Abstract
The objective was to study the optimization of fermentation conditions for fermented green jujube wine and quality analysis. This study investigated the fermentation process conditions, the changes in physicochemical indexes, antioxidant capacity and volatile compounds measured from green jujube wine during winemaking. The optimized conditions (the initial sugar, yeast addition, fermentation time and SO2 treatments) for green jujube wine were 24%, 0.3%, 8 d, 80 mg/L, respectively. The results showed that the variation trend of different substances in green jujube wine in different fermentation periods were different. In the process of alcohol fermentation, the green jujube wine had a high 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging ability, 2,2′-amino-di (2-ethyl-benzothiazoline sulphonic acid-6) ammonium salt (ABTS) free radical scavenging ability and reducing power. Furthermore, a total of 50 volatile compounds were identified in green jujube wine, in which the relative content of aldehydes, ketones, heterocyclic and aromatic compounds were significantly reduced after fermentation.
Keywords: Response surface methodology, Green jujube wine, Nutritional composition, Flavor compounds, Alcoholic fermentation
Introduction
Jujube (Ziziphus jujuba Mill.) belongs to the Rhamnaceae family, which has a history of 4000 years in China (Li et al. 2007). Jujube fruits are one of the most popular fruit consumed in Asia for its potential nutritional and nutraceutical values such as carbohydrate, phenolic compounds, saponins, alkaloids and triterpenoid acids (Song et al. 2019; Li et al. 2011) and it has the effect of increasing immunity, preventing cardiovascular diseases, preventing cancer and anti-oxidation (Zhang et al. 2010; Wang et al. 2015; Guo et al. 2018). The chemical composition of jujube fruit varies with local of cultivation, variety, and stage of maturity (Wang et al. 2018). The mature stages of jujubes were divided into white maturity, half-red maturity and red maturity (Wang et al. 2016). Furthermore, the free fraction of jujube at white maturity stage had the supreme total phenolic content (TPC), total flavonoid content (TFC), total phenolic acid contents, and antioxidant capacities (Wang et al. 2016). It was evident that white maturity green jujube had a high utilization value. The planting area of jujube have increased and the annual output has increased year by year in China. Therefore, it is urgent to adopt new ideas and develop new products to change the depressed status of jujube industry.
Moderate drinking of fruit wine contributed to beneficial effects, such as reduce risk of cardiovascular diseases and lower cognitive function losses (Neafsey and Collins 2011). Fermentation not only preserves a lot of nutrients in the fruit, but also gives it a rich taste and flavor. The flavor composition of jujube wine varies with its variety, fermentation strain and technological conditions. With the rapid development of analytical detection technology, the aroma has become an important field in food research.
Some authors had focused on processing research and biological activities of functional components in red maturity jujube. Previously, jujube wine was always produced using red maturity jujube. Eom et al. (2016) have evaluated the changes in physicochemical and antioxidant characteristics in the fermentation process of jujube wine using hot water extract of dried jujube. The results showed that the fermented jujube wine had significant antioxidant activity. Guo et al. (2018) have studied the chemical and aroma component of jujube alcohol beverage fermented with T. delbrueckii with/without enzymatic hydrolysis treatment. The results manifested that enzymatic hydrolysis elevated the aroma substances of jujube alcoholic beverage. Based on present scientific literature, there was no report on the production of green jujube wine. Therefore, the purpose of this research was to systematically optimize the fermentation process of green jujube wine, and to study the changes of various chemical components, antioxidant capacity and volatile substances of the optimized green jujube wine. We hope that the consequences of this study will provide scientific evidence for green jujube wine winemaking.
Materials and methods
Raw materials and chemicals
Green jujubes (Zizyphus jujuba cv. Muzao) were collected from in Lvliang city of Shanxi province, China in September 2018. Intact fruits of similar shape and size were collected without any physical injuries. The samples were transported to the lab and frozen at − 20 °C. Brewing highly active dry yeast was purchased from Angel Yeast Co., Ltd (Hubei, China). L-ascorbic acid (purity ≥ 99.7%), 3,5-Dioitrosalicylic acid, potassium ferricyanide and potassium persulfate were obtained from Tianjin Guangfu Technology Development Co., Ltd (Tianjin, China). Gallic acid (GA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) were provided from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). 2,6-Dichloroindophenol sodium salt (purity ≥ 95%) was obtained from Shanghai Ica Biotechnology Co., Ltd. (Shanghai, China). The Folin-Ciocalteu reagent was provided from Hefei Bomei Biotech Co., Ltd. (Anhui, China). Other reagents used were of analytical grade.
Green jujube wine preparation
Jujube pit was separated from the green jujube fruits. The ratio of the green jujube pulp and water was 1:2 (w/v), and softened in water bath at 90 °C for 8–10 min. Then, it was hydrolyzed with pectinase of 0.3% at 40 °C for 3 h. Enzyme solutions speed up juice filtration and promote clarification. Green jujube juice was collected by juice filtration with a laboratory JJ-2B tissue masher (Ronghua Instrument Manufacturing Co., Ltd., Jiangsu, China) and then filtrated through gauze. The obtained green jujube juice (soluble solid content, (SSC), 9.8°Brix; pH value, 3.81) of approximately 5 L was stored in − 18 °C storage chamber until the analysis. Yeast activation: the dry yeast is activated for 15–30 min with more than 5 times 2% sugar water during the 35–38 °C.
SSC of green jujube juice was adjusted to suitable sugar in a 500 mL glass fermentation cylinder. Potassium sulphate (80 mg/L) was added to the sterilized juice. The method of pasteurization was used to sterilize green jujube wine. The heating temperature was controlled at 80 °C and the time was 15 min. After addition of the activated yeast at suitable ratio, fermentation was started and temperature was kept at 24 ± 1 °C throughout the fermentation process. Owing to the fermentation temperature has little effect on the test results, the fermentation temperature of the green jujube wine was selected as 23–25 °C (Liu et al. 2018). After 8 days, the wine started post-fermentation, and the suitable temperature was around 20 °C for 1 month. The wine was transferred to a new glass vessel and placed in dark and at room temperature for 1 month and 2 months for aging. Wine samples were prepared on the main fermentation stage (day 0, 2, 4, 6 and 8), post-fermentation (day 38), aging 1 month (day 68) and aging 2 months (day 98) and then sealed and stored at − 20 °C until analysis.
Experimental design
A preliminary investigation of the factors affecting the taste and quality of fermented greengage wine was conducted using single factor experiments, including the initial sugar, yeast addition, fermentation time and SO2 treatments. The factors chosen were the initial sugar (18, 20, 22, 24 and 26%), yeast addition (0.1, 0.2, 0.3, 0.4 and 0.5%), fermentation time (day 3, 5, 7, 9 and 11) and the content of sulfur dioxide SO2 (0, 40, 80, 120 and 160 mg/L) and played a significant effect on the wine quality.
Twenty-nine experiments were performed according to the Box-Behnken center-united experimental design principles with 4 factors and 3 levels for each variable. As shown in Table 1, the independent variables applied in the experimental design were the initial sugar (20, 22 and 24%), yeast addition (0.2, 0.3 and 0.4%), fermentation time (day 5, 7 and 9) and the content of sulfur dioxide SO2 (40, 80, and 120 mg/L), consistent with the coded levels (− 1, 0 and 1), (− 1, 0 and 1), (− 1, 0 and 1) and (− 1, 0 and 1), respectively. The response factors were the crucial biochemical indicators, namely alcohol content (% (v/v)) and sensory evaluation (scores). The polynomial regression equation was described the second-order response as function of the experiments. The quadratic polynomial model fitted to each response value was as follows:
| 1 |
where X1, X2, X3 and X4 are the independent variables for the initial sugar (%), yeast addition (%), fermentation time (d) and SO2 treatment (mg/L), respectively; β0 = constant, β1,2,3,4 = linear coefficient, β12,13,14,23,24,34 = interaction coefficient, and β11,22,33,44 = quadratic coefficient; Y is the response value.
Table 1.
The test and results of the response surface of the green jujube wine
| Run | Independent variable | Response | ||||
|---|---|---|---|---|---|---|
| The initial sugar (%) | Yeast addition (%) | Fermentation time (d) | SO2 treatment (mg/L) | Alcohol content (% (v/v)) | Sensory evaluation (scores) | |
| 1 | 20 | 0.3 | 7 | 40 | 13.0 | 79 |
| 2 | 24 | 0.3 | 7 | 40 | 15.5 | 82 |
| 3 | 20 | 0.4 | 7 | 80 | 13.0 | 72 |
| 4 | 22 | 0.3 | 9 | 120 | 13.0 | 80 |
| 5 | 20 | 0.3 | 7 | 120 | 13.7 | 69 |
| 6 | 22 | 0.2 | 7 | 40 | 12.3 | 77 |
| 7 | 20 | 0.2 | 7 | 80 | 12.0 | 77 |
| 8 | 20 | 0.3 | 9 | 80 | 12.8 | 78 |
| 9 | 24 | 0.3 | 5 | 80 | 15.0 | 83 |
| 10 | 22 | 0.4 | 7 | 40 | 13.8 | 78 |
| 11 | 22 | 0.3 | 5 | 120 | 14.2 | 68 |
| 12 | 24 | 0.2 | 7 | 80 | 14.0 | 84 |
| 13 | 22 | 0.3 | 7 | 80 | 14.6 | 84 |
| 14 | 22 | 0.3 | 7 | 80 | 14.7 | 84 |
| 15 | 22 | 0.4 | 7 | 120 | 13.0 | 75 |
| 16 | 20 | 0.3 | 5 | 80 | 14.0 | 71 |
| 17 | 24 | 0.4 | 7 | 80 | 14.2 | 84 |
| 18 | 22 | 0.3 | 9 | 40 | 13.1 | 78 |
| 19 | 22 | 0.2 | 9 | 80 | 11.9 | 76 |
| 20 | 22 | 0.4 | 5 | 80 | 13.5 | 69 |
| 21 | 22 | 0.2 | 5 | 80 | 13.8 | 78 |
| 22 | 22 | 0.3 | 7 | 80 | 14.7 | 82 |
| 23 | 24 | 0.3 | 7 | 120 | 14.5 | 86 |
| 24 | 22 | 0.2 | 7 | 120 | 13.5 | 76 |
| 25 | 22 | 0.3 | 5 | 40 | 14.5 | 79 |
| 26 | 22 | 0.4 | 9 | 80 | 13.8 | 85 |
| 27 | 24 | 0.3 | 9 | 80 | 14.9 | 89 |
| 28 | 22 | 0.3 | 7 | 80 | 14.5 | 82 |
| 29 | 22 | 0.3 | 7 | 80 | 14.5 | 85 |
Sensory analysis
According to GB/T 15,038–2006 ‘Analytical methods of wine and fruit wine’, sensory evaluation of jujube wine was carried out by seven trained teachers and students, respectively. Mouthwash was provided to raters between the evaluations of different samples to avoid lingering aftertaste. The panelists gave scores for appearance (0–10), aroma (0–30), taste (0–40) and typicality (0–20), respectively.
Analysis of nutrients
The content of protein, amino acid nitrogen, total titratable acid (TTA), pH, reducing sugar, ascorbic acid, superoxide dismutase (SOD) activity, TPC and TFC were determined in green jujube wine during winemaking. The test of protein content in the green jujube fermented wine samples according to the Coomassie Brilliant Blue assay with minor modifications (Arnous et al. 2001). Amino acid nitrogen was titrated with single indicator formaldehyde. TTA was measured by acid–base titration method. A digital pH indicator (PHS-3C, Shanghai Tianda Instrument Co., Ltd., Shanghai, China) was used for the pH values measurements. The reducing sugar was tested by dinitrosalicyclic acid colorimetry (DNS) method (Cheung et al. 2009). L-ascorbic acid content was determined by 2,6-dichlorophenol indophenol method (Balthazar et al. 2019). SOD activity was determined by nitro blue tetrazolium (NBT) photoreduction.
The determination of TPC referred to the Folin-Ciocateu colorimetric method of Aydın and Mammadov (2017), which was slight modified. Briefly, 1 mL of wine samples, 1 mL of Folin-Ciocalteu reagent, and 3 mL of 7.5% Na2CO3 solution were blended. Then, the mixtures were kept at indoor temperature and then put in the dark for 2 h. The absorbance was measured at 765 nm wavelength, and the results were expressed as mg gallic acid equivalents (GAE)/g of wine.
The TFC was determined according to the NaNO2–AlCl3–NaOH method (Siriamornpun et al. 2015). Briefly, 2.0 mL of diluted wine sample was supplemented with 60% ethanol solution to 5.0 mL, mixed with 1 mL of 5% (m/v) NaNO2. After reaction for 6 min, 1.5 mL of 10% (m/v) AlCl3 was added. After standing for 5 min, 4 mL of NaOH (200 g/L) was then added. The final volume of mixture was added to 25 mL with 60% ethanol solution. The absorbance of the wine sample was analyzed at 510 nm. The results were indicated in mg rutin equivalents (RE)/g of wine.
Antioxidant capacity
Antioxidant activity of the green jujube wine samples during fermentation and aging process were determined based on the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) analysis with slight modifications (Loganayaki et al. 2013). ABTS radical scavenging activity was determined with slight modification of the method described by Re et al. (1999). Reducing power assay was determined based on the mildly modified method of Kwaw et al. (2018). Each experiment was repeated three times.
Headspace solid phase microextraction/gas chromatography-mass spectrometer (HS-SPME/GC–MS) analysis
Analysis of the volatile substances were implemented with using a gas chromatography (6890 N, Agilent Technologies) equipped with a mass spectrometric detector (5973, Agilent Technologies). For this assay, volatiles were separated using a headspace solid phase microextraction (75 μm, CAR/PDMS, Supelco, Bellefonte, PA, USA) and was kept for 30 min at 60 °C. Volatile substances were isolated using a HP-5MS quartz capillary column (30 m × 0.25 μm × 0.25 mm, J&W Scientific Co., Ltd., Folsom, CA, USA). Helium was served as the carrier gas at a flow rate of 1.0 mL/min. Temperature programming control: initial temperature was set at 40 °C, held for 2 min; increased to 300 °C at 6 °C/min, held for 5 min; then increased to 100 °C at 6 °C/min; finally, increased to 250 °C at 10 °C/min, and held for 5 min. Mass spectrometry parameters were set as follows: interface, quadrupole, and ion source temperature were set at 280, 150 and 230 °C, respectively; electron bombardment ion source; ionization energy was set at 70 eV; solvent delay was set at 3.5 min; full scanning mode; mass scan range of 35–500 m/z. Qualitative and quantitative analysis: the NIST Library database (Zheng et al. 2016) was used for spectrogram analysis, qualitative analysis of the detected aroma substances, and the relative content of each substance is calculated by the peak area normalization method.
Statistical analysis
All the treatments were carried out triple, and experimental date were represented by mean value ± standard deviation (SD). The analysis of variance (ANOVA) was performed using SPSS statistical 22.0 (OriginLab, Northampton, USA.). Duncan’s multiple range tests were used to compute significant differences at the 0.05 level.
Results and discussion
Model fitting
Response surface methodology (RSM) model for alcohol content and sensory evaluation
As a major parameter of for fermented fruit wine, the alcohol content plays an essential role in traditional alcoholic fermentation process (Nyanga et al. 2013). Furthermore, sensory evaluation is also an indispensable parameter of green jujube wine quality. Table 1 indicated the experimental data of the investigated results (alcohol content and sensory evaluation) detected under different fermentation conditions (the initial sugar, yeast addition, fermentation time, and SO2 treatment) for the green jujube wine, and variance analysis of the regression RSM model were given in Table 2. The especially low p values connected with the F test for two models (p < 0.0001 for alcohol content; p < 0.0001 for sensory evaluation) indicated that these factors were greatly significant. The fit of the models was identified by the high R2 for all response values (R2 > 0.90) (Tian et al. 2018). The fitted quadratic polynomial models for alcohol content (Y1) and sensory evaluation (Y2) were evaluated by RSM, only taking into account the significant terms, which were shown in Eq. (2) and in Eq. (3):
| 2 |
| 3 |
Table 2.
Variance analysis of the regression model
| Response value | Y1 alcohol content | Y2 sensory evaluation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Sum of squares | Degree of freedom | Mean square | F value | P value | Sum of squares | Degree of freedom | Mean square | F value | P value |
| Model | 22.81 | 14 | 1.63 | 35.94** | < 0.0001 | 834.55 | 14 | 59.61 | 47.83** | < 0.0001 |
| Residual | 0.63 | 14 | 0.05 | 11.00 | 1 | 1.25 | ||||
| Lack of fit | 0.59 | 10 | 0.06 | 5.95 | 0.0502 | 7.00 | 10 | 1.23 | 0.94 | 0.5755 |
| Pure error | 0.01 | 4 | 0.0001 | 4.00 | 4 | 1.30 | ||||
| R2 | 0.9729 | 0.9459 | ||||||||
Significant levels; *means significant difference (p < 0.05), **means extremely significant difference (p < 0.01)
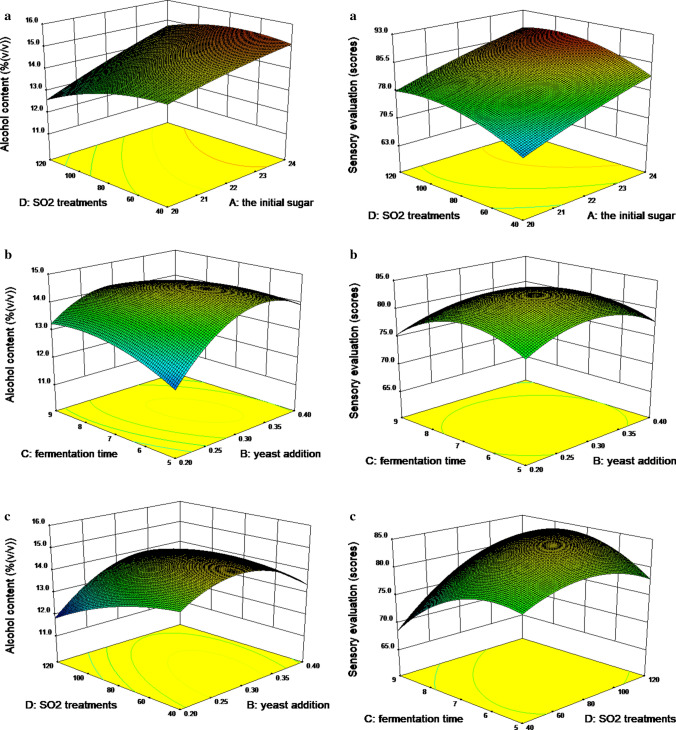
The quadratic polynomial model for alcohol content resulted in a determination coefficient (R2 = 0.9729), demonstrating that 97.29% of the change could be explained excellently, and this indicated that the model is well-matched by the relationship between the factor and the response value (Hou et al. 2019). The lack of fit associated with P-values of 0.0502, showed a non-significance of difference, demonstrating that the model fits with the data. In Fig. 1a–c, the three-dimensional response surface plots describing the interaction effect of the two factors. In response to alcohol, the one-time item the initial sugar (X1), yeast addition (X2), fermentation time (X3), interaction X1X3, X1X4, X2X3, X2X4 and quadratic term X22, X32, X42 were significant (p < 0.05). The other terms were inessential (p > 0.05). It is shown that fermentation is the result of multi-factor interaction. Through the analysis of the main factor effect, it can be concluded that the effect of the experimental factors on the alcohol content was: initial sugar > fermentation time > SO2 treatment > yeast addition. The determination coefficient of quadratic response surface model set of 0.9795 was observed for sensory evaluation, demonstrating that 97.95% of the change can be explained excellently, only 2.05% of the total variation could not be explained, and this indicated that the model is well-matched by the relationship between the factor and the response value (Hou et al. 2019). The sensory evaluation of green jujube wine ranged from 68.0 to 89.0, depended on the initial sugar, yeast addition, fermentation time and SO2 treatment and their interaction (Fig. 1a–c). In the selected levels, the one-time item initial sugar (X1), fermentation time (X3), SO2 treatment (X4) interaction X1X2, X1X4, X2X3, X3X4 and quadratic term X22, X32, X42 were significant (p < 0.05). Meanwhile, the effect of the experimental factors on the sensory evaluation was: initial sugar > fermentation time > SO2 treatment > yeast addition.
Fig. 1.
Response surface for the effect of independent variables on alcohol content and sensory evaluation
Optimization and verification of fermentation process parameters
The model optimization solution was used by the statistical software Design-Expert V8.0.6, optimum fermentation parameters for green jujube wine were 24%, 0.3%, 8 d, and 80 mg/L for initial sugar, yeast addition, fermentation time and SO2 treatment, respectively. Under these conditions, the alcohol content of the fermented green jujube wine was 15.2% (v/v), and sensory evaluation score was 88.96. In order to verify the authenticity of the test results, 3 parallel verification tests were carried out under the best process conditions. The alcohol content of the green jujube wine was 15.0% (v/v), and the sensory score was 87 scores. It was slightly different from the predicted value. It can be seen that the predicted values of the indicators were in good agreement with the experimental values, which further demonstrated that the model can accurately predict the experimental results.
Dynamic change of chemical indicators in optimized green jujube wine during winemaking
Change of basic chemical value parameters in optimized green jujube wine during winemaking
Table 3 showed the changes in the content of protein, amino acid nitrogen, TTA, pH and reducing sugar with fermentation time in the different fermentation and aging times. As summarized in Table 3, the protein content reached the maximum on day 4. After 6 days of fermentation, it showed a downward trend, which may be due to protein decomposition and release of free amino acids. At the same time, the content of free amino acids in fermentation liquid gradually increased, rising from 2.43 ± 0.02 mg/100 g at green jujube juice to 6.29 ± 0.78 mg/100 g at the end of the main fermentation. This was most probably because increased acidity caused by the rapid degradation of the protein of the wine samples, which released a large number of amino acids (Zhao et al. 2019).
Table 3.
Dynamic change of basic nutrition in optimize fermented green jujube wine during fermentation
| Time (days) | Protein/ (mg/L) | Amino acid nitrogen/(mg/100 g) | TTA /(g/L) | pH | Reducing sugar/(mg/mL) | TPC /(mg/L) | TFC /(mg/L) | Ascorbic acid/(mg/100 g) | SOD activity /(U/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.27 ± 0.01a | 2.43 ± 0.02e | 1.84 ± 0.16f | 3.81 ± 0.02a | 25.91 ± 0.50 h | 42.05 ± 0.94d | 27.19 ± 1.11a | 152.81 ± 1.80a | 27.73 ± 1.42a |
| 2 | 0.24 ± 0.03a,b | 2.44 ± 0.06e | 2.15 ± 0.18f | 3.74 ± 0.04b | 145.46 ± 0.69a | 51.10 ± 1.05b | 27.37 ± 0.65a | 51.76 ± 1.78b | 20.68 ± 1.04b |
| 4 | 0.27 ± 0.04a | 4.50 ± 0.02d | 3.71 ± 0.20e | 3.66 ± 0.02c | 99.80 ± 0.31b | 56.74 ± 0.73a | 20.74 ± 0.64c | 33.98 ± 0.22c | 22.73 ± 3.15b |
| 3 | 0.25 ± 0.02a,b | 5.16 ± 0.29c | 3.91 ± 0.41d,e | 3.62 ± 0.00d | 81.07 ± 0.36c | 44.22 ± 1.46c | 16.64 ± 1.94d | 19.46 ± 1.38d | 27.73 ± 1.42a |
| 8 | 0.23 ± 0.01b | 6.29 ± 0.78a,b | 6.03 ± 0.17a | 3.60 ± 0.01d | 63.87 ± 3.04d | 39.20 ± 0.18e | 23.73 ± 1.40b | 17.16 ± 1.53d | 23.35 ± 1.37b |
| 38 | 0.21 ± 0.02b | 6.44 ± 0.11a | 4.24 ± 0.36c,d | 3.61 ± 0.02d | 55.97 ± 1.13e | 35.69 ± 0.24f | 12.55 ± 0.77e | 12.92 ± 0.76e | 16.59 ± 3.36c |
| 68 | 0.12 ± 0.01c | 5.90 ± 0.03b | 4.95 ± 0.10b | 3.63 ± 0.01c,d | 36.28 ± 0.63f | 34.06 ± 1.22f | 7.46 ± 0.36f | 9.38 ± 1.94e | 10.68 ± 1.72d |
| 98 | 0.06 ± 0.01d | 4.95 ± 0.05c,d | 4.40 ± 0.06c | 3.61 ± 0.02d | 29.49 ± 0.47 g | 25.39 ± 1.17 g | 3.56 ± 0.12 g | 4.29 ± 0.05f | 9.09 ± 1.04d |
Values are means ± standard deviation (SD). Different letters in the same column represent significant differences (p < 0.05)
aTTA total titration acid; TPC total phenolic content; TFC total flavonoid content; SOD superoxide dismutase
Acids in food not only serve as sour ingredients, but also play an indispensable role in the processing, storage and quality of food. There were no significant changes in pH (Table 3). The TTA in the green jujube juice was 1.837 ± 0.163 g/L. Compared with green jujube juice, TTA was gradually increased with the processing of main fermentation and TTA was remained stable in the post-fermentation and aging time. The values of TTA displayed a similar tendency with previous research (Xu et al. 2019), which might be connected with the organic acids produced in the process of fermentation. The reducing sugar content was rapidly decreased in the winemaking (Table 3). This was mainly contributed to the growth and fermentation of yeast.
The content of TPC, TFC, ascorbic acid and SOD activity in the winemaking of optimized green jujube wine
The changes in ascorbic acid, SOD, TPC and TFC in the fermentation process of the green jujube wine were shown in Table 3. Ascorbic acid was largely founded in green jujube fruits (Song et al. 2019). It has been rendered to scavenge radicals and antioxidative defense mechanism in cells and tissues (Koley et al. 2016; Wang et al. 2013). The ascorbic acid content of green jujube wine provided a degraded trend throughout the whole fermentation period. It was firstly declined from 152.81 mg/100 g (day 0) to 51.76 mg/100 g (day 2), then slightly decreased, however, there was no significant variation between day 6 and day 8 (p > 0.05) (Table 3). Throughout the production process, the decrease of ascorbic acid content may be attributed to oxidation and yeast growth. These green jujube wine samples exhibited the slightly drop on the SOD activity (Table 3). We speculated that it was the green jujube fruit raw material that might induce the different metabolisms during winemaking to result in high level of SOD activity.
It has been generally accepted that polyphenols are the major antioxidants that supply fruit wines with the antioxidant capacity (Eklund et al. 2005). As can be seen from Table 3, there are great differences in the TPC of the green jujube wine in different fermentation stages, and the TPC of wine sample was 42.05 ± 0.94 mg/L before fermentation. The highest TFC was represented on day 4, reaching concentration of 56.74 ± 0.73 mg/L, and then gradually decreased. The decreased of TPC could be caused by the oxidation and hydrolysis of phenolic compounds (Czyzowska and pogorzelski 2002). Flavonoids also play a key role in antioxidant activity. The TFC change of green wine during winemaking was shown in Table 3. The variation tendency was roughly similar to that of TPC. As illustrated in the Table 3, the TFC attained a minimum level at the end of winemaking. The decrease of TFC (from 27.19 ± 1.11 mg/L to 3.56 ± 0.12 mg/L) could be attributed to the polymerization and degradation of flavonoids and the interaction between flavonoids and other phenolic compounds. Our results on changes in TPC and TFC during alcohol fermentation were consistent with the results of Lan et al. (2017). In terms of the functional characteristics of jujube wine, the composition of phenolic compounds should be further studied.
Changes in antioxidant activity of green jujube wine during winemaking
DPPH radical scavenging activity
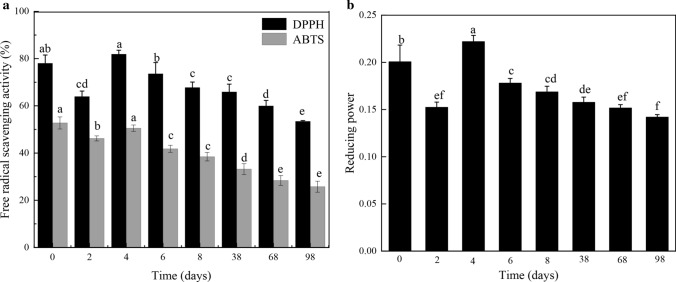
The flavonoids, polyphenols, saponins and other substances contained in the green jujube could effectively scavenge free radicals. Table 3 showed changes in the antioxidant capacity of the sample during the winemaking process. As shown in the Fig. 2a, the DPPH scavenging ability lowered firstly and then increased during fermentation, and maintained a relatively steady state in the final aging period of green jujube wine. The change of DPPH scavenging capacity was primarily related to the change of polyphenols content (Wang et al. 2016). Despite the loss, the produced green jujube wine still maintained an abundant capacity to inhibit DPPH radical.
Fig. 2.
Changes in antioxidant capacity during the brewing of green jujube wine. Different letters at the different time indicate significant differences between means (p < 0.05).
ABTS free radical scavenging ability
ABTS was oxidized to produce a stable blue-green cation ABTS plus. When a solution was added to the substance under test, the antioxidant content reacts with ABTS plus and fades the reaction system (Schaich et al. 2015). It showed that ABTS scavenging activity had the same trend with DPPH scavenging ability after fermentation and aging of green jujube wine (Fig. 2a). Compared with green jujube juice, ABTS assay decreased by 37.0% and 51.19% on day 38 and day 98, respectively. The difference in ABTS capacity was significant (p < 0.01).
Reducing power
Figure 2b showed that reducing power illustrated a slightly decrease after winemaking of green jujube wine. It is a remarkable fact that the produced green jujube wine still showed strong reducing power after fermentation and aging time. This result was similar to the antioxidant activity of products studied in fermentation stage, such as pomegranate wine Lan et al. (2017). This may be related to changes in the content of antioxidants and their synergies during fermentation.
GC–MS analysis
Aroma component was the direct influence factor of sensory evaluation of fruit wine, and was also an important index to evaluate the quality of fruit wine. Most aroma components in fruit wine were produced by yeast metabolism during fermentation. They were rich in varieties, including esters, alcohols, acids and terpenes (Styger et al. 2011). The key flavor substances in the fermentation and aging of jujube wine were identified by headspace solid phase microextraction and GC–MS. In the alcoholic fermentation process, a total of 51 kinds of volatile substances were detected, of which 21 kinds of esters, 4 kinds of alcohol, 4 kinds of acids, 5 kinds of aldehydes, 10 other kinds.
According to the GC–MS results, the samples of jujube wine (day 0, 8, 38, 68, 98) were analyzed, respectively. According to the analysis in Table 4, aromatic compounds were the main volatile substances in the samples on day 0, especially phenyl ethanol (> 40%). Phenethyl alcohol was a by-product during fermentation with roselike flavour, which was generated through the free phenylalanine-derived Ehrlich pathway (Xu et al. 2019). Phenethyl alcohol and hydroxyphenethyl alcohol were detected in the fermentation process. In addition, esters and aldehydes were the second most abundant components, especially phenylacetaldehyde (Table 4). Esters were one of the most important metabolites of alcoholic fermentation produced by yeast and impart fruity flavor (Xu et al. 2019). The biosynthesis of esters proceeds through esterification and alcoholysis (Liu et al. 2004). After the main fermentation, the relative content of heterocyclic aromatic compounds and aldehydes reduced significantly (Table 4). With the alcohol fermentation of green jujube wine, the variety of volatile substances increased. This may be due to the fact that the juice had been fermented to increase the flavor of the fruit wine. In the second month of aging, 4-Hydroxyphenethyl alcohol (14.95%), 3-Methyl-1-butanol (14.85%), 3-Methyl-1-butanol (39.04%) and Phenylacetic acid (9.27%) were major flavor substances.
Table 4.
Relative contents of aroma components in green jujube wine during fermentation and aging process
| Category | 0 d | RC | 8 d | RC | 38 d | RC | 68 d | RC | 98 d | RC |
|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | Phenethyl alcohol | 42.622 | Phenethyl alcohol | 17.250 | Phenethyl alcohol | 8.790 | Phenethyl alcohol | 10.680 | Phenethyl alcohol | 4.661 |
| 4-Hydroxyphenethyl alcohol | 3.856 | 4-Hydroxyphenethyl alcohol | 7.286 | 4-Hydroxyphenethyl alcohol | 2.937 | 4-Hydroxyphenethyl alcohol | 6.569 | 4-Hydroxyphenethyl alcohol | 14.945 | |
| Benzyl alcohol | 0.843 | Benzyl alcohol | 0.476 | 1-Butanol | 0.351 | 3-Methyl-1-butanol | 54.903 | 3-Methyl-1-butanol | 14.851 | |
| Furfuryl alcohol | 0.379 | 2,3-Butanediol | 0.585 | 2,3-Butanediol | 0.325 | |||||
| Sub-total | 47.321 | 25.391 | 12.078 | 72.737 | 34.782 | |||||
| Acids | Stearic acid | 1.585 | Stearic acid | 6.055 | 2-Ketoglutaric acid | 0.409 | Stearic acid | 0.313 | Stearic acid | 2.096 |
| 2-Ketoglutaric acid | 2.160 | Palmitic acid | 10.071 | Palmitic acid | 1.163 | Palmitic acid | 0.592 | Palmitic acid | 4.154 | |
| Phenylacetic acid | 0.966 | Butyric Acid | 0.259 | L ( +)-Lactic acid | 0.497 | Phenylacetic acid | 9.271 | |||
| Palmitic acid | 0.456 | Hexanoic acid | 0.612 | |||||||
| Oleic acid | 2.057 | |||||||||
| Sub-total | 7.224 | 16.126 | 2.443 | 1.402 | 15.521 | |||||
| Esters | Mono-Ethyl Succinate | 4.028 | Mono-Ethyl Succinate | 2.033 | Mono-Ethyl Succinate | 1.476 | Mono-Ethyl Succinate | 10.425 | Mono-Ethyl Succinate | 39.044 |
| Phenethyl acetate | 0.257 | Fema 3457 | 0.031 | Benzyl benzoate | 4.682 | Diethyl succinate | 0.272 | Diethyl succinate | 0.326 | |
| Benzyl benzoate | 0.879 | 2-Monostearin | 4.637 | Diisobutyl phthalate | 3.487 | Gamma-butyrolactone | 0.434 | Gamma-butyrolactone | 0.320 | |
| Dipropyl phthalate | 2.182 | Amyl acetate | 0.194 | Methyl hydrogen glutarate | 1.960 | |||||
| Diisobutyl phthalate | 6.299 | Ethyl 3-hydroxybutyrate | 0.234 | Ethyl lactate | 0.152 | |||||
| Ethyl phenylacetate | 0.309 | |||||||||
| 3-Phenylpropionic acid methyl ester | 0.384 | |||||||||
| Palmitic acid ethyl ester | 0.977 | |||||||||
| Ethyl oleate | 0.654 | |||||||||
| Palmitic acid ethyl ester | 1.403 | |||||||||
| Sub-total | 13.645 | 6.701 | 13.800 | 11.131 | 41.802 | |||||
| Aldehydes | Phenylacetaldehyde | 21.761 | 5-Hydroxymethylfurfural | 7.874 | Phenylacetaldehyde | 36.900 | Phenylacetaldehyde | 3.625 | Phenylacetaldehyde | 2.535 |
| 5-Hydroxymethylfurfural | 2.105 | Furfural | 0.647 | Benzaldehyde | 9.270 | Benzaldehyde | 5.370 | Benzaldehyde | 2.632 | |
| Furfural | 0.049 | |||||||||
| Sub-total | 23.866 | 8.521 | 46.170 | 8.995 | 5.216 | |||||
| Ketone | 2,3-butanedione | 0.594 | 2(5H)-Furanone | 1.713 | 2,3-butanedione | 0.480 | ||||
| 2(5H)-Furanone | 3.191 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one | 1.725 | 2(5H)-Furanone | 0.577 | |||||
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one | 0.275 | |||||||||
| Sub-total | 4.060 | 3.465 | 1.057 | |||||||
| Others | Oleamide | 3.883 | Oleamide | 16.870 | Oleamide | 8.405 | Oleamide | 2.932 | Oleamide | 0.639 |
| Octadecanamide | 1.105 | MonopalMitin | 2.297 | Octadecanamide | 0.286 | Erucylamide | 0.539 | |||
| MonopalMitin | 9.634 | Erucylamide | 11.732 | Erucylamide | 1.578 | 2,4-Di-tert-butylphenol | 1.260 | |||
| Erucylamide | 6.801 | 2,4-Di-tert-butylphenol | 2.018 | Glycine benzyl ester Hydrochl oride | 0.939 | Succinic anhydride | 0.241 | |||
| 2,4-Di-tert-butylphenol | 3.430 | |||||||||
| 2,3-Dihydrobenzofuran | 0.854 | |||||||||
| Hexadecanamide | 1.132 |
RC relative content (%)
High concentrations of aldehyde material could bring peculiar smell (Cagno et al. 2017). Aldehyde material was unstable compounds, and it can reduce alcohol or oxidize acid under the action of microorganisms, this can explain the aging process, which elaborated aldehyde material in fruit wine decreased incessantly, finally even disappear (Table 4). Fatty acids have an unpleasant soapy flavor, but they can act as precursors to the formation of esters. After yeast fermentation of jujube juice, alcohols, ketones and esters increased, while aldehydes decreased, indicating that the balance among esters, ketones and alcohols had an important effect on the flavor of fermented jujube wine. The results of this study were consistent with (Lan et al. 2017), and (Guo et al. 2018), who reported that the decrease of aldehydes could be contribute to their oxidation to the acids or reduction to the alcohols.
In a word, the final aroma components of green jujube wine were mainly constituted with esters and alcohols, including Phenethyl alcohol, 4-hydroxyphenethyl alcohol, isopentyl alcohol 3-methyl-1-butanol, and mono-ethyl Succinate, four key compounds.
Conclusion
In the present study, the fermentation parameters for fermented green jujube wine were optimized by using RSM to analyze the individual and interactive influences of the initial sugar content, yeast addition, fermentation time and SO2 treatments. Quadratic polynomial models could well predict and describe the results of alcohol content and sensory evaluation of fermented green jujube wine. The optimal parameter conditions for fermented green jujube wine were decided by initial sugar 24%, yeast addition 0.3%, fermentation time 8 d, and SO2 treatment 80 mg/L. The chemical composition and antioxidant capacity in the optimized sample were evaluated. Statistical analysis showed that green jujube wine contained high antioxidant substances and good antioxidant activity in vitro, which may indicate that green jujube wine has higher biological activities. The flavor of wine changed significantly over time due to the gradual reduction of aldehydes, ketones and heterocyclic aromatic compounds and the production of esters and alcohols.
Availability of data and material
All data generated or analysed during this study are included in this published article.
Author contributions
LY designed the study, conducted all experiments, and wrote the manuscript. NY, JW, and JD helped carry out all the experiments. GL designed the study and supervised all the experiments. All authors approved the final manuscript.
Funding
This work was financially supported by project of the Food nutrition and health quality program of Shanxi Normal University (2018YZKC-07).
Compliance with ethical standard
Conflicts of interest
The authors declare no conflict of interest.
Consent for publication
Written informed consent for publication was obtained from all participants.
Ethics approval
This article does not contain any studies with human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lu Yuan, Email: yuanlu08042@163.com.
Guifeng Li, Email: liguifeng99@163.com.
Ni Yan, Email: 18634810506@163.com.
Jianhu Wu, Email: 287718596@qq.com.
Junjie Due, Email: 182284317@qq.com.
References
- Arnous A, Makris DP, Kefalas P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J Agric Food Chem. 2001;49(12):5736–5742. doi: 10.1021/jf010827s. [DOI] [PubMed] [Google Scholar]
- Aydin C, Mammadov R. Phenolic composition, antioxidant, antibacterial, larvacidal against Culex pipiens, and cytotoxic activities of Hyacinthella lineata steudel extracts. Int J Food Prop. 2017;20(10):2276–2285. doi: 10.1080/10942912.2016.1236271. [DOI] [Google Scholar]
- Balthazar CF, Santillo A, Guimarães JT. Novel milk–juice beverage with fermented sheep milk and strawberry (Fragaria × ananassa): Nutritional and functional characterization. J Dairy Sci. 2019;102:10724–10736. doi: 10.3168/jds.2019-16909. [DOI] [PubMed] [Google Scholar]
- Cagno RD, Filannino P, Gobbetti M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int J Food Microbiol. 2017;248:56–62. doi: 10.1016/j.ijfoodmicro.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Cheung JKH, Li J, Cheung AWH, Zhu Y, Zheng KYZ, Bi CWC, Duan R, Choi RCY, Lau DTW, Dong TTX, Lau BWC, Tsim KWK. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: signaling cascade and induction of cytokines. J Ethnopharmacol. 2009;124(1):61–68. doi: 10.1016/j.jep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Czyzowska A, Pogorzelski E. Changes to polyphenols in the process of production of must and wines from blackcurrants and cherries. Part I. Total polyphenols and phenolic acids. Europ Food Res Technol. 2002;214(2):148–154. doi: 10.1007/s00217-001-0422-9. [DOI] [Google Scholar]
- Eklund P, Langvik O, Warna J, Salmi T, Willfor S, Sjoholm R. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org Biomol Chem. 2005;3(18):3336–3347. doi: 10.1039/b506739a. [DOI] [PubMed] [Google Scholar]
- Eom I, Choi J, Kim I, Kim TH, Kim S. Changes in the physicochemical and antioxidant characteristics during the fermentation of jujube wine using hot water extract of dried jujube. J Life Sci. 2016;26(11):1298–1307. doi: 10.5352/JLS.2016.26.11.1298. [DOI] [Google Scholar]
- Guo J, Yan Y, Wang M, Wu Y, Liu S-Q, Chen D, Lu Y. Effects of enzymatic hydrolysis on the chemical constituents in jujube alcoholic beverage fermented with Torulaspora delbrueckii. LWT. 2018;97:617–623. doi: 10.1016/j.lwt.2018.07.051. [DOI] [Google Scholar]
- Hou F, Mu T, Ma M, Blecker C. Optimization of processing technology using response surface methodology and physicochemical properties of roasted sweet potato. Food Chem. 2019;278:136–143. doi: 10.1016/j.foodchem.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Koley TK, Kaur C, Nagal S, Walia S, Jaggi S, Sarika, Antioxidant activity and phenolic content in genotypes of Indian jujube (Zizyphus mauritiana Lamk.) Arab J Chem. 2016;9:S1044–S1052. doi: 10.1016/j.arabjc.2011.11.005. [DOI] [Google Scholar]
- Kwaw E, Ma Y, Tchabo W, Apaliya MT, Wu M, Sackey AS, Xiao L, Tahir HE. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018;250:148–154. doi: 10.1016/j.foodchem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Lan Y, Wu J, Wang X, et al. Evaluation of antioxidant capacity and flavor profile change of pomegranate wine during fermentation and aging process. Food Chem. 2017;232:777–787. doi: 10.1016/j.foodchem.2017.04.030. [DOI] [PubMed] [Google Scholar]
- Li JW, Fan LP, Ding SD, Ding XL. Nutritional composition of five cultivars of Chinese jujube. Food Chem. 2007;103(2):454–460. doi: 10.1016/j.foodchem.2006.08.016. [DOI] [Google Scholar]
- Li J, Liu Y, Fan L, et al. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao Carbohydr Polym. 2011;84(1):390–394. doi: 10.1016/j.carbpol.2010.11.051. [DOI] [Google Scholar]
- Liu SQ, Holland R, Crow VL. Esters and their biosynthesis in fermented dairy products: a review. Int Dairy J. 2004;14(11):923–945. doi: 10.1016/j.idairyj.2004.02.010. [DOI] [Google Scholar]
- Liu G, Sun J, He X, et al. Fermentation process optimization and chemical constituent analysis on longan (Dimocarpus longan Lour.) wine. Food Chem. 2018;256:268–279. doi: 10.1016/j.foodchem.2018.02.064. [DOI] [PubMed] [Google Scholar]
- Loganayaki N, Siddhuraju P, Manian S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol Mysore. 2013;50(4):687–695. doi: 10.1007/s13197-011-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, Collins MA. Moderate alcohol consumption and cognitive risk. Neuropsychiatr Dis Treat. 2011;7(1):465–484. doi: 10.2147/NDT.S23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyanga LK, Nout MJR, Smid EJ, Boekhout T, Zwietering MH. Fermentation characteristics of yeasts isolated from traditionally fermented masau (Ziziphus mauritiana) fruits. Int J Food Microbiol. 2013;166(3):426–432. doi: 10.1016/j.ijfoodmicro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Schaich KM, Tian X, Xie J. Hurdles and pitfalls in measuring antioxidant efficacy: a critical evaluation of ABTS, DPPH, and ORAC assays. J Funct Foods. 2015;14:111–125. doi: 10.1016/j.jff.2015.01.043. [DOI] [Google Scholar]
- Siriamornpun S, Weerapreeyakul N, Barusrux S. Bioactive compounds and health implications are better for green jujube fruit than for ripe fruit. J Funct Foods. 2015;12:246–255. doi: 10.1016/j.jff.2014.11.016. [DOI] [Google Scholar]
- Song J, Bi J, Chen Q, et al. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2019;270:344–352. doi: 10.1016/j.foodchem.2018.07.102. [DOI] [PubMed] [Google Scholar]
- Styger G, Prior BA, Bauer F. Wine flavor and aroma. J Ind Microbiol Biotechnol. 2011;38(9):1145–1159. doi: 10.1007/s10295-011-1018-4. [DOI] [PubMed] [Google Scholar]
- Tian T, Yang H, Yang F, et al. Optimization of fermentation conditions and comparison of flavor compounds for three fermented greengage wines. LWT. 2018;89:542–550. doi: 10.1016/j.lwt.2017.11.006. [DOI] [Google Scholar]
- Wang C, Cheng D, Cao J, Jiang W. Antioxidant capacity and chemical constituents of Chinese jujube (Ziziphus jujuba Mill.) at different ripening stages. Food Sci Biotechnol. 2013;22(3):639–644. doi: 10.1007/s10068-013-0125-6. [DOI] [Google Scholar]
- Wang Y, Liu X, Zhang J, et al. Structural characterization and in vitro antitumor activity of polysaccharides from Zizyphus jujuba cv. Muzao RSC Adv. 2015;5(11):7860–7867. doi: 10.1039/C4RA13350A. [DOI] [Google Scholar]
- Wang B, Huang Q, Venkitasamy C, et al. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT Food Sci Technol. 2016;66:56–62. doi: 10.1016/j.lwt.2015.10.005. [DOI] [Google Scholar]
- Wang L, Fu H, Wang W, et al. Analysis of reducing sugars, organic acids and minerals in 15 cultivars of jujube (Ziziphus jujuba Mill.) fruits in China. J Food Compos Anal. 2018;73:10–16. doi: 10.1016/j.jfca.2018.07.008. [DOI] [Google Scholar]
- Xu L, Tang Z, Wen Q, et al. (2019) Effects of pulsed electric fields pretreatment on the quality of jujube wine. Int J Food Sci Technol. 2019;54(11):3109–3117. doi: 10.1111/ijfs.14226. [DOI] [Google Scholar]
- Zhang H, Jiang L, Ye S, et al. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem Toxicol. 2010;48(6):1461–1465. doi: 10.1016/j.fct.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ren J, Wang L, et al. Evolution of sensory attributes and physicochemical indexes of Gouqi fermented wine under different aging treatments and their correlations. J Food Process Preserv. 2019;43(3):e13873. doi: 10.1111/jfpp.13873. [DOI] [Google Scholar]
- Zheng H, Zhang Q, Quan J, Zheng Q, Xi W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016;205:112–121. doi: 10.1016/j.foodchem.2016.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.