1.13 ± 0.04b | 6.62 ± 0.28a | 9.60 ± 0.14b |
| Ash | 0.33 ± 0.09a | 2.27 ± 0.11b | 2.82 ± 0.11a | 3.04 ± 0.08b |
| Carbohydrates | 13.83 ± 0.11a | 28.0 ± 0.55b | 71.97 ± 1.8a | 72.07 ± 0.09a |
*Different letters in the same row per component (fresh corms or malanga flour) show significant statistical differences (p < 0.05, ANOVA)
pH values
The pH values obtained were very close to neutral, X. sagittifolium (fresh corm 6.18 ± 0.02, flour 6.54 ± 0.19) and C. esculenta (fresh corm 6.35 ± 0.34, flour 6.44 ± 0.07); besides there was no significant differences (p > 0.05, data not shown) between the values of fresh corms from Chiapas and Veracruz, as well as their flours. It is worth mentioning that malanga plants, besides coming from different geographical areas, were also from different species. The corms from Chiapas were of the species X. sagittifolium and those from Veracruz belonged to C. esculenta. Very close values in the range of 6.56 to 7.59 were reported by Falade and Okafor (2015), in fresh corms of Colocasia spp and Xanthosoma spp; while the same authors reported for corm flours pH values in the range of low acid to neutral (4–8). Pérez et al. (2007), reported pH values in flours similar to ours, 6.3 and 6.5, for C. esculenta and X. sagittifolium, respectively. The pH values found in the corms, their moisture and nutrient content, make an ideal environment for the growth of an array of microorganisms, so that their preservation should be done through refrigeration or dehydration; such is the case of the flours, that although they had pH values close to neutral their moisture is reduced which limits microbial growth.
Oxalate content
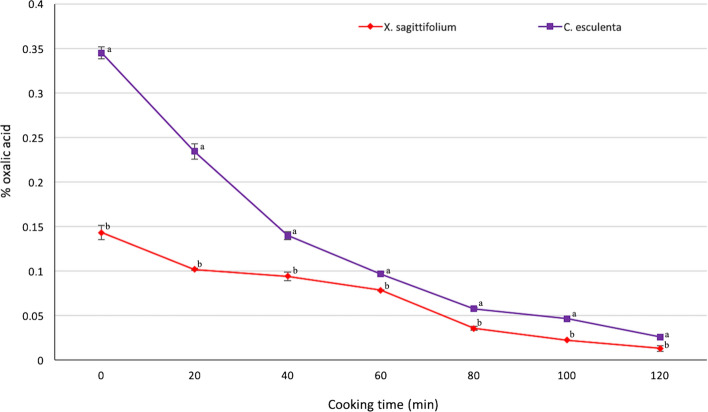
The content of soluble oxalates in fresh corms was greater than 0.143% (143 mg/100 g sample) and 0.345% (345 mg/100 g sample) in the corms of Xanthosoma sagittifolium and the corms of Colocasia esculenta, respectively (Fig. 2); when the corms were cooked (boiled) the levels of the compound decreased as time passed. It is noted that the inactivation rate was significantly different (p < 0.05) between the two species of malanga; for X. sagittifolium corms after 80 min of cooking oxalates reached levels below 0.0356% (35.6 mg oxalates/100 g sample), while C. esculenta corms in the same time reached levels below 0.0578% (57.8 mg/100 g sample). Abdulrashid and Agwunobi (2012) reported oxalate contents in raw malanga of 33.32 mg/100 g and cooked malanga of 21.70 mg/100 g. According to Boakye et al. (2018), drying (conventional or drum kiln, and solar) can efficiently reduce the content of anti-nutrients in malanga to appreciable levels; also, the fermentation of corms reduced considerably the levels of oxalate, phytates and tannins in Colocasia spp. Cooking (boiling) of Japanese taro cormels (Colocasia esculenta L. Schott) reduced the level of soluble oxalate in the cooked tissue below detectable levels (Akhtar et al. 2011). Oxalic acid does not interfere with zinc absorption and metabolism; insoluble oxalate is excreted in the feces, while soluble oxalate affects the human body by forming strong chelates with dietary calcium and other minerals making a complex unavailable for absorption and assimilation (Natesh et al. 2017). This insoluble calcium oxalate is stored as a crystal in the kidney causing a condition called kidney stone. The adverse effect of calcium absorption is greatest when the oxalate:calcium ratio is greater than 9:4 (Natesh et al. 2017). The intake and adsorption of oxalates in the population depends on the type of diet, level of cooking, amount of food ingested, bioavailability of the compound, weight and height of the person, among other factors. When the degradation capacity of oxalate is exceeded by the batteries or endogenous enzymes, caused by an excess consumption of oxalate-rich foods or metabolic disorders, it is excreted in the urine (Jáuregui-Zúñiga and Moreno 2004). Oxalobacter formigenes is believed to be primarily responsible for the breakdown of oxalate in animals and humans; its absence may predispose individuals to idiopathic calcium oxalate kidney stone disease (Stewart et al. 2004).
Fig.2.
Amount of oxalates present in malanga samples (Xanthosoma sagittifolium and Colocasia esculenta) during the cooking (boiling) process. Different letters in the same cooking time indicate statistical differences (p < 0.05, ANOVA)
There is a wide range and variability in the content of oxalates in foods; for example, broccoli varies from 0.3 to 13 mg/100 g, potatoes from 5.5 to 30 mg/100 g, and wheat bran from 58 to 524 mg/100 g (Holmes and Kennedy 2000); this variability depends on many causes, including the variety and species of plant, the type of soil in which it is grown, environmental conditions for crop development, and the age of the plant or fruit, among others. The Western diet on average provides about 50—150 mg of oxalate/day, while the vegetarian diet may contribute somewhat more. The American Dietetic Association recommends that patients with kidney stones may restrict their dietary oxalate to less than 40 to 50 mg per day (Akhtar et al. 2011). Some authors, reported that oxalate present in malanga corms, should not cause any nutritional problem because people who consume it, usually subject corms to prolonged cooking, thereby destroying the compound (Púa et al. 2019; Owusu-Darko et al. 2014).
Hydrocyanic acid (HCN)
The hydrocyanic acid (HCN) present in fresh corms of both species of malanga was minimal or negligible compared to that contained in fresh cassava (Table 3). It can be observed that the amount of HCN present in the corms of malanga evaluated were significantly different (p < 0.05) among them; moreover, it is observed that 20 min of cooking were sufficient to eliminate completely the presence of HCN in the product. These results indicate that there is no risk in consuming malanga either raw or cooked, as the Committee of CODEX on Food Contaminants established in (2013), as health reference values for cyanogenic glycosides in cassava and cassava products; acute reference dose (ARD) of 0.09 mg/kg body weight, and a provisional maximum tolerable daily intake (PMTDI) of 0.02 mg/kg body weight, as cyanide. A study by Olajide et al. (2011), managed to reduce more than 57% of the HCN present in corms of malanga (Colocasia esculenta) by cooking and fermentation; the authors also indicated that the fermentation process was more efficient. Many reports indicate that heat treatments are the most suitable methods to reduce anti-nutrients, caused by the rupture of the cell wall by the effect of heat. Consequently, the solubility of the components in the cooking or blanching water occurs. Fresh tubers generally contain compounds that are hazardous to health, often related to their HCN content (Ketiku et al. 1977); when consumed, raw and containing high amounts of HCN, during the process of absorption in the small intestine, they stop the oxidation of the protoplasm in the tissue cells and can cause dizziness, headache, unconsciousness and seizures with cerebral palsy (Drochioiu et al. 2000). Heat treatments have proven to be very efficient in reducing or eliminating these anti-nutritional factors, as is the case with HCN. The consumption of vegetables with reduced or no content of HCN does not represent any risk in decreasing the bioavailability of other nutrients or for the health of the consumer; while, for vegetables with considerable levels, cooking is recommended, before consumption.
Table 3.
HCN values determined in raw and cooked corms de malanga at different times
| Amount of HCN (mg/kg sample) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Origin of the corm or tuber | Corm o raw tuber | Cooking time (boiling) | ||||||
| Raw malanga flour | 20 min | 40 min | 60 min | 80 min | 100 min | 120 min | ||
| X. sagittifolium | 0.1584 ± 0.0228a | 3.5218 ± 0.1754a | ND | ND | ND | ND | ND | ND |
| C. esculenta |
3.5244 ± 0 .0396b |
4.4715 ± 0.1978b | ND | ND | ND | ND | ND | ND |
| Veracruz Cassava | 117.27 ± 1.3552c | ND | ND | NR | ND | NR | ND | NR |
Different letters in the same column indicate significant statistical differences (p < 0.05, ANOVA)
NR not realized, ND not detected
Biological test with mice
Figure 3A shows that the consumption of foods added with raw malanga in its different concentrations (Groups 2 and 3, 15 and 50%, respectively) did not have a significant effect (p > 0. 05) between the initial and final weight of the mice; the same behavior was observed in the weight of the mice that were fed cooked malanga (Groups 1 and 4, 15 and 50%, respectively) and the control; therefore, it can be deduced that the amount of ANF (oxalates and HCN) present in the raw malanga corms used in mice feeding did not alter the weight of the animals, since there were no significant differences during the 9 weeks of evaluation. This is consistent with feed consumption, as shown in Fig. 3B for all groups, including the control; the average feed consumption during the first four weeks increased exponentially, and during the five weeks after that it remained unchanged. It should be noted that all groups showed the same consumption pattern, without significant differences (p > 0.05) between them, which indicates that the added malanga (raw or cooked) did not promote changes in that pattern. In general, a normal behavior could be observed in all the groups of mice studied, including the control. The number of precooking activities observed in the animals before the beginning of the feeding decreased considerably during the study period (data not shown); which indicates that malanga or its components did not cause any kind of discomfort or stress in the animals.
Fig.3.
a Initial and final average weight of groups of mice during the nine weeks of feeding with cooked and raw malanga. b Average food consumed by the groups of mice during the nine weeks of study. Different letters in the initial and final average weight of the mice by groups indicate statistical differences (p < 0.05, ANOVA)
Conclusion
Raw malanga corms have certain levels of oxalates (Xanthosoma sagittifoilium: 143 mg/100 g of flesh, Colocasia esculenta: 345 mg/100 g of flesh). By cooking more than 75% of their oxalates concentration could be reduced in both products. The amount of HCN present in flour and fresh corms is very low and was completely eliminated after 20 min of boiling. The reduction of these ANF by the cooking process indicates that the consumption of cooked malanga does not pose any risk to human health; furthermore, it was validated by the fact that there was no adverse effect on the weight of the mice fed the different percentages of raw and cooked malanga during the 9 weeks, and the control group; as well as by the fact that there were no changes in the feeding pattern of these animals. Because of the above, in addition to its nutritional and functional benefits, malanga is an excellent alternative for feeding people in communities where there is food insecurity. The use of malanga in food in rural communities promotes traditional gastronomy and encourages its production.
Acknowledgements
The authors would like to thank the students who participated in the development of activities as part of the social service program of the Bachelor of Science and Food Technology, as well as the academic technicians who participated of the University of Sciences and Arts of Chiapas and the Veracruz Institute of Technology. To the sectorial fund SAGARPA-CONACYT for the financing of the project with code 2016-01-277457 called Development of technologies for the use of malanga corms (Xanthosoma saggittifolium) from the state of Chiapas and Veracruz in the 2016 call.
Abbreviations
- HCN
Hydrocyanic acid
- X. sagittifolium
Xanthosoma sagittifolium
- C. esculenta
Colocasia esculenta
- X. violaceum
Xanthosoma violaceum
- ANF
Anti-nutritional factors
- CNG
Cyanogenic glycosides
- RFPDL
Research and Functional Products Development Laboratory
- UNIDA
Research Unit and Food Development
- AOAC
Association of Official Analytical Chemists
- UV–Vis
Ultraviolet–visible
- HF
Fresh malanga
- HH
Boiled corms
- ARD
Acute reference dose
- PMTDI
Provisional maximum tolerable daily intake
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gilber Vela-Gutiérrez, Email: gilber.vela@unicach.mx.
Arturo A. Velázquez López, Email: arturo.velazquez@unicach.mx
Veymar G. Tacias Pascacio, Email: veymar.tacias@unicach.mx
Dolores G. Vidal López, Email: lolita.vidal@unicach.mx
Elizabeth León García, Email: eliibq@gmail.com.
Javier De La Cruz Medina, Email: javierdelacruz2004@yahoo.com.mx.
References
- Abdulrashid M, Agwunobi LN. Tannia (Xanthosoma sagittifolium) Cocoyam as dietary substitute for maize in broiler chicken. Greener J Agric Sci. 2012;2(5):167–171. [Google Scholar]
- Agbaire PO. Nutritional and anti-nutritional levels of some local vegetables (Vernomia anydalira, Manihot esculenta, Teiferia occidentalis, Talinum triangulare, Amaranthus spinosus) from Delta State, Nigeria. J Appl Sci Environ Manag. 2011;15(4):625–628. [Google Scholar]
- Akhtar MS, Israr B, Bhatty N, Ali A. Effect of cooking on soluble and insoluble oxalate contents in selected Pakistani vegetables and beans. Int J Food Prop. 2011 doi: 10.1080/10942910903326056. [DOI] [Google Scholar]
- Almeida JE, Bernardes ME, Fonseca RH, Vanzela EC, Amaya-Farfán J. Taioba (Xanthosoma sagittifolium) leaves: nutrient composition and physiological effects on healthy rats. J Food Sci. 2013 doi: 10.1111/1750-3841.12301. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists – AOAC . Official methods of analysis of AOAC Intl. 163. Washington: AOAC Intl; 1995. [Google Scholar]
- Boakye AA, Wireko-Manu FD, Oduro I, Ellis WO, Gudjónsdóttir M, Chronakis LS. Utilizing cocoyam (Xanthosoma sagittifolium) for food and nutrition security: a review. Food Sci Nutr. 2018 doi: 10.1002/fsn3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Ordóñez MA, Palacios-Pola G, Márquez-Montes R, Vela-Gutiérrez G (2017) Tecnologías para la conservación de cormos de malanga (Xanthosoma sagittifolium). In: Corzo SA (eds) Aportaciones a las Ciencias Alimentarias, Velázquez, MJR y. México, pp 81–89. ISBN: 978-607-606-343-9
- Committee of CODEX Alimentarius (2013) Project of maximum levels for cyanhydric acid in cassava and cassava products. www.codexalimentarius.org. Accessed 9 Aug 2020
- De la Cruz J, Vela G, Dorantes L, García HS. Efecto del etileno sobre el ACC y ACC oxidasa en la maduración de papaya “Maradol”. Rev Fitotec Mex. 2010;33(2):133–140. [Google Scholar]
- Drochioiu G, Mangalagiu I, Tataru V. Specific spectrophotometric determination of hydrocyanic acid in the environment. R Soc Chem. 2000;125:939–941. [Google Scholar]
- Faisal M, Hossain AI, Rahman S, Jahan R, Rahmatullah M. Preliminary report on oral glucose tolerance and antinociceptive activity tests conducted with methanol extract of Xanthosoma violaceum aerial parts. BMC Comp Alt Med. 2014;14:335–339. doi: 10.1186/1472-6882-14-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade KO, Okafor CA. Physical, functional, and pasting properties of flours from corms of two Cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) cultivars. J Food Sci Technol. 2015 doi: 10.1007/s13197-014-1368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RP, Kennedy M. Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int. 2000;57:1662–1667. doi: 10.1046/j.1523-1755.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- Jáuregui-Zúñiga D, Moreno CA. La biomineralización del oxalato de calcio en plantas: retos y potencial. REB. 2004;23(1):18–23. [Google Scholar]
- Ketiku A, Akinyele O, Okinnawo O. Changes in the hydrocyanic acid concentration during traditional processing of cassava into ‘gari’ and ‘lafun’. Food Chem. 1977;3:221–228. doi: 10.1016/0308-8146(78)90022-5. [DOI] [Google Scholar]
- Morales V, Santacruz S. Uso de Películas Comestibles a base de Carboximetilcelulosa y goma Xantana para la Disminución de Absorción de Grasa de Malanga Frita (Xanthosoma Sagittifolium) Rev Politécnica. 2017;40(1):1–6. [Google Scholar]
- Natesh HN, Abbey L, Asiedu SK. An overview of nutritional and antinutritional factors in green leafy vegetables. Hort Int J. 2017;1(2):1–9. [Google Scholar]
- Olajide R, Akinsoyinu AO, Babayemi OJ, Omojola AB, Abu AO, Afolabi KD. Effect of processing on energy values, nutrient and anti-nutrient components of wild cocoyam [Colocasia esculenta (L.) Schott] Corm. Pak J Nut. 2011 doi: 10.1007/s13197-014-1368-9. [DOI] [Google Scholar]
- Owusu-Darko PG, Paterson A, Omenyo EL. Cocoyam (corms and cormels)—an underexploited food and feed resource. J Agric Chem Environ. 2014;3(1):22–29. doi: 10.4236/jacen.2014.31004. [DOI] [Google Scholar]
- Pérez EE, Gutiérrez ME, Pacheco de Delahaye E, Tovar J, Lares M. Production and characterization of Xanthosoma sagittifolium and Colocasia esculenta flours. Sens Nut Qual Food. 2007 doi: 10.1111/j.1750-3841.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- Púa A, Barreto G, Zuleta J, Herrera O. Análisis de nutrientes de la raíz de malanga (Colocasia esculenta Schott) en el trópico seco de Colombia. Inf Tecnol. 2019;30(4):69–76. doi: 10.4067/S0718-07642019000400069. [DOI] [Google Scholar]
- Stewart CS, Duncan SH, Cave DR. Oxalobacter formigenes and its role in oxalate metabolism in the human gut. FEMS Microbiol Lett. 2004;230:1–7. doi: 10.1016/S0378-1097(03)00864-4. [DOI] [PubMed] [Google Scholar]
- Tuncel NB, Uygur A, Yüceer YK. The effects of infrared roasting on HCN content, chemical composition and storage stability of flaxseed and flaxseed oil. J Am Oil Chem Soc. 2017 doi: 10.1007/s11746-017-2982-2. [DOI] [Google Scholar]
- Udousoro II, Ekop RU, Udo EJ. Effect of thermal processing on antinutrients in common edible green leafy vegetables grown in Ikot Abasi, Nigeria. Pak J Nut. 2013;12(2):162–167. doi: 10.3923/pjn.2013.162.167. [DOI] [Google Scholar]