Abstract
Biological control of phytopathogen is a promising approach when compared to the use of chemical agents. In the present study, seven Streptomyces cultures showing promising anti biofilm activity against Ralstonia solanacearum was mixed individually with farmyard manure. All the Streptomyces fortified farmyard manure (SFYM) were screened for plant growth promotion and control of bacterial wilt caused by R. solanacearum on tomato. Further, the ability of SFYM on stimulating the production of defense-related enzymes in R. solanacearum-inoculated tomato plants was investigated. When compared to the control tomato plants, the SFYM-treated plants had longer shoot and root length along with higher fresh and dry weight. The maximum level of chlorophyll was observed in the plants treated with strain UP1A-1 (2.21 ± 0.18 mg g−1). Strain UP1A-1 also showed maximum of 96.8 ± 1.4% biocontrol efficacy in tomato plants challenged with R. solanacearum. In addition, the UP1A-1 treated tomato plants showed maximum accumulation of total phenolics (3.02 ± 0.09 mg g−1) after 6 days of pathogen inoculation (DPI). Similarly, tomato plants treated with UP1A-1 showed highest level of peroxides, polyphenol oxidase and phenylalanine ammonia lyase during 1–9 DPI. Findings of present study revealed that the Streptomyces culture UP1A-1 fortified farm yard manure could be applied as an eco-friendly alternative to synthetic agents for controlling bacterial wilt in tomato plants.
Keywords: Tomato bacterial wilt, Ralstonia solanacearum, Biological control, Streptomyces, farm yard manure
Introduction
Tomato (Solanum lycopersicon L.) is one of the most important vegetable crops in the solanaceae family, with a global production of 188 million tons in 2018. India, after China, is the world's second-largest tomato producer [1]. The overall area for tomato cultivation and level of production are rising every year due to its potential health advantages and economic relevance. Tomato may readily be integrated into a balanced diet as a source of nutrients since they contain numerous health-promoting components such as carotenoids, vitamins A, C and E, antioxidants and phenolic compounds [2]. However, the wilt disease in tomato plants is a challenging problem. Among the various plant diseases, bacterial wilt caused by Ralstonia solanacearum has one of the most devastating symptoms resulting in massive production losses across the world. Tomato crop losses owing to bacterial wilt disease were estimated to be between 10 and 90% in India [3].
Ralstonia solanacearum is a debilitating plant vascular pathogen with a broad host variety [4]. Because of excessive extracellular polysaccharides (EPS) development within the vascular system, this pathogen causes typical wilting symptoms by colonizing xylem tissue and modifying water fluxes in the plant. N-octanoyl homoserine lactone (C8-HSL) is a major quorum sensing (QS) molecule generated by R. solanacearum, which promotes cell growth and biofilm formation [5, 6]. Potential bacteria such as Bacillus, Pseudomonas and Streptomyces inhibit QS through inactivation of C8-HSL signals by producing enzymes like AHL-lactonases and acylases [7–11]. Many microbicidal synthetic products, such as copper derivatives, have traditionally been used to combat bacterial wilt in tomato. However, conventional agrochemicals have not been very successful in controlling bacterial wilt, and antibiotic usage may result in the development of antibiotic-resistant strains. Alternative approaches such as applying biological control agents (BCAs) have proven to be successful and are now being used more often in the field. Pseudomonas, Bacillus, Streptomyces and Trichoderma are some of the potential BCAs widely used to control bacterial wilt disease [1, 12].
Streptomyces, the well investigated genus under the phylum Actinobacteria, is being explored in agriculture sector as plant growth promoters (PGP) and BCA. Due to their remarkable antagonistic behavior by producing different bioactive agents, certain Streptomyces have been used to manage soil-borne diseases [13]. Biocontrol products such as Mycostop, Actinovate, and Rhizovit by S. griseoviridis K61, S. lidicus WYEC 108, and Streptomyces sp. DSMZ 12,424, respectively are already developed to tackle fungal diseases in plants [14, 15]. Hence, members of the genus Streptomyces remain as promising microbial resources for the development of biocontrol agents and/or biofertilizers in agriculture. Nevertheless, the genus Streptomyces has not been investigated in detail as biocontrol agents for the management of bacterial wilt in tomato plants.
Induced systemic resistance (ISR) enzymes such as phenylalanine ammonia lyase (PAL), peroxidase (POX) and polyphenol oxidase (PPO) are involved in redirecting the flow of carbon from primary to secondary metabolism in plants. In addition, they act as key enzymes in the synthesis of phenolic compounds with antimicrobial activity [16]. When a pathogen is expressed, the ISR activates several defense mechanisms including the enhanced activity of POX, PPO, and PAL. POX is a broad-spectrum resistance enzyme that plays a role in plant-pathogen interactions. POX is believed to be one of the significant enzymes of the plant's biochemical protection against pathogens, and it contributes in self-regulation of plant metabolism following infection [17]. PPO is an oxidative enzyme that catalyzes the conversion of phenolic compounds into extremely poisonous quinones, which are essential for disease tolerance in plants [18]. The PAL enzyme, which converts l-phenylalanine to trans-cinnamic acid, a key product of phenylpropanoid metabolism and a critical step in the production of salicylic acid, protects against pathogen invasion [19]. Unfortunately, the kinetic modifications in defense-related enzymes produced by Streptomyces cultures on tomato are very less understood. Hence, this work is undertaken to study the anti-biofilm and biocontrol potential of Streptomyces cultures against Ralstonia solanacearum on tomato plants.
Materials and Methods
Antagonists and Plant Pathogen
Antagonistic Streptomyces cultures were obtained from Division of Bioprospecting, Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu and India. The bacterial wilt causing pathogenic strain Ralstonia solanacearum (BRs_Gr) was used for in-vitro anti-biofilm study and pot culture study [20]. The antagonistic cultures were sub-cultured using YEME (Yeast Extract and Malt Extract) agar media at 28 °C. Similarly, the R. solanacearum was sub-cultured and maintained on Pseudomonas solanacearum medium amended with 1% TTC (2, 3, 5 triphenyl tetrazolium chloride) solution at 28 °C for further studies.
Anti-Biofilm Assay
Streptomyces cultures were grown in 20 ml of YEME broth in 100 ml conical flask at 28 °C in rotary shaker with 150 rpm for 7 days. After incubation, the supernatant was collected by centrifugation at 10,000 rpm for 15 min at 4 °C and used for biofilm inhibition study. Approximately 40 µl culture filtrate of biofilm-forming test pathogenic strain R. solanacearum (108 CFU/ml) was inoculated into sterile 96-well polystyrene microtiter plate containing 60 µl LB broth supplemented with 100 µl of Streptomyces culture supernatant. In case of control treatment, 160 µl of LB broth with 40 µl culture filtrate of R. solanacearum was used and the plate was incubated at 37 °C for 16 h. After incubation, media was discarded from the 96 well plate and biofilm inhibition was observed by staining the wells with crystal violet, further dissolving with 95% ethanol. Quantification was done by UV–Vis spectrophotometer absorbed at 530 nm and the biofilm inhibition percentage was calculated using the formula:
For microscopic imaging, anti-biofilm assay performed in 12-well polystyrene microtiter plate amended with 1 × 1 cm cover slip. The biofilm inhibition was observed by staining the cover slip with crystal violet method described above and visualized by inverted bright-field microscope at magnifications of 40 X [21].
Pot Culture Study
Preparation of Streptomyces Enriched Farm Yard Manure (SFYM)
Streptomyces enriched farm yard manure (SFYM) was prepared with sterile talc powder, carboxy methyl cellulose (CMC) and well decomposed farm yard manure (FYM) in a standard ratio. A loopful of each Streptomyces culture (UP1A-1, UP1A-4, UP2A-9, UT2A-30, UT3A-39, UT4A-49 and UT6A-57) was inoculated into 200 ml of YEME broth and incubated in rotary shaker at 150 rpm for 7 days at 28 °C. To prepare SFYM, 200 ml of 7 days old Streptomyces suspension (108 CFU/ml or absorbance at 600 nm = 0.5–0.9) was mixed with one kg of sterilized talcum powder amended with 10 g of carboxy methyl cellulose (CMC) in sterile container. The freshly prepared talc formulation was then mixed with 50 kg of well decomposed FYM and the mixture was maintained under shaded conditions for 15 days with intermittent manual turn over once a day to enrich Streptomyces population in the FYM substrate.
Evaluation of SFYM for Plant Growth Promoting Properties
A fine red soil having the following characteristics: sand 14%; silt 39.5%; clay 24.2%; pH 7.03; EC (electrical conductivity) 1.39 dSm−1; OM (organic matter) 12.52%; total organic C 2.98%; total N 3.51%; P 0.94%; total K 1.64%, was taken. Twenty one days old tomato seedlings (cv. Meghdoot 2048, Syngenta, Pvt. Ltd) were transplanted into sterilized pots (15 × 15 cm) containing sterile mixture of red soil, coco peat powder and FYM (control treatment) or SFYM (Streptomyces treatment) at 2:1:1 ratio. Each pot contained approximately 300 g of soil mixture. Three tomato plants were maintained in each pot, and the experiment was repeated five times to examine the plant growth. Plants were irrigated 2 days once. After 30 days of treatment, plant growth parameters including shoot length, root length, fresh weight (FW), and dry weight (DW) were measured.
Measurement of Total Chlorophyll
The total chlorophyll from fresh tomato leaf samples was extracted using 80% acetone and quantified using spectrophotometric assay [22].
Biocontrol Study
Twenty one days old tomato seedlings (cv. Meghdoot 2048, Syngenta, Pvt. Ltd) were transplanted into sterilized pots (15 × 15 cm) containing mixture of sterile red soil, coco peat powder and FYM (control treatment) or SFYM (Streptomyces treatment) at 2:1:1 ratio. After 7 days, 20 ml of pathogenic suspension (1 × 108 CFU/ml or absorbance at 600 nm = 0.6) was inoculated into each pots by soil drench method [23] and incubated at 28–32 °C under controlled conditions. Three tomato plants were maintained in each pot, replicated five times in order to achieve completely randomized design. The wilt development on each tomato plant was calculated at regular intervals. The wilt percentage and biocontrol efficacy were calculated by the following formula,
Sample Collection for Enzyme Estimation
Tomato leaves were collected at various time intervals (1, 3, 6, and 9 days after pathogen inoculation) to estimate the plant defense-related enzymes. One gram of tomato leaves was homogenized using 1 ml of 0.1 M sodium phosphate buffer (pH 7.0) at 4 °C and the aqueous portion was collected by centrifugation at 12,000 rpm for 20 min at 4 °C. The supernatant was collected in a fresh tube to determine plant defense-related enzymes such as peroxidase (POX) [24], polyphenol oxidase (PPO) [25], and phenylalanine ammonia lyase (PAL) [26]. Total phenolic content was estimated according to the method of Zieslin and Ben-Zaken [27].
Data Analysis
The data were presented as the mean ± SE of several independent replicates. Analysis of variance (ANOVA) done by Duncan multiple post hoc comparison tests was performed in SPSS software version of 16.0. The values of P ≤ 0.05 were considered as statistically significant.
Results and Discussion
Anti-Biofilm Activity of Streptomyces Cultures
In the present study, UP1A-1, UP1A-4, UP2A-9, UT2A-30, UT3A-39, UT4A-49 and UT6A-57 were selected based on their in-vitro plant growth promoting and antagonistic activity against R. solanacearum (data not shown).
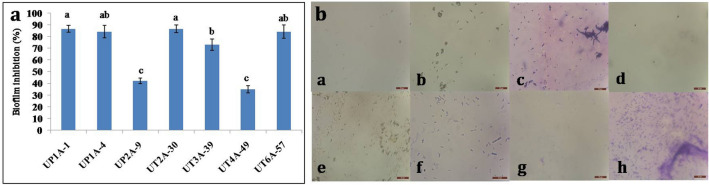
The synthesis of extracellular polymeric substances (EPS) and various proteins by R. solanacearum was commonly believed to be responsible for biofilm formation. By causing blockage in the plant xylem, biofilm formation is one of the beneficial factors for pathogenic virulence [28]. In this present study, among seven Streptomyces cultures used for biofilm inhibition against R. solanacearum, UT2A-30 and UP1A-1 showed maximum anti-biofilm activity followed by UT6A-57, UP1A-4 and UT3A-39 (Fig. 1a). The bright field microscopic observation showed prominent anti-biofilm activity by all the seven Streptomyces against R. solanacearum (Fig. 1b).
Fig. 1.
Inhibition of biofilm formation by Streptomyces cultures against Ralstonia solanacearum (a). Percentage inhibition by spectrophotometer analysis (b). Microscopic visualization a UP1A-1, b UP1A-4, c UP2A-9, d UT2A-30, e UT3A-39, f UT4A-49, g UT6A-57 and h control
Several studies reported anti-biofilm properties of bacteria and fungi against various pathogens [29–31]. Sabu et al. [32] reported that Nocardiopsis sp. can inhibit more than 90% biofilm formation by Staphylococcus capitis and Staphylococcus haemolyticus. Similarly, bioactive metabolites from Streptomyces californicus were found to inhibit more than 90% of S. aureus and MRSA biofilms [33]. In the current study, the culture supernatant of UT2A-30, UP1A-1, UT6A-57 and UP1A-4 showed more than 80% biofilm inhibition against R. solanacearum (Fig. 1a). This in-vitro study suggests that all seven Streptomyces cultures can reduce the virulence of R. solanacearum by suppressing biofilm formation.
Pot Culture Study
Promotion of Tomato Plant Growth
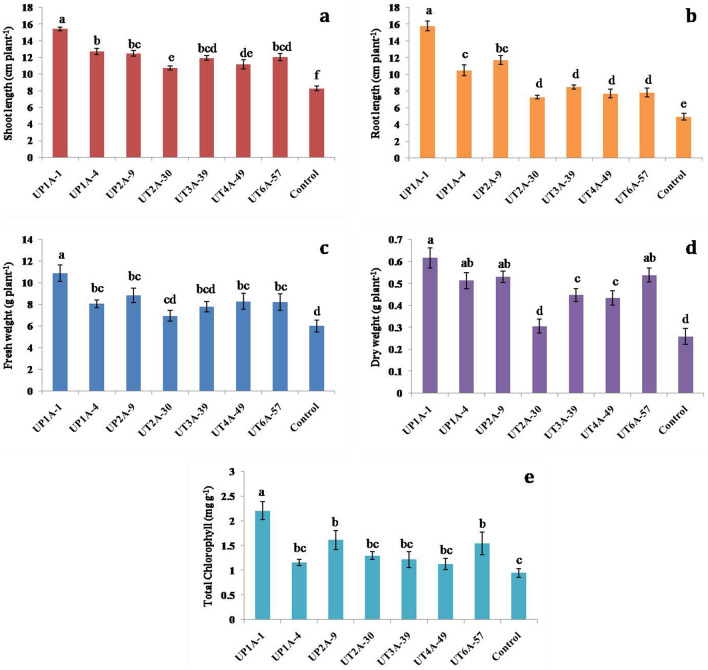
Plant growth promoting microbes are extensively researched because of their sustainable behavior in agriculture. Members of the genus Streptomyces are well renowned among Actinobacteria in terms of their biocontrol and PGP properties. It exhibits such properties on various vegetable crops including tomato, either directly by the production of phytohormones, or indirectly by antagonizing plant pathogens [34]. In this study, the growth of tomato plants evaluated by shoot length, root length, fresh weight, and dry weight was substantially higher in the SFYM treated plants than in the non-inoculated control plants. The highest shoot length was recorded in the plant treated with UP1A-1 (15.44 ± 0.21 cm plant−1) followed by UP1A-4 (12.72 ± 0.37 cm plant−1) and UP2A-9 (12.5 ± 0.32 cm plant−1) (Fig. 2a). Similarly, the application of UP1A-1 and UP2A-9 showed significant increase in the root length by 15.78 ± 0.58 and 11.72 ± 0.54 cm plant−1, respectively relative to the non-inoculated control treatment (Fig. 2b). The maximum fresh and dry weight of tomato plants was recorded in the treatment of UP1A-1, which showed 10.89 ± 0.77 and 0.62 ± 0.04 g plant−1, respectively. Next to that, UP2A-9 showed second highest fresh weight followed by UT4A-49, UT6A-57 and UP1A-4, when compared to control treatment (Fig. 2c and d). The highest level of chlorophyll was recorded on UP1A-1 (2.2 ± 0.18 mg g−1) followed by UP2A-9 (1.61 ± 0.19 mg g−1) (Fig. 2e). Our results are in agreement with the observations of Djebaili et al. [35] who reported that actinobacteria can be used for better plant growth promotion of tomato plants. Similarly, others findings also reported Streptomyces sp. used for pot culture study showing increased plant growth of tomato plants when compared to control plants [36]. These Streptomyces cultures will be the potential candidates for the development of soil nutrients and can be used as an alternate to chemical pesticides [37].
Fig. 2.
Effect of Streptomyces fortified farmyard manure (SFYM) on the growth of tomato plants under pot culture study a shoot length, b root length, c fresh weight, d dry weight, e total chlorophyll
Biocontrol Study
Streptomyces are ubiquitous in nature and can protect host from disease causing phytopathogens by secreting various antagonistic molecules. In this study, Streptomyces cultures not only enhanced growth of tomato plants but also controlled the wilt caused by R. solanacearum. The seven Streptomyces cultures amended FYM exhibited varying degrees of protection ranging from 60 to 97%. The maximum biocontrol efficacy was exhibited by UP1A-1 (96.85%) and UP2A-9 (83.1%) cultures when compared to control (Table 1). Similar to this, Streptomyces culture NEAU-HV9 isolated from soil sample was reported that can control the tomato bacterial wilt up to 82% in pot culture study [38]. Also, Streptomyces culture LD120T showed 63.6% biocontrol efficacy in pot culture study on tomato plants against R. solanacearum [12]. Our present study showed that Streptomyces culture UP1A-1 was able to control more than 90% biocontrol efficacy on tomato against R. solanacearum. This observation revealed that Streptomyces strain UP1A-1 is a potential candidate for the development of biocontrol agent against the wilt causing pathogen R. sonalacearum on tomato crops.
Table 1.
Potential of Streptomyces cultures to induce resistance against R. solanacearum in tomato plants under pot study
| Cultures | Disease incidence (%) | Biocontrol efficacy (%) |
|---|---|---|
| UP1A-1 | 2.96 ± 1.31a | 96.85 ± 1.40a |
| UP1A-4 | 21.48 ± 2.54b | 77.75 ± 2.57b |
| UP2A-9 | 16.29 ± 2.84b | 83.1 ± 2.99b |
| UT2A-30 | 28.14 ± 3.04c | 62.84 ± 4.49c |
| UT3A-39 | 17.03 ± 1.48b | 78.81 ± 2.22b |
| UT4A-49 | 31.85 ± 2.13c | 59.71 ± 2.3c |
| UT6A-57 | 19.25 ± 2.02b | 78.17 ± 2.85b |
| Control | 96.29 ± 1.77d | – |
Values are mean ± SE (n = 5). Values with the same letter within a column are not significant at p ≤ 0.05 as per Duncan multiple post hoc comparison test
Estimation of Total Phenolic and Defense Enzymes
Strains of Streptomyces were identified as intermediaries for decreasing plant disease incidence and increasing production in a variety of vegetable crops. The production of defense-related enzymes during the initial phases is important for plant disease control through cell-wall membrane and bioactive metabolites [39]. In this present study, Streptomyces-treated tomato plants showed impacted changes in the activities of defense related enzymes and total phenolic content. The maximal activity of these enzymes and total phenol occurred at different stages. Konappa et al. [40] have already demonstrated that treatment of tomato plants with antagonistic microbes develops systemic resistance through accumulation of total phenol and various plant defense enzymes against bacterial wilt. The estimation of phenol, POX, PPO, and PAL in tomato was also directly correlated with disease reduction study.
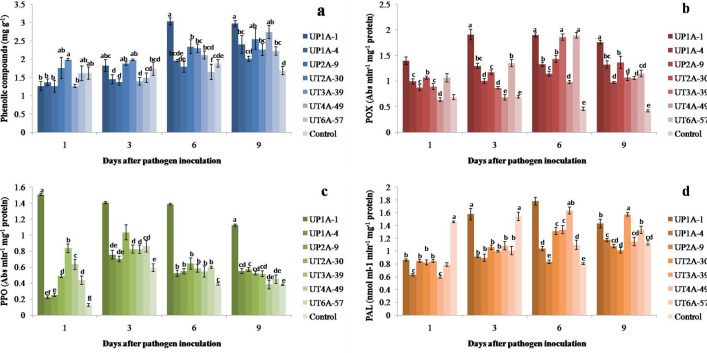
Higher phenol accumulation was observed in different Streptomyces-treated tomato plants when compared to control plant. Also tomato plants treated with UP1A-1 accumulated the maximum level of phenolic content when compared to the others at 6 DPI and 9 DPI. The accumulation of phenolic content increased after the pathogen inoculation and reached maximum level from 6 to 9 DPI in Streptomyces inoculated tomato plants while in case of control treatment, it started decreasing from 6 DPI onwards (Fig. 3a). This finding correlates with previous report where the Streptomyces and fungal pathogen inoculated Eucalyptus globulus had increased phenolic content from 1 DPI-15 DPI whereas it showed decreasing strategy in control treatment [41].
Fig. 3.
Estimation of plant defense-related enzymes of tomato plants under pot culture study a total phenolic content, b peroxidase, c polyphenol oxidase, d phenylalanine lyase activity
Peroxidase induction in plants can enhance the disease resistance through oxidation of phenolic compounds by utilizing H2O2 and lignin biosynthesis. Fortification of the cell wall by lignin biosynthesis is crucial for mechanical defense against pathogens [42]. After R. solanacearum inoculation, the activity of POX increased in Streptomyces inoculated tomato plants, whereas the POX activity decreased in control plants. The tomato plants treated with UP1A-1 induced maximum amount of POX enzyme at 3 DPI. Next to that, UT6A-57 and UT3A-39 treated tomato plants showed maximum increasing POX activity at 6 DPI (Fig. 3b). Similar studies show that Streptomyces has induced POX in tomato [43], cucumber [44] and rice [45] as well. The susceptibility of tomato plants to R. solanacearum is positively correlated with low POX activity. POX, on the other hand, plays a significant role in plant resistance to R. solanacearum when activated early [40]. In present study, at 3 DPI, tomato plants treated with UP1A-1 showed this systemic response.
Polyphenol oxidase was used to oxidize different phenolic compounds without H2O2 and employed for plant disease control. During microbial invasion, they are essential in the oxidation of polyphenols into different antimicrobial metabolites such as quinones, as well as the lignification of plant cells [46]. The activity of PPO in Streptomyces treated tomato plants were found to be higher than the control plants and it reached maximum on early stage of pathogen invention at 1 DPI (Fig. 3c). Among the Streptomyces treatments on tomato, the PPO activity was found to be higher in the plants treated with UP1A-3. The enzymes POX and PPO are involved in the activation of hypersensitive reactions in plant cells, as well as the modification of adjacent cell to prevent pathogen entry [43]. The findings of previous investigations also concur that tomato plants treated with beneficial microbes increased POX and PPO levels against the disease causing R. solanacearum pathogen [47].
The estimation of PAL enzyme was comparatively higher in control treatment than Streptomyces treated plants at 1 DPI. However, all Streptomyces treated tomato plants depicted increased PAL enzyme from 3 DPI onwards but in control treatment it showed a significantly decreasing strategy. It was observed that maximum PAL activity was induced in UP1A-1 treated tomato plants at 6 DPI followed by UT4A-49. The present study clearly observed that the PAL enzyme activity significantly increased in all Streptomyces inoculated tomato plants, whereas in control treatment it showed reduced PAL activity at the end of experiment (Fig. 3d). Induction of PAL activity by antagonistic microbes improved their biocontrol effect, according to several earlier findings [43, 47]. Such observations were also seen in this study in agreement with previous research findings showing that beneficial microbes can increase PAL enzyme in tomato infected with R. solanacearum [40, 47].
In conclusion, Streptomyces culture UP1A-1 fortified farmyard manure has the potential properties to control bacterial wilt and promote growth in tomato. Evaluation in field conditions is needed to be done to prove its further potentials.
Acknowledgements
Authors acknowledge the Sathyabama Institute of Science and Technology (SIST), Chennai, Tamil Nadu for the research facilities provided.
Authors’ Contribution
MK carried out sample collection, lab experiments, and writing of the manuscript. JJ gave design, supervision, and revision of the manuscript. RM and GV supervised the research work and revised the article. AS contributed in lab experiments and manuscript writing. BA contributed to work on particular pathogen R. solanacearum and to the experimentation. SK supervised the research work and revised the article. All authors have read and approved the final manuscript.
Funding
The authors thank the Department of Biotechnology, India (BT/PR10814/AAQ/3/669/2014) and National Centre for Polar and Ocean Research, Ministry of Earth Sciences (MoES-NCPOR/R.No.NCPOR/2019/PACER-POP/BS-08 dated 05-07-2019), for their support in the form of research grant.
Data Availability
All datasets are presented in the main manuscript.
Declarations
Conflict of interest
Authors do not have any competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guo Y, Fan Z, Yi X, et al. Sustainable management of soil-borne bacterium Ralstonia solanacearum in vitro and in vivo through fungal metabolites of different Trichoderma spp. Sustainability. 2021;13:1491. doi: 10.3390/su13031491. [DOI] [Google Scholar]
- 2.Manickam R, Chen JR, Sotelo-Cardona P, et al. Evaluation of different bacterial wilt resistant eggplant rootstocks for grafting tomato. Plants. 2021;10:1–12. doi: 10.3390/plants10010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balamurugan A, Muthamilan M, Kamalakannan A, et al. Characterization of Ralstonia solanacearum causing bacterial wilt disease of tomato in coimbatore district of Tamil Nadu, India. Int J Curr Microbiol Appl Sci. 2020;9:3010–3016. doi: 10.20546/ijcmas.2020.902.345. [DOI] [Google Scholar]
- 4.Paudel S, Dobhal S, Alvarez AM, Arif M. Taxonomy and phylogenetic research on Ralstonia solanacearum species complex: a complex pathogen with extraordinary economic consequences. Pathogens. 2020;9:1–26. doi: 10.3390/pathogens9110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 6.Kalia VC, Patel SKS, Kang YC, Lee J-K. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2018;37:68–90. doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2013;68:13–23. doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koul S, Prakash J, Mishra A, Kalia V.C. Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Indian J Microbiol. 2015;56:1–18. doi: 10.1007/s12088-015-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koul S, Kalia VC. Multiplicity of quorum quenching enzymes: a potential mechanism to limit quorum sensing bacterial population. Indian J Microbiol. 2016;57:100–108. doi: 10.1007/s12088-016-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar JS, Umesha S, Prasad KS, Niranjana P. Detection of quorum sensing molecules and biofilm formation in Ralstonia solanacearum. Curr Microbiol. 2016;72:297–305. doi: 10.1007/s00284-015-0953-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang X, Gao C, Peng C, et al. Characterization of a novel endophytic actinomycete, Streptomyces physcomitrii sp. nov., and its biocontrol potential against Ralstonia solanacearum on tomato. Microorganisms. 2020;8:1–12. doi: 10.3390/microorganisms8122025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vurukonda SSKP, Giovanardi D, Stefani E. Plant growth promoting and biocontrol activity of Streptomyces spp. As endophytes. Int J Mol Sci. 2018;19:952. doi: 10.3390/ijms19040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minuto A, Spadaro D, Garibaldi A, Gullino ML. Control of soilborne pathogens of tomato using a commercial formulation of Streptomycesgriseoviridis and solarization. Crop Prot. 2006;25:468–475. doi: 10.1016/j.cropro.2005.08.001. [DOI] [Google Scholar]
- 15.Zeng W, Wang D, Kirk W, Hao J. Use of Coniothyrium minitans and other microorganisms for reducing Sclerotinia sclerotiorum. Biol Control. 2012;60:225–232. doi: 10.1016/j.biocontrol.2011.10.009. [DOI] [Google Scholar]
- 16.Hata EM, Yusof MT, Zulperi D. Induction of systemic resistance against bacterial leaf streak disease and growth promotion in rice plant by Streptomyces shenzhenesis TKSC3 and Streptomyces sp. SS8. Plant Pathol J. 2021;37:173–181. doi: 10.5423/ppj.oa.05.2020.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agunbiade OJ, Famutimi OG, Kadiri FA, et al. Studies on peroxidase from Moringa oleifera Lam leaves. Heliyon. 2021;7:e06032. doi: 10.1016/j.heliyon.2021.e06032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Kaur I, Kariyat R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int J Mol Sci. 2021;22:1442. doi: 10.3390/ijms22031442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naz R, Bano A, Nosheen A, et al. Induction of defense-related enzymes and enhanced disease resistance in maize against Fusarium verticillioides by seed treatment with Jacaranda mimosifolia formulations. Sci Rep. 2021;11:59. doi: 10.1038/s41598-020-79306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakthivel K, Gautam RK, Kumar K, et al. Diversity of Ralstonia solanacearum strains on the Andaman Islands in India. Plant Dis. 2016;100:732–738. doi: 10.1094/PDIS-03-15-0258-RE. [DOI] [PubMed] [Google Scholar]
- 21.Thenmozhi R, Nithyanand P, Rathna J, Pandian SK. Antibiofilm activity of coral-associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol Med Microbiol. 2009;57:284–294. doi: 10.1111/j.1574-695X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 22.Ni Z, Kim E-D, Chen ZJ. Chlorophyll and starch assays. Protoc Exch. 2009 doi: 10.1038/nprot.2009.12. [DOI] [Google Scholar]
- 23.Kumar A. Methods for screening ginger (Zingiber officinale Rosc.) for bacterial wilt resistance. Indian Phytopathol. 2006;59:281–286. [Google Scholar]
- 24.Hammerschmidt R, Nuckles EM, Kuc J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Mol Plant Pathol. 1982;20:73–82. doi: 10.1016/0048-4059(82)90025-X. [DOI] [Google Scholar]
- 25.Mayer AM, Harel E, Shanl RB. Assay of catechol oxidase: a critical comparison of methods. Phytochemistry. 1965;5:783–789. doi: 10.1016/S0031-9422(00)83660-2. [DOI] [Google Scholar]
- 26.Lisker N, Cohen I, Chalutz E, Fucus Y. Fungal infections suppress ethylene-induced phenylalanine ammonia lyase activity in grape fruits. Physiol Mol Plant Pathol. 1983;22:331–338. doi: 10.1016/S0048-4059(83)81020-0. [DOI] [Google Scholar]
- 27.Zieslin N, Ben-Zaken R. Peroxidase activity and presence of phenolic substances in peduncles of rose flower. Plant Physiol Biochem. 1993;31:333–339. [Google Scholar]
- 28.Saxena P. Biofilms: architecture, resistance, quorum sensing and control mechanisms. Indian J Microbiol. 2019;59:3–12. doi: 10.1007/s12088-018-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: Acyl-Homoserine Lactone-Acylase and -Lactonase. Open Microbiol J. 2011;5:1–13. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meena H, Mishra R, Ranganathan S, et al. Phomopsis tersa as inhibitor of quorum sensing system and biofilm forming ability of Pseudomonas aeruginosa. Indian J Microbiol. 2019;60:70–77. doi: 10.1007/s12088-019-00840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huma N, Shankar P, Kushwah J, et al. Diversity and polymorphism in AHL-Lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol. 2011;21:1001–1011. doi: 10.4014/jmb.1105.05056. [DOI] [PubMed] [Google Scholar]
- 32.Sabu R, Soumya KR, Radhakrishnan EK. Endophytic Nocardiopsis sp. from Zingiber officinale with both antiphytopathogenic mechanisms and antibiofilm activity against clinical isolates. 3 Biotech. 2017;7:115. doi: 10.1007/s13205-017-0735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh R, Dubey AK. Isolation and characterization of a new endophytic actinobacterium Streptomyces californicus strain adr1 as a promising source of anti-bacterial, anti-biofilm and antioxidant metabolites. Microorganisms. 2020;8:929. doi: 10.3390/microorganisms8060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur T, Rani R, Manhas RK. Biocontrol and plant growth promoting potential of phylogenetically new Streptomyces sp. MR14 of rhizospheric origin. AMB Express. 2019;9:125. doi: 10.1186/s13568-019-0849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Djebaili R, Pellegrini M, Smati M, et al. Actinomycete strains isolated from saline soils: plant-growth-promoting traits and inoculation effects on Solanum lycopersicum. Sustainability. 2020;12:4617. doi: 10.3390/su12114617. [DOI] [Google Scholar]
- 36.Al-Dhabi NA, Esmail GA, Mohammed Ghilan AK, Arasu MV. Composting of vegetable waste using microbial consortium and biocontrol efficacy of Streptomyces sp. Al-Dhabi 30 isolated from the Saudi Arabian environment for sustainable agriculture. Sustainability. 2019;11:6845. doi: 10.3390/su11236845. [DOI] [Google Scholar]
- 37.Olanrewaju OS, Babalola OO. Streptomyces: implications and interactions in plant growth promotion. Appl Microbiol Biotechnol. 2019;103:1179–1188. doi: 10.1007/s00253-018-09577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling L, Han X, Li X, et al. A Streptomyces sp. NEAU-HV9: Isolation, identification, and potential as a biocontrol agent against Ralstonia solanacearum of tomato plants. Microorganisms. 2020;8:351. doi: 10.3390/microorganisms8030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurth F, Mailander S, Bonn M, et al. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol Plant-Microbe Interact. 2014;27:891–900. doi: 10.1094/MPMI-10-13-0296-R. [DOI] [PubMed] [Google Scholar]
- 40.Konappa NM, Maria M, Uzma F, et al. Lactic acid bacteria mediated induction of defense enzymes to enhance the resistance in tomato against Ralstonia solanacearum causing bacterial wilt. Sci Hortic. 2016;207:183–192. doi: 10.1016/j.scienta.2016.05.029. [DOI] [Google Scholar]
- 41.Salla TD, Astarita LV, Santarem ER. Defense responses in plants of Eucalyptus elicited by Streptomyces and challenged with Botrytis cinerea. Planta. 2016;243:1055–1070. doi: 10.1007/s00425-015-2460-8. [DOI] [PubMed] [Google Scholar]
- 42.Mandal S, Mitra A. Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol Mol Plant Pathol. 2007;71:201–209. doi: 10.1016/j.pmpp.2008.02.003. [DOI] [Google Scholar]
- 43.Solanki MK, Yandigeri MS, Kumar S, et al. Co-inoculation of different antagonists can enhance the biocontrol activity against Rhizoctonia solani in tomato. Antonie Van Leeuwenhoek. 2019;112:1633–1644. doi: 10.1007/s10482-019-01290-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang M, Xue J, Ma J, et al. Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Front Microbiol. 2020;11:942. doi: 10.3389/fmicb.2020.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao Z, Li Z, Fu Y, et al. Induction of defense responses against magnaporthe oryzae in rice seedling by a new potential biocontrol agent Streptomyces JD211. J Basic Microbiol. 2018;58:686–697. doi: 10.1002/jobm.201800100. [DOI] [PubMed] [Google Scholar]
- 46.Lattanzio V, Lattanzio VMT, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato F, editor. Phytochemistry: advances in research. Kerala: Research Signpost; 2006. pp. 26–67. [Google Scholar]
- 47.Jinal NH, Amaresan N. Evaluation of biocontrol Bacillus species on plant growth promotion and systemic-induced resistant potential against bacterial and fungal wilt-causing pathogens. Arch Microbiol. 2020;202:1785–1794. doi: 10.1007/s00203-020-01891-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets are presented in the main manuscript.