Abstract
Faba bean flours (germinated, soaked, cooked and raw) were evaluated for physiochemical and functional properties. The flour samples exhibited considerable amounts of carbohydrates (58.79–66.19 g/100 g) and proteins (21.9–29.1 g/100 g). Soaked faba bean (SFB) (29.1 g/100 g) and raw faba bean (RFB) (25.70 g/100 g) flour contained significantly higher amount of protein than germinated faba bean (GFB) and pressure cooked faba bean (PCFB). The physicochemical and functional composition of GFB and PCFB were improved compare to raw flour. Physical and functional properties such as water absorption index (2.97 g/g) and foaming stability (140.13 mL/100 mL) were increased by germination. The functional properties of pressure-cooked faba bean such as water solubility index (2.12 g/100 g) and water absorption capacity (2.02 g/g) were higher than other flour samples. The microstructure of legume flour samples explained that the starch granules of raw flours were smooth, oval and granular structure whereas soaked, germinated and cooked flours showed damaged starch granules. The effect of soaking, germination and pressure-cooking demonstrated significant variations in functionals characteristics of faba bean flour. Therefore, various processing conditions can be combined to obtain the desired characteristics in faba bean-based food products.
Keywords: Legume, Nutritional composition, Functional properties, Microstructure
Introduction
Legumes are the earliest plants familiarised by human and considered as the rich source of proteins, phenolic compounds, flavonoids, vitamins minerals etc. and are commonly used as a staple food in various countries (Campos–Vega et al. 2010). Faba bean (Vicia faba L.) is a rich source of protein (27–34% of the dry weight) and various bioactive compounds such as phenolic compounds and flavonoids, with high antioxidant, anti-diabetic and anti-inflammatory properties (Collado et al. 2019; Liu et al. 2019). Despite the nutritional value, pulses are not consumed widely because of the presence of antinutrients (tannins, saponins and phytic acid) cooking difficulties and intense flavour profiles (Setia et al. 2019).
Soaking and germination are the traditional methods used to enhance the nutritional quality and to reduce the anti-nutritional factors of legumes (Handa et al. 2017). Soaking is used domestically to hydrate seeds in water for a few hours and to reduce the anti-nutrients present in the legumes (Kajihausa et al. 2014; Singh et al. 2017). Soaking is done before several treatments such as germination, cooking and fermentation (Handa et al. 2017). The soaking time of 12–18 h was reported to be effective in reducing the phytic acid and proteolytic enzyme inhibitors in legumes which are partially soluble in soaked water (Embaby 2010; Kajihausa et al. 2014). Germination enhances the nutritional value, flavour, aroma and organoleptic qualities of legumes by the activation of enzymes which reduces the antinutrients and indigestible compounds in legumes (Collado et al. 2019; Saleh et al. 2017). Cooking process helps in reducing anti-nutrients like trypsin and flatulence-causing oligosaccharide (Medhe et al. 2019).
The use of legume flour is an emerging trend for the development of functional food such as bread, pasta and other bakery products (Medhe et al. 2019). The functional properties of foods such as water absorption capacity, gel hydration, swelling and viscosity are directly known to influence the food formulation (Du et al. 2014). The functional properties of the food depend on molecular size, charge distribution and three-dimensional structure of the protein (Du et al. 2014). The structure–function association of proteins, decide their interactions with themselves and with other ingredients in complex food systems (Joshi et al. 2015). Functional properties influence the behaviour of food system during manufacturing, processing, storage and consumption due to the nature, molecular structure and size of the protein (Rodsamran et al. 2018).
None of the studies has yet been reported on comparative physicochemical, functional and antinutritional properties of raw, soaked, germinated and cooked faba bean flour. Therefore, the aim of this study was to investigate the functional characteristics of faba been flour after various processing techniques, such as soaking, germination and cooking.
Materials and methods
Faba bean (BR-2) were procured from the local market in Muzaffarpur, Bihar, India harvested in December 2017. Samples were examined physically to ensure the absence of any damage or disease and milled in milling machine, passed through 70 mesh sieve and finally stored in the cold (5 °C) temperature till further use.
Processing methods
Soaking, germinating and pressure cooking
For soaking, faba beans (200 g) were soaked for 24 h in 600 mL. For cooking process, 12 h soaked beans (200 g/600 mL) were cooked with 400 mL of water in a pressure cooker (TTK Prestige, 3.5 L). The pressure cooking was done for 15 min at 120 °C and 14 psi. For germination, the beans were soaked (200 g/600 mL) for 24 h and kept further for 24 h at 25 °C in a dark place as a single grain bed. After that, all the samples were oven-dried at 60 °C till constant weight, followed by milling and passed through 70 mesh sieve and finally stored at cold (5 °C) temperature for further use.
Chemical composition of flours
Flour samples of broad beans were estimated for their moisture, ash, fat, carbohydrate and protein contents by using the standard methods of analysis (AOAC 2005).
Antinutritional components of flours
Oxalate content
Oxalate content of the flour samples was determined following the method as described by Kaushal et al. (2012) with slight modification. Samples (1 g) were dispersed in 75 mL of 3 mol/L H2SO4, with continuous stirring for an hour and filtered through Whatman filter paper 1. The filtrate (25 mL) was titrated with 0.5 mol/L KMnO4 solution while hot (80–90 °C) until a faint pink colour was prolonged for at least 30 s. The oxalate content was calculated as 1 mL of 0.5 mol/L KMnO4 as equivalent to 2.2 mg of oxalate.
Tannin content
Tannin content was determined by following the method of H.P. Makkar (2003) with slight modifications. The sample (0.25 g) was boiled in 20 mL of distilled water for 30 min, followed by filtration with Whatman No 1 filter paper. The Folin-Ciocalteu reagent (0.25 mL), Na2CO3 (1.25 mL) and distilled water (0.50 mL) were added to the filtrate (1 mL).The mixture was further incubated at room temperature (25 °C) for 40 min. The absorbance at 725 nm was measured by using UV–VIS spectrophotometer (UNICAM UV/Vis spectrophotometer, UK). The standard tannic acid was used to develop the standard curve.
Phenolic content
The phenolic content of flour sample was determined as described by Sadiq et al. (2015) with slight modification. The sample 0.5 mL (5 mg/mL in distilled water) was mixed with 2 mL of the Folin-Ciocalteu reagent followed by the addition of 4 mL of sodium carbonate (7.5%, w/v). The reaction mixture was incubated at room temperature (25 °C) for 30 min, followed by measuring the absorbance at 765 nm using UV–Visible spectrophotometer. The standard curve was prepared by using gallic acid as a reference standard, and phenolic content was expressed as mg gallic acid equivalent (GAE)/g.
Flavonoid content
The total flavonoid content was determined by the colourimetric method as described by Sadiq et al. (2015) with slight modification. The sample, 0.5 mL (5 mg/mL in distilled water) was mixed with 10% aluminium chloride hexahydrate (0.1 mL), 1 M potassium acetate (0.1 mL) and distilled water (2.8 mL). The mixture was incubated for 40 min at 25 °C, and the absorbance was read at 415 nm. The standard curve was prepared by using quercetin as a reference standard. The total flavonoid content was expressed as mg quercetin equivalent (QE)/g.
Physical properties of flours
Color characteristics
Sample colour was analyzed by Hunter Lab Spectrocolorimeter (Model TC-P III A, Tokyo Denshoku Co., Ltd., Japan) by following the method of Medhe et al. (2019). Sample (10 g) flour was filled in the glass container of the instrument and placed over the slit of the equipment. CIE lab system was used to measure the colour parameters, where L* (L* = null means black and L* = 100 means white), a* (− a* = greenness and + a* = redness) and b* (− b* = blueness and + b* = yellowness). The total colour difference (∆E) was calculated by using the following Eq. 1
| 1 |
Water absorption index (WAI) and water solubility index (WSI)
Water absorption index (WAI) and water solubility index (WSI) of flours were determined following the method of Du et al. (2014) with slight modification. The sample (0.83 g) was mixed with 10 mL of distilled water and cooked at 90 °C for 15 min in a water bath. The cooked flour paste was then cooled at room temperature (25 °C) and centrifuged (model EBA 8S, Hettich, Germany) at 2310 × g for 10 min. The supernatant was further transferred into a pre-weighed evaporating dish, and the weight of sediment was taken. The sediment was dried at 110 °C and weighed again. Water absorption index and water solubility index were calculated using Eqs. 2 and 3, respectively:
| 2 |
| 3 |
Bulk density
The bulk density of flours was determined by the method described by Medhe et al. (2019) with slight modification. Samples were gently filled into 10 mL previously weighed graduated cylinders. The bottom of each cylinder was gently tapped several times until there was no further constriction of the sample level after filling up to the 10 mL mark. Bulk density was calculated as the weight of the sample per unit volume of sample (g/mL).
Functional properties of flours
Water absorption capacity and oil absorption capacity
Water absorption and oil absorption capacity of flour samples were determined following the method described by Chandra et al. (2015) with slight modification. For water absorption, sample (0.83 g) was mixed in 10 mL of distilled water and transferred in centrifuge tubes. Then, the dispersion was stirred periodically, held for 30 min, followed by centrifugation (model EBA 8S Hettich, Germany) for 25 min at 2310 × g. For the determination of oil absorption capacity, sample (0.5 g) was assorted with 6 mL of soybean oil and centrifuged at 2310 × g for 25 min. Separated oil was removed from the tubes kept inverted for 25 min to drain the oil before reweighing. The oil and water absorption capacities were expressed as a gram of water or oil bound per gram of the sample on a dry basis. Water absorption capacity and oil absorption capacity were calculated to follow the Eqs. 4 and 5, respectively:
| 4 |
| 5 |
Least gelation concentration of flour samples
Least gelation concentration of samples was determined by the method of Joshi et al. (2015) with slight modification. Sample was prepared in different concentrations (2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 g/100 mL) and 5 mL from each concentration was placed in test tubes and heated for an hour at 98 °C in a water bath, followed by rapid cooling under running tap water. The tubes were further cooled at 4 °C for 2 h. Least gelation concentration was expressed as the concentration above which the sample did not fall or slip when the test tubes were inverted.
Foaming capacity and foaming stability
Foaming properties of flours were determined by the method described by Du et al. (2014) with slight modification. Flour sample (1.5 g) was mixed with 50 mL of distilled water and homogenised, using a homogeniser (Servodyne, Model 50,000–25) at 960 rpm for 3 min. The suspended mixture was transferred into a graduated cylinder, and the homogeniser cup was rinsed with 10 mL of distilled water and added to the graduated cylinder. The volume was recorded before and after whipping. Foaming capacity (FC) and foaming stability (FS) were calculated following the Eq. 6.
| 6 |
FS = Foam volume changes in the graduated cylinder recorded at an interval of 20, 40, 60 and 120 min of storage.
Pasting properties of flours
Pasting properties of the legume flours were evaluated following the method of Taslima et al. (2015) by using Rapid Visco Analyzer (RVA) (Model 4, Newport Scientific Pvt., Ltd. Australia). Sample (2.5 g) was placed into the canister and dispersed thoroughly in 25 mL of distilled water. The suspension was heated at 50 ºC for one min, and then the temperature was increased to 95 °C for 3.2 min and finally, the temperature was again decreased to 50 °C. All the flour samples were dispersed and homogenised with 960 rpm throughout the test.
FTIR of legume flours
Infrared spectra (500 to 4000 cm−1) of the raw, soaked, germinated and pressure-cooked legume flours were obtained by attenuated total reflectance (ATR) Fourier transform infrared (FT-IR) spectrometer (Nicolet Avatar 36) following the method of Taslima et al. (2015).
Thermal properties
Thermal properties of legume flour samples were analyzed by using differential scanning calorimeter (DSC) (SHIMADZU/DSC-60A Plus) following the method of Falade and Christopher (2015) with slight modifications. The sample (1 mg) was weighed into pierced DSC aluminium pans, and distilled water was added to make the flour: water ratio of 1:3. The pans were hermetically sealed, and samples were left to stand for an hour at 25 °C for moisture equilibration. The sealed pans were heated from 20 °C to 130 °C under nitrogen gas at a heating rate of 10 °C min−1 to gelatinize the flour samples. An empty aluminium pan was used as a reference, and the calorimeter was calibrated with indium. From the DSC thermograms, the onset temperature (To), peak temperature (Tp), conclusion temperature (Tc) and enthalpy of gelatinization (ΔHG) were determined.
Scanning electron micrographs of legume flours
Microstructure images of legume flours were observed by scanning electron microscope (SEM) (Hitachi SU8230, Japan), as described by Shrestha et al. (2018). The sample particles were sprinkled with gold over carbon tape splattered, and then examinations were observed at an accelerated voltage of 5.000 kV using SEM.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) by using SPSS version 23 (SPSS, IBM, Chicago USA) significant differences (p < 0.05) among mean observations were evaluated by Tukey’s HSD test.
Results and discussion
Chemical composition of flour
The nutrient composition of flour samples is presented in table.1. The moisture, ash, protein, fat and carbohydrate contents vary considerably among samples and wherein the range of 5.93–11.26 g/100 g, 2.29–2.96 g/100 g, 21.9–29.10 g/100 g, 1.34–1.87 g/100 g, 58.79–66.19 g/100 g, respectively. SFB was observed with higher protein content compared to other samples. The soaking resulted an increase in biological breakdown of complex compounds into simple and non-protein nitrogen compounds which contributed to an increase in total protein content. Handa et al. (2017) reported higher protein content in soaked horse gram (22.62–27.52 g/100 g), as soaking resulted in the breakdown of complex components into simple compounds. Medhe et al. (2019) observed a decrease in protein content of germinated (23.60 to 16.71 g/100 g) and cooked (23.60 to 12.70 g/100 g) moth bean, which was similar to this study. The fat content in raw flour was 1.69 g/100 g which was not significantly different from the GFB (1.82 g/100 g) and SFB (1.87 g/100 g), however, the fat content in PCFB was significantly lower than other flours (1.34 g/100 g). Cooking treatment significantly (p < 0.05) decreased the fat content in PCFB flour which was due to the breakdown and diffusion of fats into the cooking water (Mubarak 2005). The slight increase in crude fat content of GFB and SFB was due to the leaching of soluble components, causing an overall increase in concentration of the lipids in the flour (Agume et al. 2017). Setia et al. (2019) reported higher fat content of SFB and GFB bean (1.3 g/100 g) than in RFB (0.9 g/100 g). Carbohydrate content of RFB was not significantly different from SFB flour however, it was observed significantly lower (p < 0.05) than GFB and PCFB flours. During germination, the carbohydrate content was increased due to increase in crude dietary fiber and structural carbohydrates like hemicellulose and cellulose. Martin et al. (2008) also observed an increase in dietary fiber during germination of cowpea and soybean. Thakur et al. (2019) also reported that soaking of legume grains softened the outer coat which resulted an increase in cellulose, hemicellulose and fiber content of the legumes. Medhe et al. (2019) reported an increase in carbohydrate content of germinated (69.93 g/100 g) and cooked (75.01 g/100 g) moth bean as compared to raw beans (63.17 g/100 g).
Table 1.
Nutritional and antinutritional composition of faba bean flours
| Parameters | RFB | SFB | GFB | PCFB |
|---|---|---|---|---|
| Moisture (g/100 g) | 11.26 ± 0.07d | 5.93 ± 0.05a | 6.21 ± 0.05b | 8.28 ± 0.06c |
| Fat (g/100 g) | 1.69 ± 0.17b | 1.87 ± 0.01b | 1.82 ± 0.01b | 1.34 ± 0.08a |
| Protein (g/100 g) | 25.70 ± 0.6b | 29.1 ± 0.7c | 24. 9 ± 0.7b | 21.9 ± 0.3a |
| Ash (g/100 g) | 2.56 ± 0.10a | 2.96 ± 0.12b | 2.86 ± 0.13b | 2.29 ± 0.12a |
| Carbohydrate (g/100 g) | 58.79 ± 0.74a | 60.14 ± 0.72a | 64.21 ± 0.78b | 66.19 ± 0.28c |
| Oxalate (mg/100 g) | 21.78 ± 0.76 | − | − | − |
| Tannin (mg/100 g) | 0.083 ± 0.005 | − | − | − |
| TPC (mg/g) | 0.45 ± 0.02 | − | − | − |
| TFC (mg/100 g) | ND | NM | NM | NM |
Each value is a mean of triplicates ± SD of triplicates. Means with no common letters within a row significantly differ (p < 0.05)
- Not determined; RFB raw faba bean; SFB soaked faba bean; GFB germinated faba bean; PCFB pressure cooked faba bean; ND Not detectable; NM Not measured
Antinutrients and plant toxins such as phytic acid, oxalic acid, trypsin inhibitor, cyanide etc. are well known for interfering the bioavailability of some nutrients and also having toxic effect. The Table 1 also presents some of the antinutritional components of RFB flour. Oxalate content in RFB was 21.78 mg/g. An excessive amount of oxalate prevents calcium absorption due to the binding of calcium ion to oxalate and form calcium oxalate complex (Kaushal et al. 2012). Total polyphenolic content and tannin content were observed in RFB as 0.45 mg/g and 0.08 mg/g respectively, whereas, the flavonoid content was not detected.
Physical properties of flours
Color
Hunter colour values (L*, a*,b*) of flour samples are presented in Table 2. The lightness “L” values of RFB, SFB, GFB and PCFB were observed as 85.53, 86.58, 88.96 and 73.61 respectively. GFB was observed with higher L* value compared to other flours, whereas PCFB exhibited a lower L* value than others. The “L*’’ value was significantly different among different flour samples. The value of “a*” was also significantly different in legume flours. Medhe et al. (2019) observed a decrease in “ L*’’of germinated and cooked moth bean, whereas an increase in a* value was reported. The colour of flour depends on the amount and presence of flavonoids, anthocyanins and tannins (Medhe et al. 2019). During cooking the formation of brown pigments due to Maillard reaction and thermal oxidation of polyphenols increased “a” (redness) and “b” (yellowness) values of flours (Bagheri et al. 2016).
Table 2.
Physical and functional properties of flours
| Sample | RFB | SFB | GFB | PCFB |
|---|---|---|---|---|
| L* | 85. 53 ± 0.30b | 86.58 ± 0.21c | 88.96 ± 0.21d | 73.61 ± 0.57a |
| a* | 0.13 ± 0.02a | 0.85 ± 0.20c | 0.26 ± 0.03b | 1.53 ± 0.10d |
| b* | 11.74 ± 0.14a | 15.94 ± 0.01c | 13.42 ± 0.03b | 15.88 ± 0.18c |
| ΔE | – | 4.40 ± 0.29b | 3.51 ± 0.19a | 15.65 ± 0.62c |
| BD (g/ml) | 0.60 ± 0.01b | 0.58 ± 0.01b | 0.55 ± 0.01a | 0.71 ± 0.00c |
| WAI (g/g) | 2.79 ± 0.08a | 2.77 ± 0.03a | 3.13 ± 0.10b | 2.97 ± 0.08ab |
| WSI (g/100 g) | 0.09 ± 0.05a | 0.57 ± 0.57ab | 1.35 ± 0.20c | 2.12 ± 0.17c |
| WAC (g/g) | 1.10 ± 0.08a | 1.23 ± 0.04a | 1.11 ± 0.07a | 2.02 ± 0.09b |
| OAC (g/g) | 0.58 ± 0.13a | 0.60 ± 0.03a | 0.61 ± 0.13a | 0.59 ± 0.0a |
| FC (mL/100 mL) | 1.77 ± 0.03c | 1.57 ± 0.03b | 1.77 ± 0.01c | 1.37 ± 0.01a |
| FS (mL/100 mL) | ||||
| 20 min | 110.16 ± 0.15a | 130.13 ± 0.15c | 140.13 ± 0.15d | 120.1 ± 0.10b |
| 40 min | 110.16 ± 0.15a | 130.13 ± 0.15c | 140.13 ± 0.15d | 120.1 ± 0.10b |
| 60 min | 99.5 ± 0.50a | 120.16 ± 0.15b | 129.7 ± 0.60c | 99.4 ± 0.60a |
| 120 min | 64.86 ± 0.32a | 109.4 ± 0.52c | 118.76 ± 0.68d | 80.83 ± 0.76b |
L*, a*,b* are colour values. WAI: Water absorption index, WSI: Water solubility index, BD: Bulk density, WAC: Water absorption capacity, OAC: Oil absorption capacity, FC: Foaming capacity, FS: Foaming stability. Each value is a mean of triplicates ± SD of triplicates. Means with no common letters within a row significantly differ (p < 0.05)
- Not detectedp; RFB raw faba bean; SFB soaked faba bean; GFB germinated faba bean; PCFB pressure cooked faba bean
Water absorption index (WAI) and water solubility index (WSI)
The WAI of different flours was in the range of 2.77 to 3.13 g/g, whereas the highest value was observed for GFB (3.13 g/g) and lowest value for SFB (2.77 g/g). WAI is unintegrated with hydrophilicity and gelation capacity of biomacromolecules, such as starch and protein in flour (Kaur et al. 2007). Chauhan et al. (2015) observed an increase in WAI of amaranth after germination (3.48 to 4.20 g/g), which was similar to this study. Breakdown of polysaccharide molecules and changes in the quality of protein and protein content increased the water absorption of germinated bean (Cahuhan et al. 2015).
WSI value was in the range of 0.09 to 2.12 g/100 g for different flour samples, whereas the highest value was observed in PCFB (2.12 g/100 g) and the lowest value observed in RFB (0.09 g/100 g). The increase in WSI of GFB than RFB, was associated with the formation of low molecular weight compounds by amylases and proteases during germination (Chauhan et al. 2015). High WSI index of PCFB was associated with the breakdown of starch due to thermal treatment and release of soluble polysaccharides (Rashid et al. 2015).
Bulk density
The bulk density of flours was in the range of 0.55–0.71 g/mL, whereas the highest value and lowest value were obtained for RFB (0.85 g/mL) and PCFB (0.71 g/mL) flour respectively. The bulk density of RFB and SFB was not significantly different (p > 0.05) whereas GFB and PCFB were significantly (p < 0.05) different from other samples.
The bulk density of flours depends on the moisture content of grain (Subramanium and Viswannathan 2007). The higher bulk density of the flour sample suggested the denser structure of flours (Du et al. 2014). Low bulk density flours are used in the preparation of baby food formulations (Devisetti et al. 2014). Chauhan et al. (2015) reported a lower bulk density in germinated (0.55 g/mL) compare to raw amaranth (0.61 g/mL).
Functional properties of flours
Water and oil absorption capacity
The WAC of the flours were ranged from 1.10 to 2.02 g/g, whereas the WAC of PCFB (2.02 g/g) flour was the highest, and RFB (1.10 g/g) exhibited the lowest WAC (Table 2). The WAC of RFB, SFB and GFB were not significantly (p < 0.05) different, whereas PCFB showed significantly higher WAC (p < 0.05). WAC was increased in SFB due to breakdown of proteins with increased activity of proteases, whereas, PCFB exhibited significantly high WAC due to thermal degradation of proteins and starch (Rashid et al. 2015). Handa et al. (2017) also observed higher WAC in soaked horse gram, and germinated horse gram compare to raw horse gram. The affinity between water molecules and polar amino acids residues of the proteins caused differences in WAC of flour (Devisetti et al. 2014).
OAC plays a vital role in the improvement of mouth feel and maintenance of the flavour of food products (Du et al. 2014). The OAC of flours ranged from 0.58 to 0.61 g/g. The higher OAC was observed for GFB (0.61 g/g) and the lowest for RFB (0.58 g/g). There was no significant (p < 0.05) difference observed among all flour samples. Chauhan et al. (2015) reported an increase in OAC after germination in amaranth because of change in protein during germination and also increased the capacity to hold fat globule by lipophilic protein. OAC is mainly affected by total protein content which is composed of hydrophobic and hydrophilic parts which can interact with both water and oil, therefore; it enhanced the OAC (Chandra et al. 2015).
Least gelation concentration
Least gelation concentration (LGC) of different flour samples was in the range of 6 to 16% as shown in Table 3. LGC with lower value indicates the better swelling ability of the flour and gelation ability of the protein ingredient (Chandra et al. 2015). RFB, SFB and GFB formed a gel at concentration 6–8% (w/v) whereas, PCFB formed a gel at a higher concentration 10% (w/v). Swelling and hydration of starch and predominatly amorphous region of starch form a gel. Strength of gel depends on the annealing of amylose and amylopectin, moisture treatment and the intragranular binding force of swollen starch granules due to heat (Devisetti et al. 2014). Medhe et al. (2019) reported that gel formation of germinated and cooked moth bean exhibit higher gel concentration compared to raw one because of changes in quality of protein, fat and carbohydrate during germination and cooking.
Table 3.
Least gelation concentration of flours
| Concentrations (% w/v) | RFB | SFB | GFB | PCFB |
|---|---|---|---|---|
| 2 | − | − | − | − |
| 4 | − | − | − | − |
| 6 | + | + | + | − |
| 8 | + | + | + | − |
| 10 | + | + | + | + |
| 12 | + + | + | + | + |
| 14 | + + | + | + + | + |
| 16 | + + | + + | + | + + |
| 18 | + + | + + | + + | + + |
| 20 | + + | + + | + + | + + |
- No gelation; + gel; + + firm gel; RFB raw faba bean; SFB soaked faba bean; GFB germinated faba bean; PCFB pressure cooked faba bean
Foaming capacity and stability
Table.2 represents the foaming capacity and stability of faba bean flour samples. The foaming capacity of flours ranged from 1.37 to 1.77 g/g. Foaming capacity of RFB (1.77 mL/100 mL) and GFB (1.77 mL/100 mL) was significantly higher (p < 0.05) than SFB (1.57 mL/100 mL) and PCFB (1.37 mL/100 mL). Foaming stability of GFB was significantly higher than all other flours whereas, RFB exhibited lowest FS. GFB exhibited high foaming capacity and stability due to the formation of soluble proteins during germination (El-Adawy et al. 2003). Medhe et al. (2019) also reported higher foaming stability of germinated moth bean compare to the raw sample.
Pasting properties
Pasting properties play an important role in the selection of food binder and thickener. Pasting properties mainly depend on the rigidity of starch granules which affects starch granule swelling potential and leaching amount of amylose in the solution (Medhe et al. 2019). The pasting temperature of RFB (57 °C) was higher than all other flours, however there was not significant difference in pasting temperatre of all samples. SFB was observed with lower (54.33 °C) pasting temperature than all treatments. Pasting temperature corresponds to minimum temperature required for cooking and it also indicates the temperature at which viscosity starts to increase during thermal treatment (Yadav et al. 2018). The lower pasting temperature of SFB and GFB was attributed to degradation of starch which resulted a decrease in final viscosity of SFB and GFB. Chauhan et al. (2015) also found a decrease in pasting temperature in germinated (77.13 °C) amaranth compare to raw (78.25 °C) one. SFB and GFB observed a higher peak viscosity compare to other samples. Setia et al. (2019) reported that the higher peak viscosity found in soaked faba bean. Trough viscosity of RFB and GFB flour samples was observed significantly different, but it was not significantly different for SFB and PCFB. Breakdown viscosity of processed flours was lower than the raw flour. Chauhan et al. (2015) also observed a decrease in the breakdown of germinated amaranth compare to raw. Low breakdown of flour, indicates that flour exhibits good paste stability and strong shearing (Du et al. 2014). Set back value was found higher in RFB compare to other samples. Chauhan et al. (2015) reported the same trend in raw and germinated amaranth.
Thermal properties
Flours are a blend of the fibres, lipids, proteins, starch molecules and other components which overall affect the heat capacity of the flour (Chávez-Murillo et al. 2018). The onset temperature ranged from 63.43 to 80.12 °C for flour samples, as shown in Table 4. The highest onset temperature was observed in PCFB and lowest in RFB flour. The processing treatments such as pressure cooking transformed the inter-crystalline amorphous form of starch and these structural changes in starch granules caused an increase in onset gelatinization temperature (To) (Sharanagat et al. 2019). The peak gelatinisation temperature ranged from 71.34 to 97.55 °C, whereas the highest in PCFB, and the lowest one was observed in RFB. The gelatinisation temperature differs in flour because of size, form and distribution and internal structure of starch granules (Kaur et al. 2007). Wani et al. (2017) reported higher gelatinisation temperatures in the microwave and pan-roasted chestnut flours because of high temperature while roasting. Conclusion temperature ranged from 80.85 to 112.12 °C whereas the highest one was observed in PCFB and the lowest one in RFB. Setia et al. (2019) reported raw, soaked and germinated faba bean onset temperature (66.2,65.9–65.9 °C), peak temperature (72.9, 70.8–71.1 °C), conclusion temperature (85.2,84.0–82.3 °C) and enthalpy (5.0, 5.0–4.5 J/g) respectively.
Table 4.
Pasting properties of legume flours
| Parameters | RFB | SFB | GFB | PCFB |
|---|---|---|---|---|
| Peak viscosity (RVU) | 2157.94 ± 1.50ab | 2165.08 ± 1.95c | 2160.32 ± 1.25b | 2154.22 ± 1.96a |
| Trough viscosity (RVU) | 2124.75 ± 2.68a | 2144.80 ± 3.08c | 2134.55 ± 2.50b | 2147.27 ± 1.23c |
| Break down (RVU) | 34.53 ± 1.27b | 24.39 ± 0.55a | 25.08 ± 0.72a | 25.87 ± 0.92a |
| Final viscosity (RVU) | 2147.72 ± 1.84bc | 2145.47 ± 0.64b | 2133.33 ± 0.64a | 2149.69 ± 0.58c |
| Set back (RVU) | 6.27 ± 0.42b | 4.46 ± 0.36a | 5.74 ± 0.37b | 4.61 ± 0.17a |
| Pasting temp (°C) | 57.00 ± 1.71a | 54.33 ± 1.42a | 54.88 ± 2.51a | 55.2 ± 1.28a |
| TO (°C) | 63.43 | 66.59 | 65.06 | 80.12 |
| TP (°C) | 71.34 | 72.46 | 72.83 | 97.55 |
| TC (°C) | 80.85 | 82.69 | 92.29 | 112.12 |
| H (J/g) | 0.78 | 0.96 | 2.22 | 2.13 |
| TC – TO (°C) | 17.42 | 16.1 | 27.23 | 32 |
To: onset gelatinisation temperature, Tp: peak gelatinisation, Tc: conclusion, H: enthalpy. Each value is a mean of triplicates ± SD of triplicates. Means with no common letters within a row significantly differ (p < 0.05)
RFB raw faba bean; a SFB soaked faba bean; GFB germinated faba bean; PCFB pressure cooked faba bean
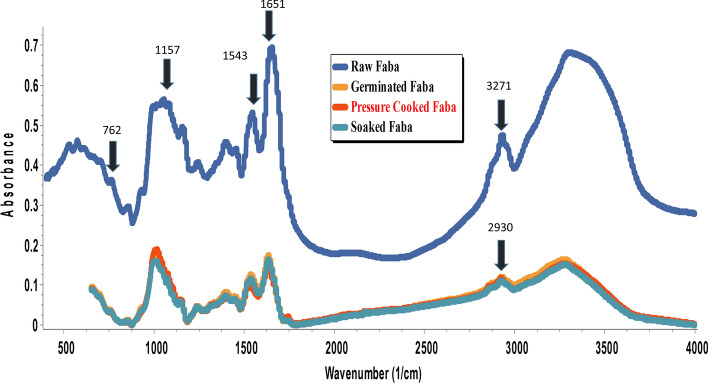
FTIR analysis of flours
Figure 1 illustrates the FT-IR spectra of RFB, SFB, GFB and PCPM. The peaks were observed in the range of specific spectral regions in raw, soaked, germinated and pressure cooked faba bean. The peaks observed in the range of 3271, 2930, 1651, 1543, 1449, 1398, 1240, 1157, 1079 and 762 cm−1. There were some spectral changes observed in flour samples. There was no difference observed in the carbohydrate region (1200- 900 cm−1) among the samples. The peaks in the range of 1600–1100 cm−1 were found in all flour samples and corresponded to amide groups, i.e. amide I and amide II, which indicated the presence of lysine, valine, phenylalanine and tyrosine. The peak at 1651 cm−1 was corresponded to oxalate and peak intensity was reduced in treated flour samples (Handa et al. 2017). There was slight difference observed in SFB, GFB and PCFB compare to RFB in protein region (1700–1600 and 1570 -1534 cm−1) which was attributed to degradation of proteins during processing treatments.
Fig. 1.
FTIR spectra of RFB, SFB, GFB and PCFB. RFB = raw faba bean, SFB = soaked faba bean, GFB = germinated faba bean, PCFB = pressure cooked faba bean
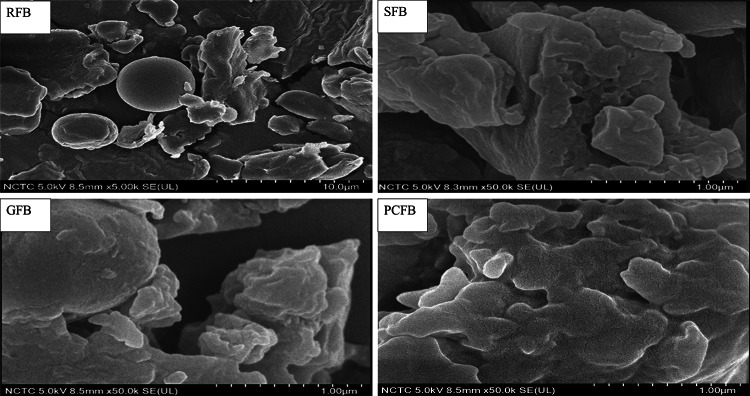
Microstructure of faba bean flour
Figure 2 illustrates the morphological micrographs of different flour samples. The flours of RFB were spherical, granular, smooth and oval close together without gaps. Some granules were damaged and irregular in shape. After germination and cooking significant changes were found in starch, i.e. granules were damaged. Compared with raw samples, the geminated and cooked flours showed many branching structures that connected to form a network. Medhe et al. (2019) found rough and damaged starch granules of geminated and cooked moth bean flour compared to raw flour. Shrestha et al. (2018) reported changes in starch such as irregular shapes and an amorphous mass of cohesive structure in culled banana starch after heat treatment.
Fig. 2.
Scanning electron microscopic structures of RFB, SFB, GFB and PCFB. RFB = raw faba bean, SFB = soaked faba bean, GFB = germinated faba bean, PCFB = pressure cooked faba bean
Conclusions
The soaking, germination, and pressure cooking were found improving significantly the physiological and functional properties of faba bean flour. The soaking and germination improved the overall properties of flour. The low setback of SFB, GFB and PCFB suggests its use to develop the food-based products, in which starch stability is desired at low temperature. The germination improved effects on functional and physiochemical properties including the enhanced WAI and WSI. Soaking and germination enhanced the functional properties, such as WAC and OAC, which will be useful for flavour retention and improvement of palatability. This study confirms the great potential of this under-utilized faba bean to be used in the food industry for the formulation of new functional food products or as a replacement in food products due to their improved nutritional physiochemical and functional properties.
Abbreviations
- RFB
Raw faba bean
- SFB
Soaked faba bean
- GFB
Germinated faba bean
- PCFB
Pressure cooked faba bean
- WAI
Water absorption index
- WSI
Water solubility index
- WAC
Water absorption capacity
- OAC
Oil absorption capacity
Author contributions
Dr. SRK performed all the experiments and compiled the data. Dr. AKA supervised the project and helped in writing manuscript. Dr. MBS helped in experimental design, data analysis and manuscript drafting.
Funding
Not applicable.
Data availability
Data available on request from authors.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable (include appropriate approvals or waivers).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Association of Official Analytical Chemists . Official methods of analysis of the association of analytical chemists. Washington, DC: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Agume ASN, Njintang NY, Mbofung CMF. Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods. 2017;6(2):12. doi: 10.3390/foods6020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri H, Kashaninejad M, Ziaiifar AM, Aalami M. Novel hybridized infrared-hot air method for roasting of peanut kernels. Innov Food Sci Emerg Technol. 2016;37:106–114. doi: 10.1016/j.ifset.2016.08.014. [DOI] [Google Scholar]
- Campos-Vega R, Loarca-Piña G, Oomah BD. Minor components of pulses and their potential impact on human health. Food Res Int. 2010;43(2):461–482. doi: 10.1016/j.foodres.2009.09.004. [DOI] [Google Scholar]
- Collado E, Klug TV, Martínez-Hernández GB, Artés-Hernández F, Martínez-Sánchez A, Aguayo E, Gómez PA. Nutritional and quality changes of minimally processed faba (Vicia faba L.) beans during storage: effects of domestic microwaving. Postharvest Biol Technol. 2019;151:10–18. doi: 10.1016/j.postharvbio.2019.01.008. [DOI] [Google Scholar]
- Chauhan A, Saxena DC, Singh S. Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT-Food Sci Technol. 2015;63(2):939–945. doi: 10.1016/j.lwt.2015.03.115. [DOI] [Google Scholar]
- Chávez-Murillo CE, Veyna-Torres JI, Cavazos-Tamez LM, de la Rosa-Millán J, Serna-Saldívar SO. Physicochemical characteristics, ATR-FTIR molecular interactions and in vitro starch and protein digestion of thermally-treated whole pulse flours. Food Res Int. 2018;105:371–383. doi: 10.1016/j.foodres.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Chandra S, Singh S, Kumari D. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. J Food Sci Technol. 2015;52(6):3681–3688. doi: 10.1007/s13197-014-1427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devisetti R, Yadahally SN, Bhattacharya S. Nutrients and antinutrients in foxtail and proso millet milled fractions: evaluation of their flour functionality. LWT-Food Sci Technol. 2014;59(2):889–895. doi: 10.1016/j.lwt.2014.07.003. [DOI] [Google Scholar]
- Du SK, Jiang H, Yu X, Jane JL. Physicochemical and functional properties of whole legume flour. LWT-Food Sci Technol. 2014;55(1):308–313. doi: 10.1016/j.lwt.2013.06.001. [DOI] [Google Scholar]
- El-Adawy TA, Rahma EH, El-Bedawey AA, El-Beltagy AE. Nutritional potential and functional properties of germinated mung bean, pea and lentil seeds. Plant Foods Hum Nutr. 2003;58(3):1–13. doi: 10.1023/B:QUAL.0000040339.48521.75. [DOI] [Google Scholar]
- Embaby HES. Effect of heat treatments on certain antinutrients and in vitro protein digestibility of peanut and sesame seeds. Food Sci Technol Res. 2010;17(1):31–38. doi: 10.3136/fstr.17.31. [DOI] [Google Scholar]
- Falade KO, Christopher AS. Physical, functional, pasting and thermal properties of flours and starches of six Nigerian rice cultivars. Food Hydrocoll. 2015;44:478–490. doi: 10.1016/j.foodhyd.2014.10.005. [DOI] [Google Scholar]
- Handa V, Kumar V, Panghal A, Suri S, Kaur J. Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. J Food Sci Technol. 2017;54(13):4229–4239. doi: 10.1007/s13197-017-2892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AU, Liu C, Sathe SK. Functional properties of select seed flours. LWT-Food Sci Technol. 2015;60(1):325–331. doi: 10.1016/j.lwt.2014.08.038. [DOI] [Google Scholar]
- Kaushal P, Kumar V, Sharma HK. Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta), rice (Oryza sativa) flour, pigeon pea (Cajanus cajan) flour and their blends. LWT-Food Sci Technol. 2012;48(1):59–68. doi: 10.1016/j.lwt.2012.02.028. [DOI] [Google Scholar]
- Kajihausa OE, Fasasi RA, Atolagbe YM. Effect of different soaking time and boiling on the proximate composition and functional properties of sprouted sesame seed flour. Niger Food J. 2014;32(2):8–15. doi: 10.1016/S0189-7241(15)30112-0. [DOI] [Google Scholar]
- Liu C, Damodaran S, Heinonen M. Effects of microbial transglutaminase treatment on physiochemical properties and emulsifying functionality of faba bean protein isolate. LWT. 2019;99:396–403. doi: 10.1016/j.lwt.2018.10.003. [DOI] [Google Scholar]
- Maninder K, Sandhu KS, Singh N. Comparative study of the functional, thermal and pasting properties of flours from different field pea (Pisum sativum L.) and pigeon pea (Cajanus cajan L.) cultivars. Food Chem. 2007;104(1):259–267. doi: 10.1016/j.foodchem.2006.11.037. [DOI] [Google Scholar]
- Makkar HP. Quantification of tannins in tree and shrub foliage: a laboratory manual. Berlin: Springer Science & Business Media; 2003. [Google Scholar]
- Martín-Cabrejas MA, Díaz MF, Aguilera Y, Benítez V, Mollá E, Esteban RM. Influence of germination on the soluble carbohydrates and dietary fiber fractions in non-conventional legumes. Food Chem. 2008;107(3):1045–1052. doi: 10.1016/j.foodchem.2007.09.020. [DOI] [Google Scholar]
- Medhe S, Jain S, Anal AK. Effects of sprouting and cooking processes on physicochemical and functional properties of moth bean (Vigna aconitifolia) seed and flour. J Food Sci Technol. 2019;56(4):2115–2125. doi: 10.1007/s13197-019-03692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarak AE. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005;89(4):489–495. doi: 10.1016/j.foodchem.2004.01.007. [DOI] [Google Scholar]
- Nasrin TAA, Noomhor A, Anal AK. Physico-chemical characterization of culled plantain pulp starch, peel starch, and flour. Int J Food Prop. 2015;18(1):165–177. doi: 10.1080/10942912.2013.828747. [DOI] [Google Scholar]
- Rashid S, Rakha A, Anjum FM, Ahmed W, Sohail M. Effects of extrusion cooking on the dietary fibre content and Water Solubility Index of wheat bran extrudates. Int J Food Sci Technol. 2015;50(7):1533–1537. doi: 10.1111/ijfs.12798. [DOI] [Google Scholar]
- Rodsamran P, Sothornvit R. Physicochemical and functional properties of protein concentrate from by-product of coconut processing. Food Chem. 2018;241:364–371. doi: 10.1016/j.foodchem.2017.08.116. [DOI] [PubMed] [Google Scholar]
- Setia R, Dai Z, Nickerson MT, Sopiwnyk E, Malcolmson L, Ai Y. Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res Int. 2019;122:263–272. doi: 10.1016/j.foodres.2019.04.021. [DOI] [PubMed] [Google Scholar]
- Saleh HM, Hassan AA, Mansour EH, Fahmy HA, El-Bedawey AEFA. Melatonin, phenolics content and antioxidant activity of germinated selected legumes and their fractions. J Saudi Soc Agric Sci. 2017;18:294–301. [Google Scholar]
- Sharanagat VS, Suhag R, Anand P, Deswal G, Kumar R, Chaudhary A, Nema PK. Physico-functional, thermo-pasting and antioxidant properties of microwave roasted sorghum [Sorghum bicolor (L.) Moench] J Cereal Sci. 2019;85:111–119. doi: 10.1016/j.jcs.2018.11.013. [DOI] [Google Scholar]
- Sadiq MB, Hanpithakpong W, Tarning J, Anal AK. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind Crops Prod. 2015;77:873–882. doi: 10.1016/j.indcrop.2015.09.067. [DOI] [Google Scholar]
- Shrestha S, Sadiq MB, Anal AK. Culled banana resistant starch-soy protein isolate conjugate based emulsion enriched with astaxanthin to enhance its stability. Int J Biol Macromol. 2018;120:449–459. doi: 10.1016/j.ijbiomac.2018.08.066. [DOI] [PubMed] [Google Scholar]
- Singh B, Singh JP, Shevkani K, Singh N, Kaur A. Bioactive constituents in pulses and their health benefits. J Food Sci Technol. 2017;54(4):858–870. doi: 10.1007/s13197-016-2391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Viswanathan R. Bulk density and friction coefficients of selected minor millet grains and flours. J Food Eng. 2007;81(1):118–126. doi: 10.1016/j.jfoodeng.2006.09.026. [DOI] [Google Scholar]
- Thakur S, Scanlon MG, Tyler RT, Milani A, Paliwal J. Pulse flour characteristics from a wheat flour miller's perspective: a comprehensive review. Compr Rev Food Sci Food Safety. 2019;18(3):775–797. doi: 10.1111/1541-4337.12413. [DOI] [PubMed] [Google Scholar]
- Wani IA, Hamid H, Hamdani AM, Gani A, Ashwar BA. Physico-chemical, rheological and antioxidant properties of sweet chestnut (Castanea sativa Mill.) as affected by pan and microwave roasting. J Adv Res. 2017;8(4):399–405. doi: 10.1016/j.jare.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav U, Singh N, Kaur A, Thakur S. Physico-chemical, hydration, cooking, textural and pasting properties of different adzuki bean (Vigna angularis) accessions. J Food Sci Technol. 2018;55(2):802–810. doi: 10.1007/s13197-017-2994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from authors.