Abstract
Locusts are known for their herbivorous diet that constitutes a nuisance to agriculture worldwide, in Morocco these insects are considered a real threat and are widely distributed in the country. These insects are equipped with a digestive system that allows them to digest huge amounts of plant tissue. To understand the mechanisms allowing this voracity, the current study has focused on the diversity of gut microbiome using biochemical and molecular analysis tools, different bacterial isolates were identified and studied. The present study results showed the presence of four important bacterial families that are present in the intestine of these insects, namely Micrococcaceae, Dermabacteraceae, Bacillaceae, and Pseudomonadaceae. The results of Gram staining showed that 2 of 11 isolates were Gram-negative bacteria, however, only 9 bacterial strains were catalase positive. While, 3 strains (Pseudomonas stutzeri S12, Kocuria rhizophila, and Bacillus thuringiensis S4 and S8) had pectinase activity, while only one strain (Pseudomonas stutzeri S12) had cellulase activity.
Keywords: Locusts, Gut microbiome, Middle Atlas, PCR, Acridoidae
Introduction
Orthoptera are one of the most important groups of pests with more than 12,000 recorded species, of which 500 are reported to be phytophagous pests that threaten agricultural production and heavily affect crop production [1]. Thus, locusts are known for their voracity, high fecundity, as well as the great mobility over distances [2]. Given its geographical location and the prevailing climatic conditions, North Africa and particularly Morocco have experienced numerous locust invasions since the 1970s [3].
Few species are considered to be significant crop pests. However, some species, thanks to their gregarious behavior, can become harmful when climatic conditions are conducive to their development [4]. Furthermore, the Caelifera group contains the greatest number of pests among locusts [5].
The three most prevalent locust species in our study region using the atlas are Dociostaurus marocanus, Odaepoda fuscoscincta, and Caliptamus barbarous. D. marocanus is a locust species widely studied in view of its invasion capacities and the damage it inflicts on crops. It is one of the main locust pests in Morocco [6]. Its proliferation is accompanied by important phasic ethological and morphological modifications [7]. O. fuscoscincta is a species that is very responsive at the level of the Middle Atlas and characterized by its graminivorous diet [8]. C. barbarus is considered a non-migrating locust or grasshopper, however, it can still be harmful to crops [9].
In addition, one of the least studied aspects about the Orthoptera locusts of Morocco, in general, and those of the Middle Atlas, in particular, is that of their intestinal microflora or microbiome [10]. The study of the intestinal microbiome will allow both the understanding of the trophic preferences of these arthropods [11], the identification of locust control mechanisms in the event of possible invasions [12], and the expansion of certain avenues of research and innovation, such as the discovery of bacterial strains with power in bio-industry, biotransformation, and biodegradation.
The objective of this study was to isolate and study the biochemical and molecular characteristics of bacteria in the intestinal microbiome of three locust species (D. marocanus, O. fuscoscincta, and C. barbarus) in the Moroccan Middle Atlas.
Material and Methods
Sampling Area
This study was carried out in three geographical sites in the Moroccan Middle Atlas, namely Sefrou (MEZDOU station, 33.743122; − 4.831745), Guigou (TIJMA station, 33,4,632,950; − 4,8,610,880), and El-Hajeb (AJAABOU station, 33,5,889,690; − 5,2,613,990) (Fig. 1) between April and July 2019. Sampling was carried out using an entomological net, sampling was done at the places where vegetation was of low height with a coverage of less than 80%. Sampling was done in the morning between 9 and 11 h. When the insects were captured, they were gently placed in boxes containing holes to allow sufficient ventilation and taken to the laboratory for further study.
Fig. 1.
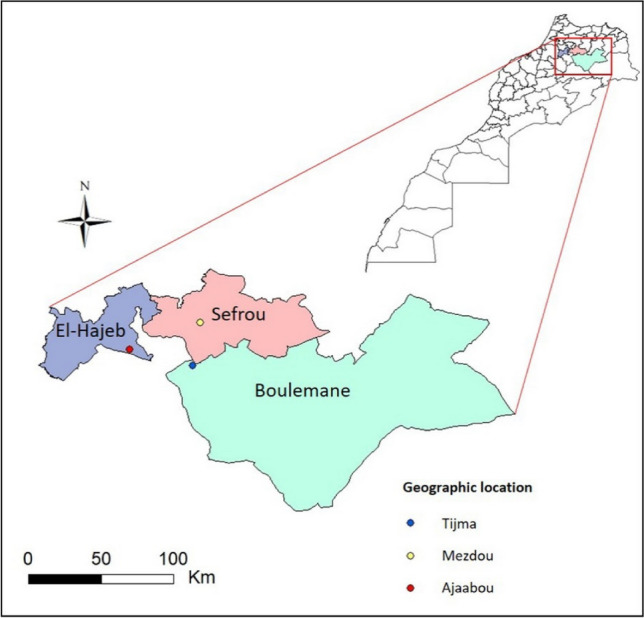
Geographical location of the studied area, in the middle atlas of Morocco
Identification of Insects
To study the intestinal microflora of locusts from the Middle Atlas of Morocco, three of the most frequent species at each of the sites of the present study were chosen. Species identification was carried out according to the determination keys of Chopard and Defaut [13–18], considering certain databases, such as that of Louveaux et al. [19].
Study of the locust intestinal microbiome.
Isolation and Purification of the Microbiome of the Studied Locusts
Dissection of the Insect and Isolation of its Intestine
Each insect was kept at 4 °C for 10 min before being handled to avoid any regurgitation of the contents of the intestine. The insects were handled by first breaking the cervical membrane and cutting the ventral nerve cord. The insects were disinfected by dipping them in ethanol for 2 min, followed by washing with sterile distilled water. The opening of the body cavity was performed by a longitudinal ventral incision. To ensure separation of the different intestinal segments without creating flows of their contents, double ligatures between the three parts of the intestine were performed. The contents of each ligation were then suspended in 3 ml of distilled sterile water and homogenized. A series of dilutions was then made from the stock solution, and a 100 µl volume of each dilution was distributed in petri dishes containing Luria–Bertani (LB) medium and incubated at 37 °C for 24 h. The resultant bacterial colonies with different morphological aspects were selected and purified. Then, morphological, biochemical, and molecular identification was undertaken on the obtained isolates.
Digestive Tract Physiology of the Investigated Species
The observation of the digestive tract of the three individuals with a binocular magnifying glass revealed the presence of a rectilinear tube occupying almost the entire body, extending from the oral cavity to the anus and covered with a fatty layer. The digestive tract of these species showed a linear succession of three parts usually found in most insects (anterior intestine or Stomodeum, middle intestine or Mesenteron, and posterior intestine or Proctodeum) [20]. However, the size of these parts differed from one species to another; that of Calliptamus barbarus was larger than that of the other two species by about 3.4 cm. The stomodeum had a very narrow esophagus and continued with a crop and gizzard of almost the same size.
The anterior limit of the gizzard consisted of a slight strangulation, separating it from the crop. The mesenteron had six gastric caeca. The point of insertion of the Malpighi tubes marked the passage between the midgut and posterior intestine. The latter ended to form a rectum that widened again to give rise to the rectal ampulla.
Biochemical Characterization
Gram Stain and Catalase Test
The phenotypic and biochemical characteristics of the isolated bacteria were studied using conventional bacteriological methods. Gram staining was carried out as described by [21], and the catalase test was done by adding a bacterial colony to a drop of hydrogen peroxide.
Pectinase Activity
Pectinase activity was tested by culturing bacterial isolates in pectin supplemented agar [22]. After growth of the bacterial isolates for 5 days at 28 °C, the plates were soaked with 2% (w/v) hexadethyltrimethylammonium bromide (CTAB) solution for 30 min, and rinsed with 1 M NaCl solution to visualize a halo around the bacterial colony that indicated pectinase production.
Cellulase Activity
Cellulase activity was tested by culturing bacterial colonies in a minimal medium supplemented with 2% (v/v) carboxymethyl cellulose (CMC) for 5 days at 28 °C, according to Rangjaroen et al. [22]. After staining with Congo Red for 5 min and subsequent rinsing with 5 M NaCl plus 0.1% (v/v) acetic acid, the generation of a clear halo around a colony indicated cellulase production.
Molecular Identification of Bacterial Isolates
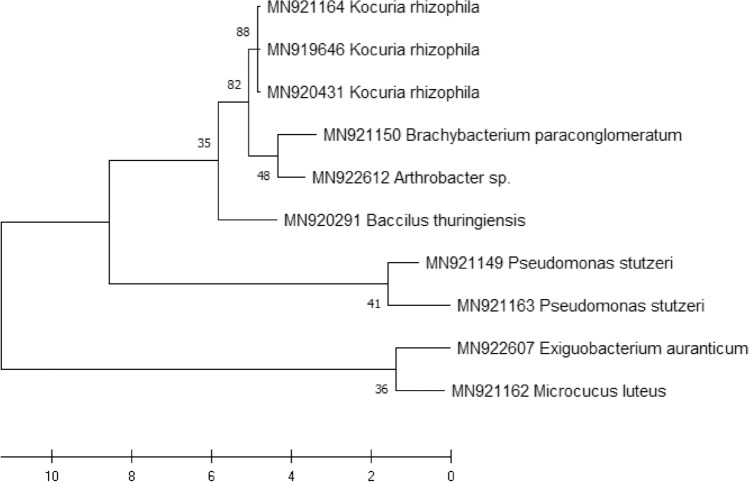
Isolates with different morphological aspects were cultured in LB medium and incubated at 28 ± 1 °C for 24 h in the dark. A colony was recovered for genomic DNA extraction. Standard protocols were used to obtain genomic DNA. Amplification of the 16 s rRNA region of the bacteria was performed using universal primers (27F/1492R) [23] according to the following program: 5 min at 94 °C, 35 cycles of 94 °C for 1 min, 52 °C for 1 min, and 72 °C for 1 min, followed by a final step of 10 min at 72 °C. The amplicon was revealed in 1% electrophoresis gel, and PCR products were then sequenced. The sequences were edited and aligned using BioEdit software (version 7.0.5.3) and checked for similarity in Genbank using the Blast program, before being deposited. Phylogenetic analysis was performed using the sequences published in GenBank. The resulting sequences were used to generate a phylogenetic tree to reorganize the isolates into major clusters using MEGA-X software. Maximum likelihood analysis was used to estimate the phylogenetic relationships, and the inferred trees were evaluated by 1000 bootstrap.
Results
Identification of Insects
The three studied species were: O. fuscocincta from the region of SEFROU at the site "MEZDOU", D. maroccanus from GUIGOU at "TIJMA", and Calliptamus barbarus from EL HAJEBE at "AJAABOU".
Locust Species Studied
O. fuscocincta (Orthoptera; Acrididae): We found the concave shape of the vertex in this species to be among the most discriminating criteria for rigorous identification. The pronotum was rough and initially narrow [13]; the median carina of the pronotum was well marked and interrupted by the typical furrow [14]. The color of this species was brown ochre to gray. The lateral carina was reduced or absent, while the median carina of the prozone was slightly protruding and wide at the base [24]. The black band on the wing was not or only slightly extended in the forefield; the wing background color was yellow or greenish blue [19] (Fig. 2a).
Fig. 2.
The studied locusts: a Oedipoda fuscocincta, b Dociostaurus maroccanus, c Calliptamus barbarous
Dociostaurus maroccanus (Orthoptera; Acrididae): This species had a narrowed pronotum anterior to the middle, a triangular vertex, a lateral carina interrupted in the middle forming an X-shaped pattern, and three triangular spots on the superior edge of the posterior femurs. This species was reported as polyphagous [25]. which has been linked to nature and the number of sensilla [26]. but without questioning the nature of the microbiome of this insect (Fig. 2b).
Calliptamus barbarus (Orthoptera; Acrididae): This species had a subconical head and fastigium of the vertex, merging with the frontal edge, which was characterized by femurs with a large black spot on the internal face [14]. These femurs were generally large, and the basal lobe was shorter than the superior lobe [27], with a smooth-edged pallium in the male [28] (Fig. 2c).
Morphological and Biochemical Characterization
Gram Staining and Catalase Test
Gram staining results showed that 2 of 11 isolated bacteria were Gram negative (Pseudomonas stutzeri S9 and Pseudomonas stutzeri S12 strains), while the remaining 10 bacterial strains were Gram positive. Nine bacterial strains showed positive catalase activity (Table 1).
Table 1.
Biochemical characterization of 11 bacterial isolates
| Bacterial species | Gram | Catalase | Pectinase | Cellulase |
|---|---|---|---|---|
| Brachybacterium Paraconglomeratum S1 | + | − | − | − |
| Brachybacterium paraconglomeratum S2 | + | − | − | − |
| Kocuria rhizophila S4 | + | + | + | − |
| Microcucus luteus S5 | + | + | − | − |
| Kocuria rhizophila S6 | + | + | − | − |
| Exiguobacterium aurantiacum S7 | + | + | − | − |
| Bacillus thuringiensis S8 | + | + | + | − |
| Pseudomonas stutzeri S9 | − | + | − | − |
| Arthrobacter sp S10 | + | + | − | − |
| Streptomyces flavoviridis S11 | + | + | − | − |
| Pseudomonas stutzeri S12 | − | + | + | + |
Pectinase Activity
Evaluation of the ability of bacterial strains to degrade pectin showed that three (Pseudomonas stutzeri S12, Kocuria rhizophila S4, and Bacillus thuringiensis S8) of the 11 isolated bacteria strains possess this activity (Table 1).
Cellulase Activity
Among the 11 isolated bacteria, one strain (Pseudomonas stutzeri S12) possessed the ability to produce cellulase (Table 1).
Molecular Characterization
The 16S rRNA sequences were deposited in the NCBI GenBank database with the access numbers listed with the origin of isolation of each orthoptera in Table 2.
Table 2.
Distribution of bacterial strains in the gut of the three locust species
| Locusts species | Physiological part of the intestine | Bacterial species | Accession number |
|---|---|---|---|
| Oedipoda fuscocincta | Stomodeum | Pseudomonas stutzeri S9 | MN921149 |
| Mesenteron | Arthrobacter sp S10 | MN922612 | |
| Kocuria rhizophila S11 | MN920431 | ||
| Proctodeum | Pseudomonas stutzeri S12 | MN921163 | |
| Dociostaurus maroccanus | Stomodeum | Microcucus luteus S5 | MN921162 |
| Mesenteron | Kocuria rhizophila S4 | MN919646 | |
| Proctodeum | Brachybacterium paraconglomeratum S1 | MN921150 | |
| Brachybacterium paraconglomeratum S2 | MN919645 | ||
| Calliptamus barbarus | Stomodeum | Bacillus thuringiensis S8 | MN920291 |
| Mesenteron | Exiguobacterium aurantium S7 | MN922607 | |
| Proctodeum | Kocuria rhizophila S6 | MN921164 |
The percentage of similarity was ≥ 96%; two isolates showed a similarity with Pseudomonas stutzeri. Two isolates were similar to Brachybacterium paraconglomeratum, and three bacterial isolates were similar to Kocuria rhizophila. The rest isolates were identified as Bacillus thuringiensis, Microcucus luteus, and Exiguobacterium aurantium, as well as one isolate belonging to the genus Arthrobacter.
Four families (Micrococcaceae, Dermabacteraceae, Bacillaceae, and Pseudomonadaceae) were found associated with the digestive tract microbiome of the studied locusts. The phylogenetic tree (Fig. 3) demonstrated the diversity, represented by the digestive tract of these arthropods.
Fig. 3.
Phylogenetic tree of bacteria isolated from the microbiome of the three studied insects. The maximum likelihood method was implemented using the two-parameter Kimura model with MEGA-X software. Phylogenetic trees were evaluated by bootstrap analysis based on 1000 replicates
Discussion
The objective of our study was to characterize the intestinal microbiome of three locust orthoptera in the Moroccan Middle Atlas, specifically Oedipoda fuscocincta, Calliptamus Barbarus, and Dociostaurus maroccanus. These species have been the subject of numerous systematic, ecological, and biodynamic studies, but they have never been studied for their intestinal microflora. Thus, the interest of the present study was to fill this gap to understand the mechanisms that influence digestion and to comprehend the trophic preferences of these locust species [29]. Furthermore, recent studies have demonstrated the impact of certain bacterial strains on insect immunity [30]. These models can provide ideas for possible locust control interventions.
The present work identified the microbiome community of 3 locust species in three compartments of the digestive tract (Stomodeum, Mesenteron, and Proctodeum). This study was carried out through isolations, followed by molecular identification by sequencing of the gene encoding the 16 s rRNA. The results showed the association of 11 strains distributed in 8 bacterial species, belonging to 4 families, namely Micrococcaceae, Dermabacteraceae, Bacillaceae, and Pseudomonadaceae, with the digestive tract of the insects of our study, confirming its microbial diversity. Other studies have shown significant microbial diversity in some orthoptera and their location in the digestive tract [29], which has also been reported in other insects [31][31].
Among these strains, only two were gram negative, namely Pseudomonas stutzeri S9 and Pseudomonas stutzeri S12, and three had pectinase activity, namely Kocuria rhizophila S4, Bacillus thuringiensis S8, and Pseudomonas stutzeri S12. Pseudomonas stutzeri S12 was the only strain with cellulase activity. Numerous studies have demonstrated the major role of microorganisms in food digestion, particularly in insects (Babendreier et al. [33]; Song et al. [34]). Additionally, MsangoSoko et al. [35] showed that the intestine of lepidoptera is rich in bacteria with important cellulolytic activity.
Similarly, our results showed that Pseudomonas stutzeri S12 was characterized by positive catalase, cellulase, and pectinase activity, and Arthrobacter sp. S10 exhibited positive catalase activity. The enzymatic activities of these bacteria can have roles in digestion but can also enable the insects to have other competencies, such as the ability to resist insecticides. Almeida et al. [36] showed that Pseudomonas sp. and Arthrobacter sp. isolated from the digestive system of Spodoptera frugiperda were endowed with insecticide resistance activity. Another study showed that the presence of a complex of bacteria, including Brachybacterium Paraconglomeratum, allowed Bombyx mori (Lepidoptera: Bombycidae) to acquire resistance to insecticides [37].
Among the studied locust microbiome strains, we identified Microcucus luteus S5, Bacillus thuringiensis S8. The latter has been reported to have an important role in sexual attractiveness in Bactrocera cucurbitae (Diptera: Tephritidae), Hadapad et al. [38] showed that these bacteria are capable of producing constituents that promote male mating attractiveness.
The feeding behavior of insects is highly linked to microbiome composition [32]. In addition, Joern [39] reported that locust diets can be influenced by abiotic factors such as the microclimate in the insect's habitat and the biochemical nature of plant substances that may have a phagostimulant or repellent role, consequently, influencing trophic preferences.
Conclusion
This preliminary study was done to identify the composition of the microbiome of certain locust orthoptera in the Moroccan Middle Atlas. Our results showed that the locust gut microbiome is characterized by various bacterial species. In addition, the enzymatic activities of the gut microbiome of these insects influenced their digestive properties and determined their trophic preferences.
The study of the intestinal microbiome, in general, and in locusts, in particular, will allow us to answer questions concerning the mechanisms of action of the bacterial flora on eating behavior, sexual attractiveness, and the mechanisms of insecticide resistance in possible locust control campaigns.
To understand all the mechanisms of action of the gut microbiome of these insects and to deepen our knowledge, further studies should focus on (i) increasing the number of microbial isolates characterized; (ii) identifying the modes of action of the microbiome on consumption choices and trophic preferences of these orthoptera; and (iii) resistance of certain orthoptera to pesticides through these microbes and the possible influence on sexual behavior.
Author Contributions
ZA and EL originally formulated the idea, ZA and LA developed methodology, ZA, EH and TK conducted fieldwork, RN generated sequencing data and molecular analyses, ES and ZA analysed data, and ZA, RN and ES wrote the manuscript.
Funding
No funding was received for conducting this study.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbassi K, Atay-Kadiri Z, Ghaout S. Caractérisation des populations de Schistocerca gregaria (Forskål 1755), durant la recrudescence de 1995 au sud du Maroc. J Orthoptera Res. 2003;12:63–69. doi: 10.1665/1082-6467(2003)012[0063:CDPDSG]2.0.CO;2. [DOI] [Google Scholar]
- 2.Kamil Usmani M, Usmani S (2018) Locusts. Pests their manag. Springer, Singapore 825–69
- 3.Piou C, Jaavar Bacar MEH, Babah Ebbe MAO, Chihrane J, Ghaout S, Cisse S, et al. Mapping the spatiotemporal distributions of the Desert Locust in Mauritania and Morocco to improve preventive management. Basic Appl Ecol Urban Fischer. 2017;25:37–47. doi: 10.1016/j.baae.2017.10.002. [DOI] [Google Scholar]
- 4.Zhang L, Lecoq M, Latchininsky A, Hunter D. Locust and grasshopper management. Annu Rev. 2019;64:15–34. doi: 10.1146/annurev-ento-011118-112500. [DOI] [PubMed] [Google Scholar]
- 5.Brahimi D, Mesli L, Rahmouni A (2019) First data onorthoptera fauna diversity in the arid region of naâma ( south west of Algeria ) orthoptérologique dans la région aride de naama ( sud-ouest de l ’ algérie ) The locust activity developed in the vast region the most serious invasions that the. ISSN 2507-7627. 2019;9:1292–301
- 6.El Ghadraoui L, Petit D, El Yamani J (2003) Le site Al-Azaghar (Moyen-Atlas, Maroc): un foyer grégarigène du criquet marocain Dociostaurus maroccanus (Thunb 1815). Bull l’Institut Sci 25:81–6
- 7.Defaut BD (2020) Révision taxinomique des Orthoptères du Maghreb. 3: biométrie des stades phasaires de Dociostaurus maroccanus (Thunberg) en région paléarctique occidentale , et recherche de sous-espèces. 2009:25–40
- 8.Essakhi D, El Harchli EH, Benjelloun M, Maazouzi N, Mansouri I, Azzouzi A, et al. Contribution A L’Étude Du Régime Alimentaire Des Orthoptères Acridiens Dans Le Moyen Atlas (Maroc) Int J Eng Sci. 2015;5:60–66. [Google Scholar]
- 9.Çiplak B. Locust and grasshopper outbreaks in the near east: Review under global warming context. Agronomy. 2021;11:111. doi: 10.3390/agronomy11010111. [DOI] [Google Scholar]
- 10.Rangberg A, Diep DB, Rudi K, Amdam GV. Paratransgenesis: an approach to improve colony health and molecular insight in honey bees (Apis mellifera)? Integr Comp Biol Oxf Acad. 2012;52:89–99. doi: 10.1093/icb/ics089. [DOI] [PubMed] [Google Scholar]
- 11.Ibanez S, Lavorel S, Puijalon S, Moretti M. Herbivory mediated by coupling between biomechanical traits of plants and grasshoppers. Funct Ecol. 2013;27:479–489. doi: 10.1111/1365-2435.12058. [DOI] [Google Scholar]
- 12.Tan SQ, Yin Y, Cao KL, Zhao XX, Wang XY, Zhang YX, et al. Effects of a combined infection with Paranosema locustae and Beauveria bassiana on Locusta migratoria and its gut microflora. Insect Sci. 2021;28:347–354. doi: 10.1111/1744-7917.12776. [DOI] [PubMed] [Google Scholar]
- 13.Chopard L (1943) Faune de l’empire française Orthoptéroides de l’Afrique du nord
- 14.Chopard L. Faune de france: Orthoptéroides. Bull la Société Entomol Fr. 1951;56:361. [Google Scholar]
- 15.Defaut B. Diagnoses d’Orthoptéroïdes nouveaux ou nouvellement décrits au Maroc. L’Entomologiste (Paris) 1987;43:109–112. [Google Scholar]
- 16.Defaut B (1988) Détermination des Orthoptéroïdes Ouest-Paléarctiques. 4. Catantopidae: le genre Calliptamus Serville 1831, en France, Espagne et Maroc. 5. Acrididae: les genres Acrida L. 1758, Truxalis F. 1775 et Ochrilidia Stal 1873, en France, Espagne et Maroc. L’Entomologiste (Paris). 44:337–45
- 17.Defaut B (1988) La détermination des Orthoptèroïdes ouest-paléarctiques. VI: Caelifera: Acrididae (suite). VII: Ensifera. VIII: Mantodea. Trav du Lab d’écobiologie des arthropodes édaphiques. 6
- 18.Defaut B. Actualisation taxonomique et nomenclaturale du «Synopsis des Orthoptères de France». Matériaux Entomocénotiques. 2001;6:107–112. [Google Scholar]
- 19.Louveaux A, Mouhim A, Roux G, Gillon Y, Barral H (1996) Influence du pastoralisme sur les populations acridiennes dans le massif du siroua (Maroc). Rev. d’Ecologie (La Terre la Vie) p 139–51
- 20.Amutkan Mutlu D (2021) The morphology and histology of the female reproductive system of Pseudochorthippus parallelus parallelus (Zetterstedt, 1821)(Orthoptera, Acrididae) and the histochemical features of the yolk granules. Microsc Res Tech. Wiley Online Library [DOI] [PubMed]
- 21.Claus D. A standardized Gram staining procedure. World J Microbiol Biotechnol Citeseer. 1992;8:451–452. doi: 10.1007/BF01198764. [DOI] [PubMed] [Google Scholar]
- 22.Rangjaroen C, Rerkasem B, Teaumroong N, Noisangiam R, Lumyong S. Promoting plant growth in a commercial rice cultivar by endophytic diazotrophic bacteria isolated from rice landraces. Ann Microbiol. 2015;65:253–266. doi: 10.1007/s13213-014-0857-4. [DOI] [Google Scholar]
- 23.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Defaut B. Révision préliminaire des Oedipoda ouest-paléarctiques (Caelifera, Acrididae, Oedipodinae) Matériaux orthoptériques et entomocénotiques. 2006;11:23–48. [Google Scholar]
- 25.Guerrero A, Ramos VE, López S, Alvarez JM, Domínguez A, Coca-Abia MM, et al (2018) Enantioselective synthesis and activity of all diastereoisomers of (E)-Phytal, a Pheromone Component of the Moroccan Locust, Dociostaurus maroccanus. J Agric Food Chem. American Chemical Society 67:72–80 [DOI] [PubMed]
- 26.Zaim A, Petit D, Elghadraoui L. Dietary diversification and variations in the number of labrum sensilla in grasshoppers: which came first? J Biosci. 2013;38:339–349. doi: 10.1007/s12038-013-9325-8. [DOI] [PubMed] [Google Scholar]
- 27.Dirsh VM (1961) A preliminary revision of the families and subfamilies of Acridoidea. Bull Br Museum (Natural Hist Entomol) 10:351–419
- 28.Jago ND (1963) A revision of the genus Calliptamus Serville (Orthoptera: Acrididae). order of the Trustees of the British Museum
- 29.Wang J-M, Bai J, Zheng F-Y, Ling Y, Li X, Wang J, et al (2020) Diversity of the gut microbiome in three grasshopper species using 16S rRNA and determination of cellulose digestibility. PeerJ [Internet]. PeerJ Inc. [cited 2021 Aug 27]; 8:e10194. Available from: https://peerj.com/articles/10194 [DOI] [PMC free article] [PubMed]
- 30.Lu HL, St Leger RJ (2016) Insect immunity to entomopathogenic fungi. Adv Genet. 94:251–85. [DOI] [PubMed]
- 31.Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse drosophila species: ecological context of a host-microbe model system. PLOS Genet Publ Lib Sci. 2011;7:e1002272. doi: 10.1371/journal.pgen.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Teh B, Sun C, Hu S, Lu X, Boland W (2016) Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera littoralis. Nat Publ Gr Nature Publishing Group; 1–14 [DOI] [PMC free article] [PubMed]
- 33.Babendreier D, Joller D, Romeis J, Bigler F, Widmer F. Bacterial community structures in honeybee intestines and their response to two insecticidal proteins. FEMS Microbiol Ecol. 2007;59:600–610. doi: 10.1111/j.1574-6941.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 34.Song XB, Peng AT, Ling JF, Cui YP, Cheng BP, Zhang LH. Composition and change in the microbiome of Diaphorina citri infected with Candidatus Liberibacter asiaticus in China. Int J Trop Insect Sci. 2019;39:283–290. doi: 10.1007/s42690-019-00036-3. [DOI] [Google Scholar]
- 35.MsangoSoko K, Bhattacharya R, Ramakrishnan B, Sharma K, Subramanian S (2021) Cellulolytic activity of gut bacteria isolated from the eri silkworm larvae, Samia ricini, (Lepidoptera: Saturniidae). Int J Trop Insect Sci. Springer; 1–10
- 36.Almeida LGD, Moraes LABD, Trigo JR, Omoto C, Cônsoli FL (2017) The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria A potential source for biotechnological exploitation. Oliveira PL, editor. PLoS One. 12:e0174754 [DOI] [PMC free article] [PubMed]
- 37.Chen B, Zhang N, Xie S, Zhang X, He J, Muhammad A et al (2020) Gut bacteria of the silkworm Bombyx mori facilitate host resistance against the toxic effects of organophosphate insecticides. Environ Int. Elsevier; 143:105886 [DOI] [PubMed]
- 38.Hadapad AB, Prabhakar CS, Chandekar SC, Tripathi J, Hire RS. Diversity of bacterial communities in the midgut of Bactrocera cucurbitae (Diptera: Tephritidae) populations and their potential use as attractants. Pest Manag Sci. 2016;72:1222–1230. doi: 10.1002/ps.4102. [DOI] [PubMed] [Google Scholar]
- 39.Joern A (2019) Behavioral responses underlying ecological patterns of resource use in rangeland grasshoppers. Integr Pest Manag Rangel. CRC Press p 137–161