Abstract
Background
To perform an updated systematic review with meta-analysis on trials focusing on patient-reported outcome measures (PROMs), nerve conduction studies (NCS) result and cross sectional area (CSA) measurements of those who underwent PRP injection for mild to moderate CTS, versus a control.
Conclusion
This study indicates that there may be a potential role for the use of PRP in the non-operative management of mild to moderate CTS results in improvements in pain scores, functional outcomes as well as CSA measurements of the MN at short-term follow-up. However, PRP does not result in improvements in NCS.
Level of evidence
II; Systematic Review & Meta-Analysis of Prospective Trials;
Keywords: PRP, Platelet rich plasma, CTS, Carpal tunnel syndrome, Median neuropathy
1. Introduction
Carpal tunnel syndrome (CTS) is the most common mono-neuropathy accounting for approximately 90% of peripheral entrapment neuropathies.1 Estimates of prevalence range from 4% up to 20% in the industrial populations.2, 3, 4, 5, 6, 7 CTS is theorized to be the result of gradual swelling, causing an hourglass compression and ischemic degradation of the median nerve (MN) as it traverses the carpal tunnel.8 Clinical findings often, but not always, reflect the individual's degree of neural damage, which explains the variation of presenting symptoms from mild to severe pain, with/without accompanying neurological symptoms and signs.9 At present, a myriad of treatments exist for CTS, with non-surgical management being the first line for milder cases, and surgery reserved for those who have failed/relapsed following non-operative management.10, 11, 12
There is growing evidence in the literature to support the use of platelet-rich plasma (PRP) injections for those with CTS undergoing non-operative management.13 PRP is an autologous blood-derived biologic product consisting of concentrated platelets, which possesses many growth factors and cytokines, which are believed to reduce inflammation by augmented cellular proliferation, migration and angiogenesis.14,15 PRP has been shown to enhance neural tissue repair in previous in-vivo studies, resulting in Schwann cell proliferation and migration following PRP injection. A previous systematic review and meta-analysis by Catapano et al. found that PRP resulted in significant improvements patients with mild-moderate CTS.13 However, the included studies did not report either the results of nerve conduction studies (NCS) or the cross sectional area (CSA) of the MN in each of the included RCTs.
The purpose of this study was to perform an updated systematic review with meta-analysis on trials focusing on patient-reported outcome measures (PROMs), NCS results and CSA measurements of those who underwent PRP injection for mild-moderate CTS, versus a control. Our hypothesis was that PRP would result in moderate improvements in PROMs, NCS and CSA at short-term follow-up.
2. Methods
2.1. Search strategy
Two independent reviewers performed a systematic review of MEDLINE, EMBASE and Scopus databases in June 2020. This search was carried out based on the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The following keywords were utilized for the search: (carpal tunnel OR carpal tunnel syndrome OR cts OR median neuropathy) AND (platelet-rich plasma OR prp). A senior author reviewed discrepancies in inclusion or exclusion of studies.
2.2. Inclusion and exclusion criteria
The inclusion criteria for studies included the following: 1) prospective trials examining non-operative management of CTS with PRP versus a control, 2) written in English, 3) paper published in a peer-reviewed journal, and 4) full text must have been available. The exclusion criteria for studies included the following: 1) non-randomized group used, 2) papers not published in English language, 3) papers published without peer-review, 4) retrospective studies, 5) review articles, 6) case reports, and 7) laboratory or cadaveric studies. No timeline was applied to the search to exclude studies. Using these inclusion and exclusion criteria, the titles and abstracts of each of the returned papers were screened with the full texts of potentially relevant studies subsequently reviewed. Each study's references list was then reviewed for additional articles.
2.3. Data analysis
Under the guidance of the senior author, data extraction from included studies was carried out independently by the same two reviewers. For each included study, the level of evidence (LOE) was assessed and evaluated based on the criteria established by Oxford Centre of Evidence Based Medicine.16 The Cochrane Collaboration risk of bias tool was used in order to evaluate risk; a study was deemed to be ‘low risk’ when every single item was scored as ‘low risk’. Studies were evaluated as moderate risk of bias when one or two items were classified as ‘high risk’ or ‘unclear risk’. Studies were deemed to be high risk if more than two items were scored as ‘high risk’.17 A comparison was formulated of study outcomes of CTS with PRP versus control groups.
2.4. Statistical analysis
Qualitative statistical analysis was performed using the Statistical Package for the Social Sciences (IBM Corp. Released 2013. IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY: IBM Corp.). Meta-analysis of results was performed on the studies using Review Manager ((RevMan) [Macintosh]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.) A p-value of <0 0.05 was deemed to be statistically significant.
3. Results
3.1. Literature search
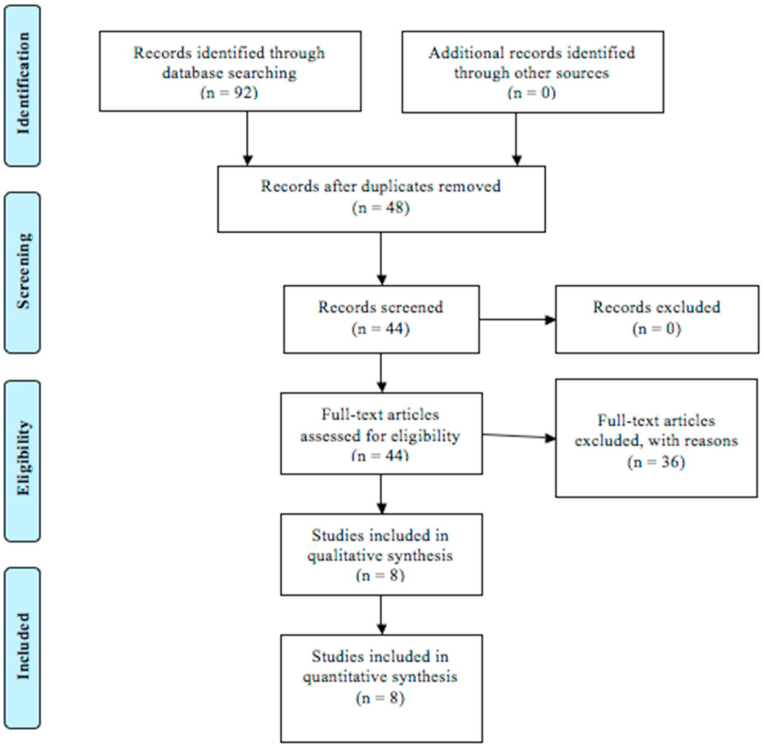
The search resulted in a total of 92 studies. After removal of 48 duplicates, the abstracts of remaining articles were assessed using the inclusion and exclusion criteria. The PRISMA Flow Chart is illustrated in Fig. 1.
Fig. 1.
PRISMA flow chart.
3.2. Study characteristics
A total of eight studies18, 19, 20, 21, 22, 23, 24, 25 with 404 patients (85.7% females) were included. All studies were prospective trials published focusing on the use of PRP in the non-operative management of CTS versus a control, with a mean follow-up time of 3.9 months (1.0–6.0). The patient demographics and specifics of the PRP injections are illustrated in Table 1, Table 2 respectively.
Table 1.
Study charcteristics & patient demographics.
| Author | LOE | PRP | N PRP | Female (%) | Age ± SD (Yrs) | Control | N Control | Female (%) | Age ± SD (Yrs) | F/U (Mo) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atwa et al., 2018 | II | PRP | 18 | 16 (88.9%) | 38.5 ± 8.0 | CS | 18 | 16 (88.9%) | 36.6 ± 8.8 | 3 | High |
| Güven et al., 2019 | II | PRP & Splint | 20 | N/R | 47.5 | Splint | 20 | N/R | 50 | 1 | High |
| Malahias et al., 2018 | I | PRP US | 26 | N/R | 60.5 ± 14.4 | NS | 24 | N/R | 57.2 ± 16.1 | 3 | Low |
| Raeissadat et al., 2018 | I | PRP & Splint | 21 | 21 (100%) | 51.2 ± 9.8 | Splint | 20 | 20 (100%) | 47.2 ± 7.1 | 3 | High |
| Senna et al., 2019 | I | PRP US | 43 | 35 (81.4%) | 38.3 ± 6.4 | CS US | 42 | 36 (85.7%) | 40.7 ± 9.4 | 3 | Moderate |
| Shen et al., 2019 | I | PRP US | 26 | 25 (96.2%) | 56.8 ± 1.7 | Dex | 26 | 22 (82.6%) | 58.5 ± 2.1 | 6 | High |
| Uzun et al., 2017 | II | PRP | 20 | 16 (80%) | 48.8 ± 5.8 | CS | 20 | 16 (80%) | 48.5 ± 6.1 | 6 | High |
| Wu et al., 2017 | I | PRP US | 30 | 27 (90%) | 57.9 ± 1.5 | Splint | 30 | 25 (83.3%) | 54.3 ± 1.3 | 6 | High |
CS; Corticosteroid, Dex; Dextrose, F/U; Follow-Up, Mo; Months, N; Number, NS; Normal Saline, PRP; Platelet Rich Plasma, SD; Standard deviation, Yrs; Years.
Table 2.
Platelet-rich plasma injection characteristics.
| Author | Preparation Kit | LR/LP | Centrifuge Time | Activating Agent |
|---|---|---|---|---|
| Atwa et al., 2018 | N/R | N/R | 3000 rpm (3 min) then 4000 rpm (15 min) | Calcium Chloride |
| Güven et al., 2019 | N/R | N/R | 100 g (15 min) then 1600 g (10 min) | N/R |
| Malahias et al., 2018 | N/R | N/R | Double Spin (Time & rpm N/R) | N/R |
| Raeissadat et al., 2018 | Rooyagen Kit (Arya Mabna Tashkis Corp) | LP | 1600 rpm (12 min) then 3500 rpm (7 min) | Sodium Citrate & Autologous Thrombin |
| Senna et al., 2019 | Special PRP Kit (GD Medical Pharma) | N/R | 3000 rpm (3 min) then 4000 rpm (15 min) | Calcium Chloride |
| Shen et al., 2019 | Regen Kit (Geosmatic) | LR | 3400 rpm (15 min) | Sodium Citrate & Autologous Thrombin |
| Uzun et al., 2017 | N/R | N/R | 4000 rpm (10 min) | Sodium Citrate |
| Wu et al., 2017 | Regen Kit (Geosmatic) | LR | 3400 rpm (15 min) | Sodium Citrate & Autologous Thrombin |
LP; Leukocyte-Poor, LR; Leukocyte-Rich, Min; Minute, N/R; Not reported, Plt Conc; Platelet Concentration, PRP; Platelet-Rich Plasma, RPM; Rounds Per Minute.
*Note: Platelet Concentration was not reported in any of the included studies.
3.3. Patient reported outcomes measures
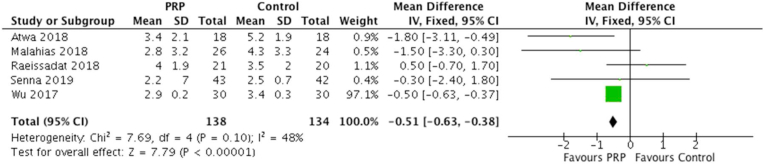
A total of five studies reported visual analogue scale (VAS) scores at three months, with 138 patients treated with PRP injection and 134 controls. For the group treated with PRP, the mean VAS score was 2.9 verses 3.5 in the control group. This difference achieved statistical significance (MD = −0.51 [0.95% CI -0.63 to −0.38]; I2 = 48%; P < 0.0001). The forest plot demonstrating VAS score at 3 months is illustrated in Fig. 2.
Fig. 2.
Forest Plot of VAS scores at 3 Months.
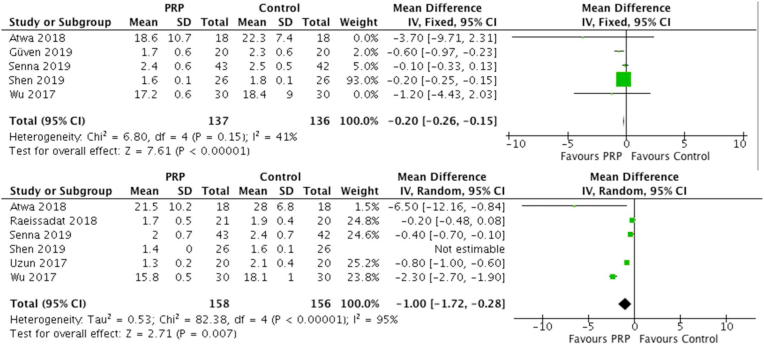
A total of five studies reported symptomatic severity scores (SSS) from the Boston carpal tunnel questionnaire (BCTQ) at 1 month, with 137 patients treated with PRP injection and 136 controls. For PRP, the mean SSS was 7.52 verses 8.46 in the control. There was a statistically significant difference between the groups (MD = −0.20 [0.95% CI -0.26 to −0.15]; I2 = 41%; P < 0.00001). Six studies reported SSS at 3 months, with 158 patients treated with PRP injection and 156 controls. In the PRP group, the mean SSS was 7.97 verses 8.14 in the control group. This difference was statistically significant (MD = −1.00 [0.95% CI -1.72 to −0.28]; I2 = 95%; P < 0.00001). The forest plot demonstrating SSS scores at 1 and 3 months are illustrated in Fig. 3.
Fig. 3.
Forest Plots of SSS scores at 1 and 3 Months.
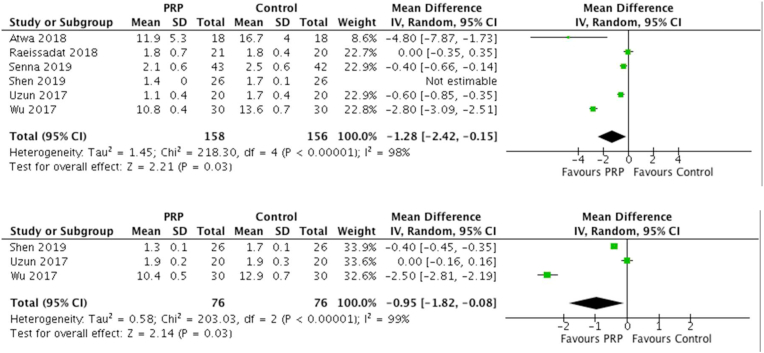
A total of six studies reported functional status scale (FSS) at three months, with 158 patients treated with PRP injection and 156 controls. For PRP, the mean FSS score was 4.59 verses 5.95 in the control group. There was a statistically significant difference between the groups (MD = −1.28 [0.95% CI -2.42 to −0.15]; I2 = 99%; P = 0.03). Overall, three studies reported FSS at six months, with 76 patients treated with PRP injection and 76 controls. With PRP, the mean FSS score was 5.05 verses 6.17 in the control. There was a statistically significant difference between the groups (MD = −0.95 [0.95% CI -1.81 to −0.08]; I2 = 99%; P = 0.03). The forest plot demonstrating FSS scores at 3 and 6 months are illustrated in Fig. 4. A summary of patient reported outcome measures is further illustrated in Table 3.
Fig. 4.
Forest Plots of FSS scores at 3 and 6 Months.
Table 3.
Patient-reported outcome measures.
| Outcome | N Studies | N PRP | PRP Mean | Control N | Control Mean | P-Value |
|---|---|---|---|---|---|---|
| FSS 1 Mo | 5 | 137 | 5.05 | 136 | 6.17 | 0.57 |
| FSS 3 Mo | 6 | 158 | 4.59 | 156 | 5.95 | 0.03a |
| FSS 6 Mo |
3 |
76 |
5.05 |
76 |
6.17 |
0.03a |
| SSS 1 Mo | 5 | 137 | 7.52 | 136 | 8.46 | <0.00001a |
| SSS 3 Mo | 6 | 158 | 7.97 | 156 | 8.14 | <0.00001a |
| SSS 6 Mo |
3 |
158 |
6.64 |
156 |
7.58 |
0.23 |
| VAS 1 Mo | 3 | 91 | 2.9 | 90 | 3.4 | 0.51 |
| VAS 3 Mo | 5 | 138 | 2.9 | 134 | 3.5 | <0.0001a |
C; Control, FSS; Functional Status Scale, Mo; Month, N; Number, N/R; Not reported, PRP; Platelet-Rich Plasma, SSS; Symptomatic Severity, VAS; Visual Analogue Scale.
denotes statistical significance.
3.4. Nerve conduction studies
A total of five studies reported distal motor latency (DML) from the NCS at 1 month, with 137 patients treated with PRP injection and 136 controls. For PRP, the mean DML was 4.8 m/s verses 4.8 m/s in the control. There was a non-statistically significant difference between the groups (MD = 0.08 [0.95% CI -0.11 to 0.27]; I2 = 72%; P = 0.39). Additionally, 5 studies reported DML at 3 months, with 136 patients treated with PRP injection and 136 with a control. With PRP, the mean DML was 4.7 m/s verses 4.7 m/s in the control. There was a non-statistically significant difference between the groups (MD = 0.04 [0.95% CI -0.12 to 0.20]; I2 = 79%; P = 0.60). Overall, 3 studies reported DML at 6 months, with 76 patients treated with PRP injection and 76 with a control. With PRP, the mean DML was 4.9 m/s verses 5.1 m/s in the control. There was a non-statistically significant difference between the groups (MD = 0.20 [0.95% CI -0.12 to 0.52]; I2 = 96%; P = 0.22).
A total of five studies reported sensory nerve conduction velocity (SNCV) from the NCS at 1 month, with 137 patients treated with PRP injection and 136 with a control. With PRP, the mean SNCV was 36.4 m/s verses 34.3 m/s in the control. There was a non-statistically significant difference between the groups (MD = 2.02 [0.95% CI -3.14 to 7.19]; I2 = 99%; P = 0.44). Additionally, 5 studies reported SNCV at 3 months, with 137 patients treated with PRP injection and 137 with a control. With PRP, the mean SNCV was 42.3 m/s verses 42.6 m/s in the control. There was a non-statistically significant difference between the groups (MD = −0.52 [0.95% CI -1.93 to 0.88]; I2 = 87%; P = 0.46). Overall, 3 studies reported SNCV at 6 months, with 76 patients treated with PRP injection and 76 with a control. With PRP, the mean SNCV was 33.5 m/s verses 34.3 m/s in the control. There was a non-statistically significant difference between the groups (MD = −0.85 [0.95% CI -2.44 to 0.74]; I2 = 89%; P = 0.30). A summary of nerve conduction studies is further illustrated in Table 4.
Table 4.
Nerve conduction studies.
| Outcome | N Studies | N PRP | PRP Mean | Control N | Control Mean | P-Value |
|---|---|---|---|---|---|---|
| DML (m/s) 1 Mo | 5 | 137 | 4.8 | 136 | 6.17 | 0.39 |
| DML (m/s) 3 Mo | 5 | 136 | 4.7 | 136 | 4.7 | 0.60 |
| DML (m/s) 6 Mo |
3 |
76 |
4.9 |
76 |
5.1 |
0.22 |
| SNCV (m/s) 1 Mo | 5 | 137 | 36.4 | 136 | 34.2 | 0.44 |
| SNCV (m/s) 3 Mo | 5 | 137 | 42.3 | 137 | 42.6 | 0.46 |
| SNCV (m/s) 6 Mo | 3 | 76 | 33.5 | 76 | 34.3 | 0.30 |
C; Control, DML; Distal Motor Latency, m/s; Metres per Second, Mo; Month, N; Number, N/R; Not reported, PRP; Platelet-Rich Plasma, SNCV; Sensory Nerve Conduction Velocity.
3.5. Cross sectional area
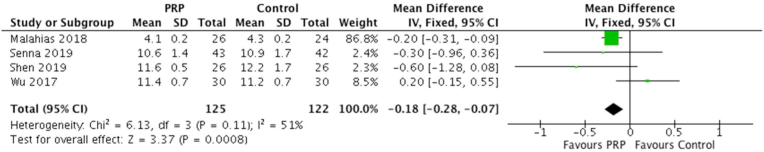
A total of 4 studies reported CSA at 3 months, with 125 patients treated with PRP injection and 122 with a control. With PRP, the mean CSA score was 9.65 mm2 verses 9.95 mm2 in the control. There was a statistically significant difference between the groups (MD = −0.18 [0.95% CI -0.28 to −0.07]; I2 = 51%; P = 0.0008). The forest plot demonstrating CSA at 3 months is illustrated in Fig. 5. A summary of the cross sectional areas are further illustrated in Table 5. The supplementary appendix comprises analyses not included in included in the accompanying figures.
Fig. 5.
Forest plot of CSA at 3 Months.
Table 5.
Cross sectional area.
| Outcome | N Studies | N PRP | PRP Mean | Control N | Control Mean | P-Value |
|---|---|---|---|---|---|---|
| CSA 1 Mo | 4 | 119 | 11.85 | 118 | 11.59 | 0.69 |
| CSA 3 Mo | 4 | 125 | 9.65 | 122 | 9.95 | 0.0008a |
C; Control, CSA; Cross Sectional Area, Mo; Month, N; Number, N/R; Not reported, PRP; Platelet-Rich Plasma.
denotes statistical significance.
4. Discussion
The most important finding in this study was that, when compared to controls, the use of PRP in CTS resulted in significant improvements in both symptoms and function at short-term. While PRP resulted in significantly smaller CSA versus controls, there was no corresponding improvement in either motor or sensory results of NCS. Therefore, there is high quality evidence to validate the use of PRP in short-term symptomatic management of mild to moderate CTS, however longer term studies are required to further evaluate the longevity of these effects when compared to controls.
Pain levels were significantly lower in the short-term following PRP injection when compared to a control, with significantly lower VAS scores reported at 3 months post-intervention. However, there was only one randomized control trial (Wu et al.25), which reported VAS scores at six months in patients who received PRP versus a night splint control. The findings of our meta-analysis are contrary to the previous systematic review and meta-analysis performed by Catapano et al.,13 who that found no significant differences in VAS scores at short-term follow-up when compared to various controls. These results are promising, as the inclusion of further studies in our review has further informed the literature on this topic. Although the results of this study are predominantly positive, the relatively short-term follow-up of the included studies poses a question as to the longevity of the effect of PRP in treating patients with mild to moderate CTS. Furthermore, the fact that there is a difference in conclusion between these two recent meta-analysis (due to the increased number of trials on the topic), this highlights the current paucity of evidence in relation to the use of PRP in the non-operative management of CTS. This therefore suggests that further studies are not only necessary to validate our conclusions, but also to focus on longer-term follow-up are warranted on the topic.
First described by Levine et al.26 in 1993, the BCTQ has emerged as a standardized, patient-based outcome measure of symptom severity and functional status. This tool focuses on nine activities of daily living which are deemed likely to cause symptoms of MN compression in patients suffering from CTS.27 This meta-analysis supports previous literature that injecting PRP in the management of CTS results in significant improvements in symptomology and functionality when compared to controls.13,28 Previous in vivo and in vitro laboratory studies have demonstrated that PRP results in reduced inflammation of soft tissues.29,30 Our meta-analysis found significant improvements in both BCTQ scores (SSS and FSS) up to 6 months follow-up in those who received PRP as a non-operative management option when compared to a control. However, discrepancies in reported PRP varieties used between the included studies of this meta-analysis limit in depth evaluation of the effects of leukocyte-rich (LR) or –poor (LP) PRP at present.
Although the pathophysiology of CTS is believed to be relatively well-understood,8,31 the most appropriate treatment at any given point of the disease spectrum remains the subject of debate.32, 33, 34, 35 The symptoms associated with CTS are believed to occur secondary to sustained pressure causing ischemia of the MN. This ischemia progresses to demyelination and axonal loss in severe cases.9,36 Therefore, a larger CSA of the MN (typically measured using US or magnetic resonance imaging) is often believed to correspond to more severe symptoms as well as poorer clinical functionality.37, 38, 39, 40 The measurement of the CSA at the point of entrance of the MN at the carpal tunnel has been previously demonstrated to represent the highest sensitivity and specificity for CTS in patients with more mild or moderate disease.37 Our meta-analysis found that the use of PRP in patients with mild to moderate CTS resulted in smaller CSA measurements of the MN at 3 months follow-up, corresponding to significantly improved reported pain and symptomology scores. Although only two included studies reported CSA measurements at 6 months limited our ability to preform meta-analysis at this time of follow-up, Wu et al.25 reported significant reductions in CSA measurements using US at 6 month post-intervention in patients when compared to those used a night splint as a control.
Severe CTS has been shown to result in demyelination, deranged action potentials and ultimate axonal loss of the MN.41 However, previous in-vivo studies have demonstrated that the injection of PRP possesses the opportunity to enhance neural tissue repair, resulting in Schwann cell proliferation, functioning and migration following PRP injection.42 Despite the potential of PRP as a viable treatment option for patients with mild to moderate symptoms of CTS, our study found that no significant improvement was seen in NCS results (both DML and SNCV) in those who received PRP versus a control. However, two prospective trials previously reported significant improvements in both DML and SNCV in those who received PRP injections versus a corticosteroid (CS) control at up to 3 months follow-up.18,22 Despite these positive findings, numerous RCTs have failed to see significant improvements in NCS results following PRP injection in the management of mild to moderate CTS when compared to controls.19,23 Although the clinical findings of this study illustrate majorly promising evidence supporting the role of PRP as a non-operative treatment of CTS, limited evidence exists for its use in benefitting NCS results.43
4.1. Limitations
The authors acknowledge this study is subject to the innate limitations of being a systematic review, with the limitations in all included studies being inherent to the study as a consequence. There are many confounding factors of the included studies, none more so than the use of numerous controls including splinting alone, injection of normal saline, dextrose or corticosteroid or even no therapy, as well as the lack of standardized description of preparation and injection technique of PRP for those in the experimental arm in each of the included studies. Furthermore, Chahla et al.44 previously proposed that standardization of the preparation and administration of PRP may improve reporting amongst studies; this would benefit this study in reducing potential heterogeneity amongst studies include in meta-analyses similar to our study. While we initially attempted to stratify our results based on LR or LP variations of PRP, analysis was limited due to the heterogeneity. Furthermore, it would be of benefit to subgroup these further based on the number of platelets, growth factors, and other bioactive cytokines. Although these are substantial limitations in the reported PRP preparation and characteristics, the heterogeneity was low across the outcome measures, with consistent outcome measure such as VAS, SSS, FSS, DML, SNCV and CSA reported across a multitude of included studies at similar time frames of follow-up.
5. Conclusion
This study indicates that there may be a potential role for the use of PRP in the non-operative management of mild to moderate CTS results in improvements in pain scores, functional outcomes as well as CSA measurements of the MN at short-term follow-up. However, PRP does not result in improvements in NCS suggesting that muscle loss may occur in patients with electrophysiological evidence of deteriorating nerve function, despite PRP treatment.
Declaration of competing interest
None.
References
- 1.Ghasemi-Rad M., Nosair E., Vegh A., et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. Jun 28 2014;6(6):284–300. doi: 10.4329/wjr.v6.i6.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yung M., Evanoff B.A., Buckner-Petty S., Roquelaure Y., Descatha A., Dale A.M. Applying two general population job exposure matrices to predict incident carpal tunnel syndrome: a cross-national approach to improve estimation of workplace physical exposures. Scand J Work Environ Health. May 1 2020;46(3):248–258. doi: 10.5271/sjweh.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maghsoudipour M., Moghimi S., Dehghaan F., Rahimpanah A. Association of occupational and non-occupational risk factors with the prevalence of work related carpal tunnel syndrome. J Occup Rehabil. Jun 2008;18(2):152–156. doi: 10.1007/s10926-008-9125-4. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan S.J., Glickel S.Z., Eaton R.G. Predictive factors in the non-surgical treatment of carpal tunnel syndrome. J Hand Surg Eur. Feb 1990;15(1):106–108. doi: 10.1016/0266-7681(90)90061-8. [DOI] [PubMed] [Google Scholar]
- 5.Masear V.R., Hayes J.M., Hyde A.G. An industrial cause of carpal tunnel syndrome. J Hand Surg Am. Mar 1986;11(2):222–227. doi: 10.1016/s0363-5023(86)80055-7. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb C.A. The clinical practice guideline on carpal tunnel syndrome and workers' compensation. J Hand Surg. Jun 2016;41(6):723–725. doi: 10.1016/j.jhsa.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Dias J.J., Burke F.D., Wildin C.J., Heras-Palou C., Bradley M.J. Carpal tunnel syndrome and work. J Hand Surg Eur. Aug 2004;29(4):329–333. doi: 10.1016/j.jhsb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Aboonq M.S. Pathophysiology of carpal tunnel syndrome. Neurosci. Jan 2015;20(1):4–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Werner R.A., Andary M. Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol : Off J Int Feder Clin Neurophysiol. Sep 2002;113(9):1373–1381. doi: 10.1016/s1388-2457(02)00169-4. [DOI] [PubMed] [Google Scholar]
- 10.Padua L., Coraci D., Erra C., et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. Nov 2016;15(12):1273–1284. doi: 10.1016/s1474-4422(16)30231-9. [DOI] [PubMed] [Google Scholar]
- 11.Tang C.Q.Y., Lai S.W.H., Tay S.C. Patient-reported outcomes of carpal tunnel release surgery in patients with bilateral severe carpal tunnel syndrome. J Hand Surg Eur. Nov 2017;42(9):932–936. doi: 10.1177/1753193417721456. [DOI] [PubMed] [Google Scholar]
- 12.Irwin L.R., Beckett R., Suman R.K. Steroid injection for carpal tunnel syndrome. J Hand Surg Eur. Jun 1996;21(3):355–357. doi: 10.1016/s0266-7681(05)80202-5. [DOI] [PubMed] [Google Scholar]
- 13.Catapano M., Catapano J., Borschel G., Alavinia S.M., Robinson L.R., Mittal N. Effectiveness of platelet-rich plasma injections for nonsurgical management of carpal tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. May 2020;101(5):897–906. doi: 10.1016/j.apmr.2019.10.193. [DOI] [PubMed] [Google Scholar]
- 14.Civinini R., Macera A., Nistri L., Redl B., Innocenti M. The use of autologous blood-derived growth factors in bone regeneration. Clin Cases Min Bone Metabol. 2011;8(1):25–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Middleton K.K., Fu F.H., Im H.J., Wang J.H. HGF mediates the anti-inflammatory effects of PRP on injured tendons. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group OLoEW. The Oxford 2011 levels of evidence. Oxf Cent Evid Based Med. Available from: http://www.cebm.net/index.
- 17.Robertson C., Ramsay C., Gurung T., Mowatt G., Pickard R., Sharma P. Practicalities of using a modified version of the Cochrane Collaboration risk of bias tool for randomised and non-randomised study designs applied in a health technology assessment setting. Res Synth Method. Sep 2014;5(3):200–211. doi: 10.1002/jrsm.1102. [DOI] [PubMed] [Google Scholar]
- 18.Atwa E.T., Esh A.M., Abd Et Al I.T., Awad Y.M. Platelet-rich plasma versus corticosteroid injections for carpal tunnel syndrome: clinical and electrophysiological study. Egypt Rheumatol. 2019/07/01/2019;41(3):237–241. doi: 10.1016/j.ejr.2018.07.008. [DOI] [Google Scholar]
- 19.Güven S.C., Özçakar L., Kaymak B., Kara M., Akıncı A. Short-term effectiveness of platelet-rich plasma in carpal tunnel syndrome: a controlled study. J Tissue Eng Regen Med. May 2019;13(5):709–714. doi: 10.1002/term.2815. [DOI] [PubMed] [Google Scholar]
- 20.Malahias M.A., Roumeliotis L., Nikolaou V.S., Chronopoulos E., Sourlas I., Babis G.C. Platelet-rich plasma versus corticosteroid intra-articular injections for the treatment of trapeziometacarpal arthritis: a prospective randomized controlled clinical trial. Cartilage. Oct 20 2018 doi: 10.1177/1947603518805230. 1947603518805230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raeissadat S.A., Karimzadeh A., Hashemi M., Bagherzadeh L. Safety and efficacy of platelet-rich plasma in treatment of carpal tunnel syndrome; a randomized controlled trial. BMC Muscoskel Disord. Feb 13 2018;19(1):49. doi: 10.1186/s12891-018-1963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senna M.K., Shaat R.M., Ali A.A.A. Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin Rheumatol. Dec 2019;38(12):3643–3654. doi: 10.1007/s10067-019-04719-7. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y.P., Li T.Y., Chou Y.C., et al. Comparison of perineural platelet-rich plasma and dextrose injections for moderate carpal tunnel syndrome: a prospective randomized, single-blind, head-to-head comparative trial. J Tissue Eng Regen Med. Nov 2019;13(11):2009–2017. doi: 10.1002/term.2950. [DOI] [PubMed] [Google Scholar]
- 24.Uzun H., Bitik O., Ö Uzun, Ersoy U.S., Aktaş E. Platelet-rich plasma versus corticosteroid injections for carpal tunnel syndrome. J Plast Surg Hand Surg. Oct 2017;51(5):301–305. doi: 10.1080/2000656x.2016.1260025. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y.T., Ho T.Y., Chou Y.C., et al. Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: a prospective randomized, single-blind controlled trial. Sci Rep. Dec 2017;7(1):94. doi: 10.1038/s41598-017-00224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine D.W., Simmons B.P., Koris M.J., et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Jt Surg Am. Nov 1993;75(11):1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Leite J.C., Jerosch-Herold C., Song F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Muscoskel Disord. Oct 20 2006;7:78. doi: 10.1186/1471-2474-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malahias M.A., Chytas D., Mavrogenis A.F., Nikolaou V.S., Johnson E.O., Babis G.C. Platelet-rich plasma injections for carpal tunnel syndrome: a systematic and comprehensive review. Eur J Orthop Surg. Jan 2019;29(1):1–8. doi: 10.1007/s00590-018-2278-8. [DOI] [PubMed] [Google Scholar]
- 29.Takamura M., Yasuda T., Nakano A., Shima H., Neo M. The effect of platelet-rich plasma on Achilles tendon healing in a rabbit model. Acta Orthop Traumatol. Jan 2017;51(1):65–72. doi: 10.1016/j.aott.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middleton K.K., Barro V., Muller B., Terada S., Fu F.H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150–163. [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeffer G.B., Gelberman R.H., Boyes J.H., Rydevik B. The history of carpal tunnel syndrome. J Hand Surg. Feb 1988;13(1):28–34. doi: 10.1016/0266-7681(88)90046-0. [DOI] [PubMed] [Google Scholar]
- 32.Gerritsen A.A., de Vet H.C., Scholten R.J., Bertelsmann F.W., de Krom M.C., Bouter L.M. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. Jama. Sep 11 2002;288(10):1245–1251. doi: 10.1001/jama.288.10.1245. [DOI] [PubMed] [Google Scholar]
- 33.Weintraub M.I. Splinting vs surgery for carpal tunnel syndrome. Jama. Jan 22-29 2003;289(4):422. doi: 10.1001/jama.289.4.422-a. author reply 422-3. [DOI] [PubMed] [Google Scholar]
- 34.Menkes D.L. Splinting vs surgery for carpal tunnel syndrome. Jama. Jan 22-29 2003;289(4):420–421. doi: 10.1001/jama.289.4.420-c. author reply 421-2. [DOI] [PubMed] [Google Scholar]
- 35.Sassi S.A., Giddins G. Gender differences in carpal tunnel relative cross-sectional area: a possible causative factor in idiopathic carpal tunnel syndrome. J Hand Surg Eur. Jul 2016;41(6):638–642. doi: 10.1177/1753193415625404. [DOI] [PubMed] [Google Scholar]
- 36.Phalen G.S., Kendrick J.I. Compression neuropathy of the median nerve in the carpal tunnel. J Am Med Assoc. Jun 1 1957;164(5):524–530. doi: 10.1001/jama.1957.02980050014005. [DOI] [PubMed] [Google Scholar]
- 37.Hammer H.B., Hovden I.A., Haavardsholm E.A., Kvien T.K. Ultrasonography shows increased cross-sectional area of the median nerve in patients with arthritis and carpal tunnel syndrome. Rheumatology. May 2006;45(5):584–588. doi: 10.1093/rheumatology/kei218. [DOI] [PubMed] [Google Scholar]
- 38.Park J.S., Won H.C., Oh J.Y., Kim D.H., Hwang S.C., Yoo J.I. Value of cross-sectional area of median nerve by MRI in carpal tunnel syndrome. Asian J Surg. Jun 2020;43(6):654–659. doi: 10.1016/j.asjsur.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Healy C., Watson J.D., Longstaff A., Campbell M.J. Magnetic resonance imaging of the carpal tunnel. J Hand Surg. May 1990;15(2):243–248. doi: 10.1016/0266-7681(90)90131-m. [DOI] [PubMed] [Google Scholar]
- 40.Funahashi T., Suzuki T., Fujita N. Three-dimensional MRI of the median nerve in the carpal tunnel. J Hand Surg Eur. Aug 13 2020 doi: 10.1177/1753193420948406. 1753193420948406. [DOI] [PubMed] [Google Scholar]
- 41.Yip C.W., Lo Y.L. Finding relief at the end of the (Carpal) tunnel: electrophysiological clues. J Neurosci Rural Pract. Oct 2013;4(4):377–378. doi: 10.4103/0976-3147.120191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng C., Zhu Q., Liu X., et al. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J Tissue Eng Regener Med. May 2016;10(5):428–436. doi: 10.1002/term.1756. [DOI] [PubMed] [Google Scholar]
- 43.Zhang D., Earp B.E., Blazar P. Utility of 'baseline' electrodiagnostic studies for carpal tunnel release. J Hand Surg Eur. Mar 2019;44(3):273–277. doi: 10.1177/1753193418815546. [DOI] [PubMed] [Google Scholar]
- 44.Chahla J., Cinque M.E., Piuzzi N.S., et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Jt Surg Am. Oct 18 2017;99(20):1769–1779. doi: 10.2106/jbjs.16.01374. [DOI] [PubMed] [Google Scholar]