Summary
The maturation and functional characteristics of human induced pluripotent stem cell (hiPSC)-cortical neurons has not been fully documented. This study developed a phenotypic model of hiPSC-derived cortical neurons, characterized their maturation process, and investigated its application for disease modeling with the integration of multi-electrode array (MEA) technology. Immunocytochemistry analysis indicated early-stage neurons (day 21) were simultaneously positive for both excitatory (vesicular glutamate transporter 1 [VGlut1]) and inhibitory (GABA) markers, while late-stage cultures (day 40) expressed solely VGlut1, indicating a purely excitatory phenotype without containing glial cells. This maturation process was further validated utilizing patch clamp and MEA analysis. Particularly, induced long-term potentiation (LTP) successfully persisted for 1 h in day 40 cultures, but only achieved LTP in the presence of the GABAA receptor antagonist picrotoxin in day 21 cultures. This system was also applied to epilepsy modeling utilizing bicuculline and its correction utilizing the anti-epileptic drug valproic acid.
Keywords: induced pluripotent stem cells, cortical neurons, long-term potentiation, maturation, epilepsy
Graphical abstract
Highlights
-
•
Characterization of human cortical neuronal differentiation to a mature phenotype
-
•
Microelectrode evaluation of development from a mixed to pure excitatory population
-
•
Utilization of defined culture stage to create an epilepsy model
-
•
Manipulation of immaturity with inhibitors for maintaining long-term potentiation
In this article, Hickman and colleagues used controlled hiPSC-cortical neuron maturation to develop models capable of evaluating functional deficits in long-term potentiation, which is the basis for many neurodegenerative diseases. This model has the potential for patient-specific personalized medicine and can be used for both the investigation of pathogenesis and subsequent preclinical assaying of potential drug treatments.
Introduction
Functionally mature human cortical neuron in vitro models are needed for investigating numerous CNS diseases, including Alzheimer disease (AD), Parkinson disease, Huntington disease, epilepsy, brain injury (Russo et al., 2015), and then for subsequent drug development (Caneus et al., 2020; Carter and Chan, 2012; Martínez-Morales and Liste, 2012). The use of induced pluripotent stem cells (iPSCs) provides phenotypic relevance in vitro because of the physiological accuracy of using human cells as opposed to animal models by eliminating the species gap (Engle et al., 2018). IPSC lines can be established through the reprogramming of adult cells such as fibroblasts and blood cells. IPSCs can be propagated indefinitely and their pluripotency easily lends itself to the differentiation of multiple cell types. This is particularly useful for modeling CNS diseases, because of the characteristic lack of proliferation in primary neurons, the difficulty harvesting and maintaining human neurons in vitro, and the ability to separately differentiate neurons, astrocytes, and microglia. The establishment of iPSC-derived cortical neuronal cultures without glia would enable the further investigation of neuronal behavior where astrocytes and microglia could be used as variables to understand their influence in disease phenotypes.
To date, only few studies have reported successful differentiation of cortical neurons from either embryonic stem cells (ESCs) or iPSCs (Cao et al., 2017; Espuny-Camacho et al., 2013; Odawara et al., 2016a; Qi et al., 2017), and only one demonstrated synapse formation in the in vitro system, but in serum-containing medium (Odawara et al., 2016a) or in astrocyte co-culture (Odawara et al., 2018). Synapse formation and subsequent neuronal interactions, especially induction of long-term potentiation (LTP), is the typical hallmark indicating functional circuit formation as seen previously in slice cultures and animal models (Abraham and Huggett, 1997; Huh et al., 2016; Hwang et al., 2017). The ability of neurons to maintain elevated firing rates for extended time periods following a high-frequency electrical stimulation protocol is correlated with learning and memory (Dragoi et al., 2003). Cortical neural circuits composed of mature cortical neurons could be more effective in modeling CNS functional impairment such as cognitive deficits in AD or dementia, both as pure cultures and with the addition of glia. One report of the induction of LTP in iPSC-derived cortical neuronal culture used co-culture rat primary cortical astrocytes during differentiation, which is undefined and introduces nonhuman components to the process (Frega et al., 2017). A new study has demonstrated LTP in cultures of human iPSC-derived cortical neurons and primary astrocytes but did not extensively characterize the neuronal maturity (Caneus et al., 2020). Undefined animal components during the differentiation procedure would limit the use of the cortical neurons in applications for clinical cell therapy and human cell-based preclinical pharmaceutical studies. This study aimed to develop a protocol for differentiating cortical neurons from human iPSCs in a defined xeno-free, serum-free system, and to establish an in vitro functional human cortical neural model, free from glial cells, and with the maturity level necessary to recapitulate LTP function to model human cortical dysfunction.
In studies of iPSC-derived cortical neurons there is a knowledge gap concerning the level of maturation necessary for the accurate recapitulation of physiological function for the modeling of relevant diseases, which in many cases is determined by the level of their synaptic and functional characteristics. Additionally, in an effort to establish the use of iPSC-cortical neurons to a level of in vivo competency, confirmation of the exact neuronal subtype present in a culture model was necessary. Reports on the classification of iPSC-derived cortical neurons as excitatory or inhibitory has been cursorily examined, such as in Cao et al. (2017), but has not been well explored longitudinally, and neither has the state of this maturation on the ability of these neurons to accurately model CNS diseases free of glial cell contributions. Similarly, modeling epilepsy can be challenging due to its primary symptom of erratic firing of neuronal networks. The randomness of these symptomatic episodes causes difficulty with in vivo studies. The employment of iPSC-cortical neurons to study this neurological disease would enable not only more accurate toxicology and efficacy modeling but also the possibility of patient-specific treatments due to the ability to isogenetically modify iPSCs (Russo et al., 2015).
This study presents a unique protocol for differentiating iPSCs into cortical neurons, as well as a maturation process for obtaining functionally mature cortical neuron circuits, which was free of astrocytes and microglia influence, and was competent for synapse formation and LTP induction. The report describes both the maturation of iPSC-derived cortical neurons and the inhibitory/excitatory subtype classification that was characterized longitudinally by immunocytochemistry and patch clamp electrophysiological as well as multi-electrode array (MEA) analysis. The iPSC-derived cortical neuron on MEA model system was further applied to model aspects of epilepsy and its treatment. This human-based defined cortical neuron-on-a-chip functional system could be a valuable platform for modeling other human CNS diseases and especially for preclinical pharmacological evaluation of therapeutic drugs.
Results
Differentiation of cortical neurons from hiPSCs
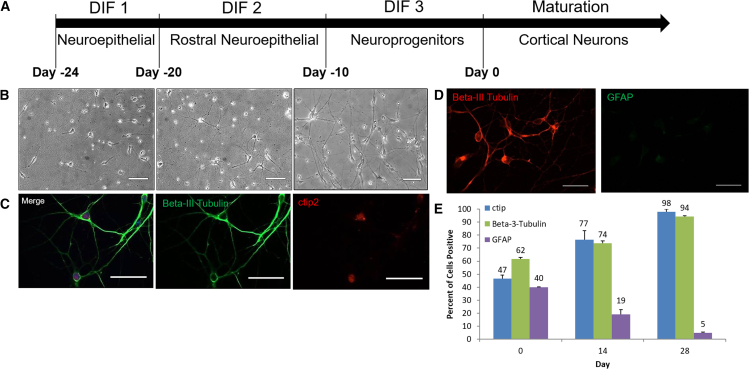
The differentiation of cortical neurons from human iPSCs (hiPSCs) consists of four major steps (Figure 1A). To initiate the differentiation, two small molecule inhibitors of SMAD signaling, LDN193189 and SB431542, were utilized for the induction of early neural lineages (Chambers et al., 2009) (DIF1). Then DKK-1, an antagonist of Wnt/β, which can induce telencephalic specification (Nicoleau et al., 2013), and DMH-1, a highly selective small molecule BMP inhibitor comparable with Noggin that can promote neurogenesis of hiPSCs, were used (Neely et al., 2012) (DIF 2). It was postulated that the combination of these two molecules would facilitate the induction of the rhombomeric neuroepithelia. Next, DKK-1 was withdrawn and cyclopamine was introduced (DIF 3). Cyclopamine, a small molecule SHH inhibitor, has been shown to specify the dorsal cortical fate and enhance the generation of cortical glutamate neurons (Cao et al., 2017). The differentiation was completed by culturing these cells in the presence of trophic factors BDNF, GDNF, cAMP, ascorbic acid, and laminin, which support the survival and maturation of cortical neurons (Bardy et al., 2015; Bonafina et al., 2018; Gorski et al., 2003; Radner et al., 2013).
Figure 1.
Characterization of hiPSC-cortical neuron maturation via phase microscopy, immunocytochemistry, and flow cytometry
(A) Timeline detailing the differentiation process from hiPSCs to cortical neurons.
(B) Phase images depicting cell morphology on day 7 (left), day 14 (middle), and day 40 (right).
(C and D) Immunocytochemistry of cortical neurons stained for β-III tubulin and ctip2 (day 40) in (C) and for β-III tubulin and GFAP (day 28) in (D).
(E) Graph of flow cytometry data on days 0, 14, and 28 illustrating neuron maturation by the expression of layer V cortical neuron marker ctip2, neuronal marker β-III tubulin, and glial cell marker GFAP. Error bar: SEM. N = 3 independent experiments. Scale bar, 50 μm.
Following differentiation, the iPSC-cortical neurons were characterized to confirm the identity and maturation level of the cortical neurons and for the presence of glial cells. Figure 1B indicates the morphological progression of the iPSC-cortical neuronal cultures. On day 7 (left), axonal and dendritic extensions from the neurons were observed, while on days 14 (middle) and 40 (right), robust neuronal process extension was observed, and networking morphology was present. On day 40, immunocytochemistry (ICC) using the layer V cortical neuron marker ctip2 (Gunhanlar et al., 2018), along with the neuronal marker β-III tubulin, to highlight neuronal processes, confirmed cortical neuron identity (Figure 1C). To quantify the percentage of the neuronal component of the differentiated culture, cultures at day 40 of maturation were stained with the neuronal marker β-III tubulin as well as the neuroprogenitor and glial cell marker GFAP (Ahmed et al., 2012). The results indicated that the neuronal percentage in the culture was greater than 95% (Figure 1D). Longitudinal expression of these markers together with the ctip2 marker was further quantified using flow cytometry, revealing that the expression of the markers ctip2 and β-III tubulin on days 0, 14, and 28 increased over time, being >90% at the last time point, in agreement with the ICC results, while the expression of GFAP decreased over time to almost undetectable levels (Figure 1E). These analyses indicated that this differentiation protocol generated a close-to-pure cortical neuronal culture if sufficient maturation time was utilized that enables the study of neuronal-only characteristics.
Characterization of terminally differentiated hiPSC-cortical neurons via immunocytochemistry
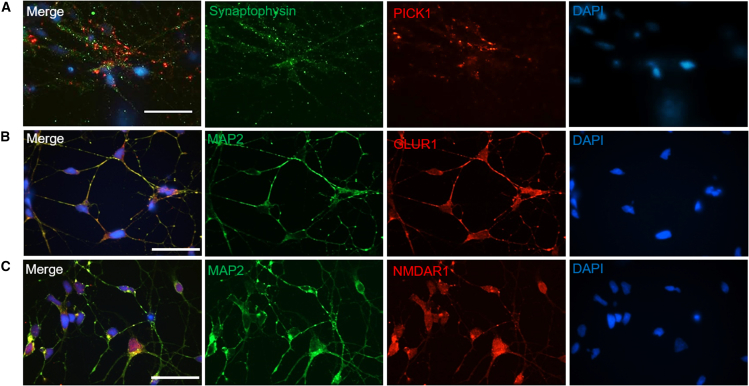
Following the successful differentiation to cortical neurons, immunocytochemistry was performed to further characterize the synapse formation by staining with the synaptic terminal protein synaptophysin and post-synaptic markers including GluR1 (a subunit of the AMPA glutamatergic receptor), the NMDA receptor, and the receptor-associated molecule PICK1 (protein interacting with C kinase 1). Day 40 cultures were utilized for this ICC analysis (Figure 2). Synaptic connectivity was observed via the co-localization of the pre-synaptic marker synaptophysin with the post-synaptic marker PICK1 (Figure 2A). Additionally, the expression of NMDA and AMPA receptors was demonstrated using MAP2 co-stained with the markers NMDAR1 and GLUR1, respectively (Figures 2B and 2C). The existence of NMDA and AMPA receptors on the neuronal cell bodies was also indicative of terminal differentiation and suggested functional and physiological maturity at this time point.
Figure 2.
Characterization of mature hiPSC-cortical neurons (day 40) via immunocytochemistry
(A–C) Immunocytochemistry of day 40 cortical neurons stained for pre-synaptic marker synaptophysin and post-synaptic marker PICK1 (A), for neuronal microtubule marker MAP2 and AMPA receptor marker GLUR1 (B), and for neuronal microtubule marker MAP2 and NMDA receptor marker NMDAR1 (C). Scale bar, 50 μm.
Characterization of hiPSC-cortical neurons via whole-cell patch clamp electrophysiology
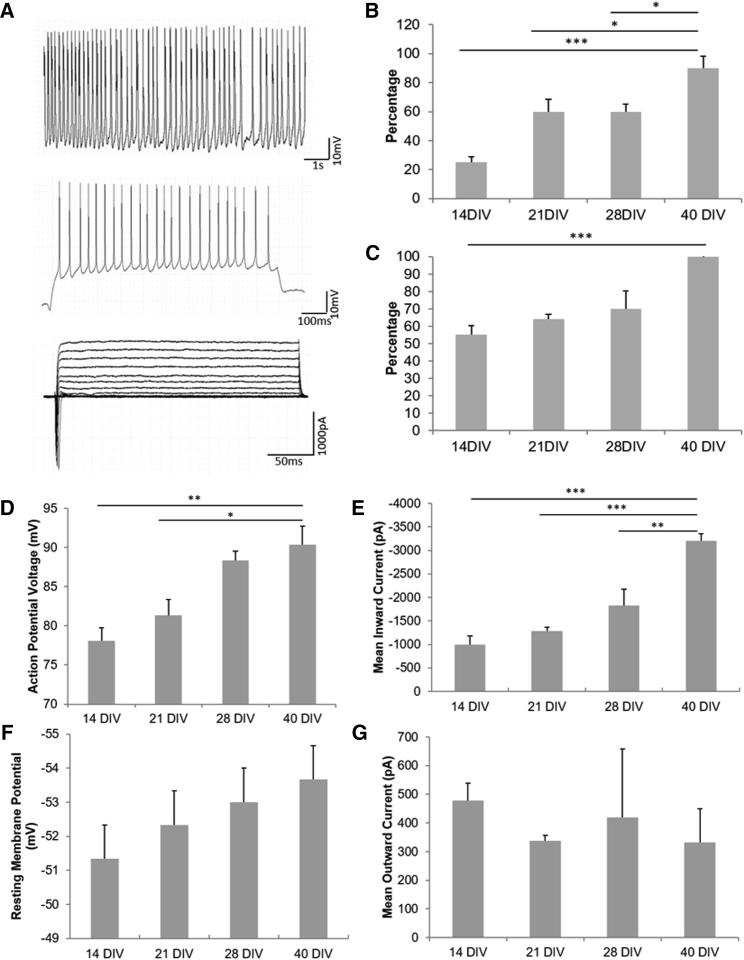
Whole-cell patch clamp was used to determine the electrical properties of the iPSC-cortical neurons at days 14, 21, 28, and 40. Specifically, the cells were examined for ionic currents, induced repetitive firing, and spontaneous firing. At day 40, the cortical neurons expressed voltage-gated Na+ and K+ channels as indicated by inward Na+ currents and outward K+ currents (Figure 3A). As the culture progressed, there was an increase in the percentage of cells showing spontaneous and repetitive firing, indicative of increasing cortical neuron excitability and functional maturation in the culture over time (Figures 3B and 3C). Additionally, other functional parameters that increased significantly over time included the action potential amplitude (Figure 3D) and the Na+ inward currents (Figure 3F), while any change of the K+ current over time is not obvious (Figure 3G), suggesting the increase of action potential amplitude can be attributed mostly to the increase in Na+ current rather than the change in the K+ current (Ghatak et al., 2019; Prè et al., 2014). Analysis of the resting membrane potential demonstrated a progressive hyperpolarization as the culture matured (Figure 3E).
Figure 3.
Electrophysiological characterization of hiPSC-cortical neurons via whole-cell patch clamp
(A) Representative traces of spontaneous firing (top), depolarization-induced repetitive firing (middle), and Na+ and K+ currents (bottom) recordings from day 40 neurons.
(B and C) Percentage of cells experiencing spontaneous firing (B) and repetitive firing (C), indicating maximal activity at day 40.
(D and E) Quantification of action potential voltage (D) and mean inward Na+ current (E), indicating maximum level at day 40.
(F and G) Quantification of resting membrane potential (F) and mean K+ outward current (G). Statistical analysis was performed using one-way ANOVA followed by Tukey's test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Error bar: SEM. N = 9 cells total from three independent experiments.
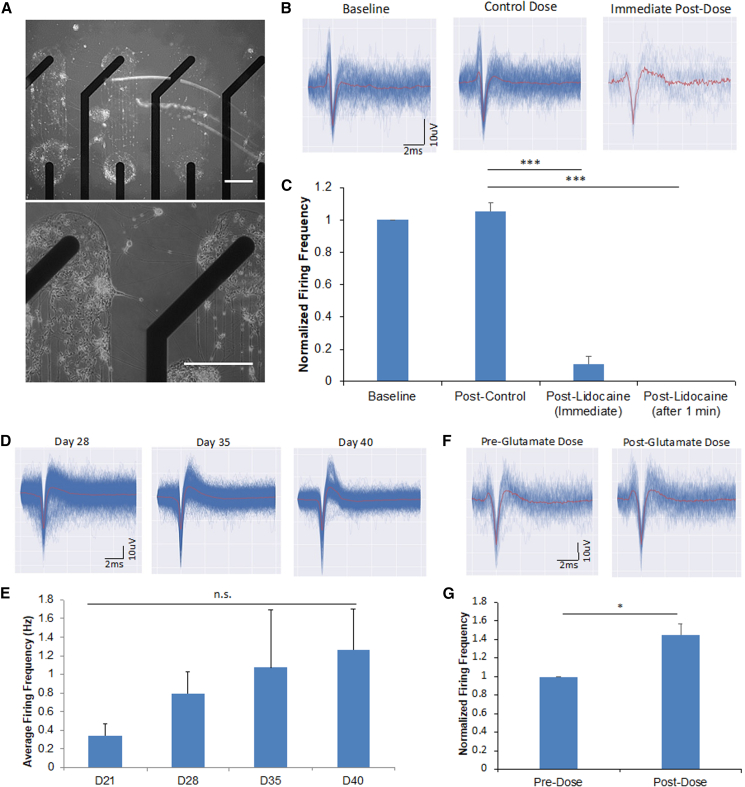
Recording of neural activity on MEAs
Microelectrode array (MEA) recording technology has been a powerful tool to monitor populational neural activity non-invasively and to analyze neurocircuit activity. To adapt these electrically active cortical neurons into an MEA system, the iPSC-cortical neurons were plated on surface patterned MEAs. Phase images in Figure 4A demonstrate an example of the patterned distribution of cortical neurons on these MEAs. Neurons were observed to successfully form patterns on electrodes, with neuronal bodies localized on the electrode surface, and axon bundles forming between the adjacent electrodes. Recording of spontaneous neural activity gave rise to representative traces as in Figure 4B. To confirm these traces were generated by neural activity, lidocaine, a Na+ channel inhibitor and well-characterized local anesthetic, was used to block activity. Addition of lidocaine quickly reduced, and subsequently eliminated, neuronal signaling within approximately 2 min (Figures 4B and 4C).
Figure 4.
MEA neuronal patterning and signal validation
(A) Phase images showing neuronal patterning on electrodes at day 40.
(B) Representative waveforms of spontaneous activity on a single electrode on day 75 at baseline, immediately following vehicle control, and with 1 mM lidocaine.
(C) Graphical representation of neuronal firing frequency during the lidocaine dosing experiment outlined in (B).
(D) Representative waveforms of spontaneous activity on a single electrode on days 28, 35, and 40 indicating increasing maturation.
(E) Quantification of MEA recordings indicating neuronal firing frequency during cortical neuron maturation over 40 days.
(F) Representative waveforms of spontaneous activity on a single electrode on day 75 during pre (left) and post dosage (right) with 100 μM glutamate.
(G) Graph representation of neuronal firing frequencies during the glutamate dosing experiment outlined in (F). Statistical analysis was performed using one-way ANOVA followed by Tukey's test or Student's t test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Error bar: SEM. Scale bar, 50 μm.
This MEA system was then utilized to investigate the progressive changes in neural activity by measuring spontaneous activity on days 21, 28, 35, and 40. The waveforms measured displayed lengthening of amplitude, especially in the positive direction to emphasize the biphasic properties as well as the sharpening of the waveform average over time (Figure 4D). Quantification of spontaneous activity indicated an increase of neuronal activity with increasing culture time, consistent with the patch clamp results, although it was non-significant (Figure 4F). Neuronal activity remained consistent past day 40 of maturation, and, as a result, it enabled experimentation on mature neurons from older cultures. Subsequent MEA experiments were performed based on the presence of NMDA and AMPA receptors found in previous ICC experiments, by the addition of the chemical stimulator glutamate to increase neural activity. As in Figures 4E and 4G, addition of glutamate increased the frequency of neural activity within the time window of 35–40 days in vitro (DIV). The silencing and enhancement of neural activity by lidocaine and glutamate, respectively, confirmed the validity of the MEA recording system as well as establishing the expected functionality of the terminally differentiated cortical neurons (Onizuka et al., 2004; Putrenko and Schwarz, 2011).
Formation of synaptic circuits and induction of LTP on MEAs
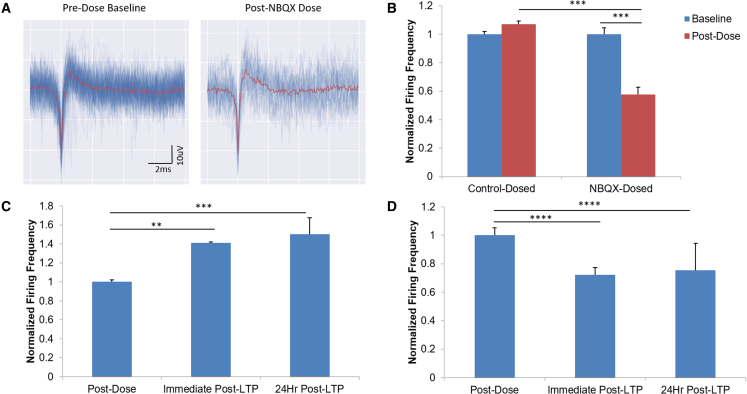
To evaluate neural circuit activity of the patterned cortical neurons on the MEAs, NBQX, an AMPA receptor blocker (Libbey et al., 2016), was applied to the culture and the activity was analyzed. Figure 5A depicts representative waveform traces from baseline recordings (left) and then following dosage with NBQX (right). As in Figure 5B, addition of NBQX induced significant reduction of recorded activity, while there was no significant change in vehicle control. This result revealed that a portion of activity recorded is from inputs through neuronal synapses, or there were functional synapses formed in the iPSC-cortical neural culture.
Figure 5.
Multi-electrode array recordings confirming synaptic connectivity via dosage with NBQX and LTP induction
(A) Representative waveforms of spontaneous activity on a single electrode on day 75 during pre (left) and post dosage (right) with 25 μM NBQX.
(B) Graphical representation of neuronal firing rates normalized to baseline activity before and after control 0.1% DMSO or 25 μM NBQX dose.
(C) Graph of normalized firing rates of control dose of 0.1% DMSO and following LTP stimulation immediately and 1 h after dosage, normalized to post-dose, pre-LTP baseline.
(D) Graph of normalized firing rates for 25 μM NBQX dosed and following LTP stimulation immediately and 1 h after dosage, normalized to post-dose, pre-LTP baseline. Statistical analysis was performed using a Kruskal-Wallis test followed by Dunn's multiple comparisons test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Error bar: SEM. N = 10 electrodes from three individual experiments.
Since LTP formation is an important property of cortical activity as well as a key mechanism for neural plasticity and a strong cellular basis for cognition, the capability of inducing LTP in these glial cell free cultures was investigated. In order to induce LTP, a paradigm of high-frequency stimulation (HFS) was applied to all the electrodes and the spontaneous activity of these neurons was quantified before, right after, and 1 h post HFS (Caneus et al., 2020). As in Figure 5C, there was a significant increase in neural activity after the HFS, and this increase was maintained for at least 1 h. This result indicated LTP formation in this iPSC-neural culture, as previously reported in other neuronal cultures (Caneus et al., 2020; Odawara et al., 2016a). As validation, similar experiments in the presence of the AMPA receptor antagonist NBQX were conducted in which glutamatergic synaptic activity was blocked and no LTP could be induced. As expected, HFS did not induce any increase in activity (Figure 5D), but rather caused the same decrease following the NBQX dosage observed in Figure 5B, confirming that the increase in neuronal activity following HFS was synapse based.
Characterization of excitatory and inhibitory features in mature and immature iPSC-cortical neurons
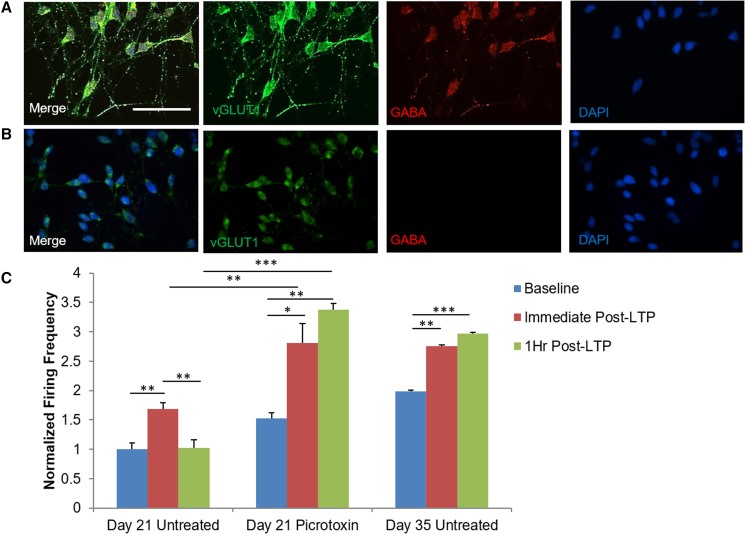
To characterize the identity of these iPSC-cortical neurons as excitatory or inhibitory (Figure 6), the vesicular glutamate transporter 1 (VGlut1) was used as a marker for the glutamatergic neurons while GABA was used to distinguish the GABAergic neuronal phenotype. Immunocytochemical staining revealed that, on day 21 (Figure 6A), neurons were positive for both the excitatory neuron marker VGlut1 and the inhibitory neuron marker GABA. Subsequently, at day 40 (Figure 6B), the neurons only stained positive for VGlut1, indicating full maturation to an excitatory phenotype as the culture progressed. This is consistent with the increased spontaneous activity measured by patch clamp and MEA recordings in the latter days of maturation (days 35–40). It has been previously reported that GABA is the predominantly expressed neurotransmitter in the early stages of brain development (Maric et al., 2001). Consequently, GABA is imperative for neuroprogenitor proliferation and migration in the early stages of development (Wang and Kriegstein, 2009), with GABA being cited to have excitatory properties of neurotransmission at the immature stage of development before the shift to the utilization of glutamate as the excitatory neurotransmitter (Le Magueresse and Monyer, 2013). This observation implied a reduced capability of inducing LTP in younger cultures. This hypothesis was then tested in culture at day 21 compared with that at day 35. As in Figure 6C, with additional waveforms depicted in Figure S1, LTP could be induced in day 21 culture, but did not persist after 1 h. However, 1-h LTP persistence was achieved with the addition of picrotoxin to block inhibitory signals. Neurons past day 35 in culture were characterized as purely glutamatergic in Figure 6, LTP was seen to persist at 1 h following LTP induction without inhibiting the GABAergic signaling. This result reproduced the previous findings that modulating the percentage of inhibitory signaling in cortical neuron circuits is an important parameter for successful induction of persistent LTP (Steele and Mauk, 1999).
Figure 6.
Confirmation of excitatory and inhibitory character in mature and immature iPSC-cortical neurons
(A and B) Immunocytochemistry of cortical neurons stained for VGlut1 (green) and GABA (red) indicating the neurons expressed both excitatory and inhibitory markers at day 21 (A), but were typically glutamatergic at D 40 (B).
(C) Quantitative MEA analysis of LTP induction for day 21 and day 35 neurons. LTP induction in day 21 neurons did not persist after 1 h when dosed with vehicle control but did so when dosed with 100 μM picrotoxin prior to LTP induction, while it was similar to those observed in innate day 35 neurons without any picrotoxin addition. All the data were normalized to the day 21 undosed baseline. Statistical analysis was performed using one-way ANOVA followed by Tukey's test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Error bar: SEM. Scale bar, 50 μm, N = 10 electrodes from three individual experiments.
The induction and quieting of epileptiform activity in immature cortical neurons
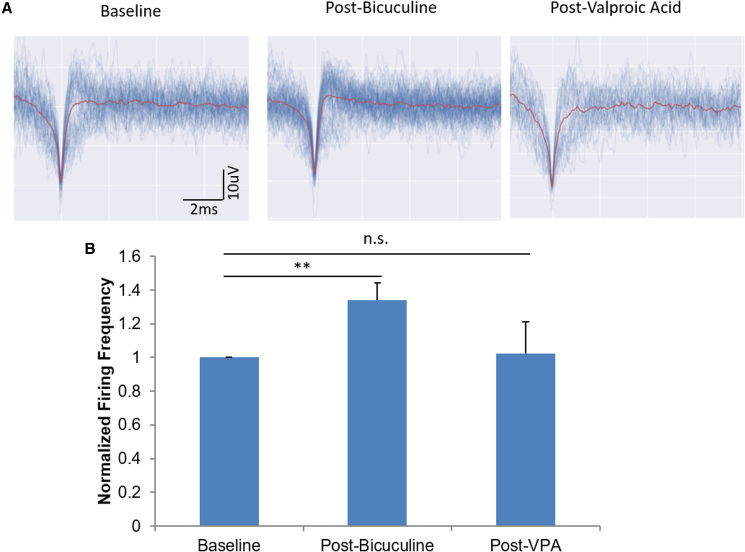
The above analysis indicated a significant amount of GABAergic signaling in cultures younger than day 40, as well as the efficiency of modulating the neurocircuit activity by regulating the GABAergic pathway. This iPSC-cortical neuron culture at earlier days thus potentially could provide an appropriate platform for modeling epilepsy or seizures, in which one or more parts of the brain has a burst of abnormal electrical signals that interrupt normal brain activity. Additionally, the usage of mixed inhibitory and excitatory neuronal co-cultures for CNS diseases, such as epilepsy, has previously been reported in the literature (Grainger et al., 2018; Nadadhur et al., 2017). To explore this potential application, neural activity was modulated chemically to induce and quiet epileptiform activity in immature, day 21 cortical neurons (Figure 7). Neural activity was enhanced by addition of 100 μM bicuculine through blocking of GABAergic signaling, and subsequently quieted following the addition of the epilepsy suppression drug valproic acid (1 mM). Representative waveforms are shown in Figure 7A depicting baseline activity, the increase in firing following the addition of bicuculine, and subsequent elimination of activity following dosage with valproic acid. Figure 7B is a graphical representation of the quantified firing frequencies normalized to the baseline activity, further confirming these results. This result demonstrated the applicability of this system for modeling epilepsy.
Figure 7.
The induction and quieting of epileptiform activity via dosage with bicuculine and valproic acid in immature (21 DIV) cortical neurons
(A) Waveform representations of baseline activity (left), 100 μM bicuculline-dosed (middle), and 1 mM valproic acid dosed (right) systems.
(B) Graphical representation of the immature firing rates normalized to baseline. Statistical analysis was performed using one-way ANOVA followed by Tukey's test; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Error bar: SEM. N = 10 electrodes from three individual experiments.
Discussion
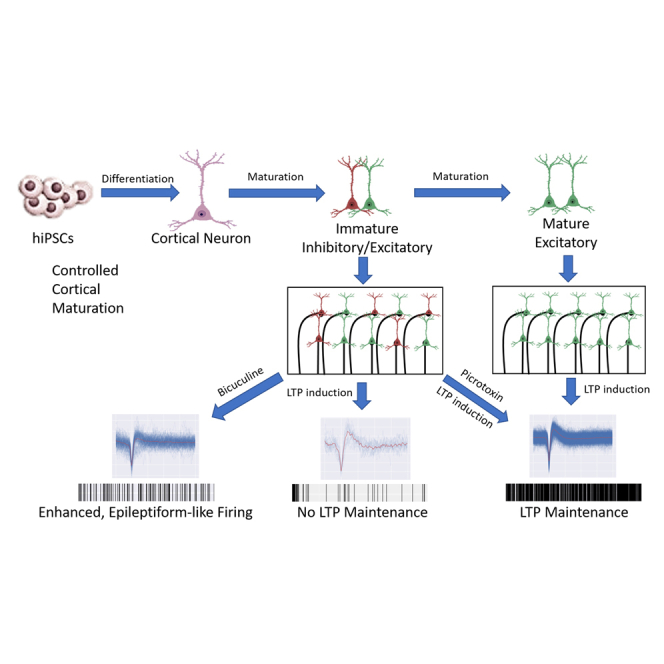
This study aimed to establish a functional model of mature hiPSC-cortical neurons in a serum-free environment that could be used as a platform for in vitro preclinical drug testing. By integrating MEA technology, this system demonstrated the capability for LTP induction and neural disease modeling for epilepsy. A primary application would be that this system can be tailored to evaluate iPSC induced, patient-specific phenotypes for treatments for personalized medicine applications, as we show the ability to control the level of maturation and manipulate the phenotype in a controlled fashion. The neuronal culture was characterized using immunocytochemistry, flow cytometry, and whole-cell patch clamp as well as on MEAs in an organ-on-a-chip system to determine functional maturity as the culture progressed to terminal differentiation that was free of glial cells. Neuronal activity on MEAs demonstrated the capability for LTP induction, as well as being conducive to both chemical and electrical modulation, for the modeling of neural diseases and drug testing. Astrocytes and microglia can be added selectively in the cultures in future experiments to understand their influence on neuronal behavior and disease phenotypes.
This study demonstrated the longitudinal maturation process of iPSC-cortical neurons in vitro through the characterization of excitatory/inhibitory neuronal components, electrophysiological activity of individual neurons, and neuron circuits. Immunocytochemistry and flow cytometry characterization during the maturation process indicated the gradual increase of the percentage of cortical neurons (by ctip2 and β-III tubulin) and the decrease of neuroprogenitors (by GFAP). By day 28, more than 90% of the cells were cortical neurons. Based on patch clamp analysis, the percentage of neurons firing spontaneously and those firing repetitive action potentials under depolarization increased over time, reaching ≥90% by day 40 (Figure 3). Correspondently, the firing frequencies recorded by MEA analysis demonstrated gradual increases during a similar time period in culture. Our success in the differentiation of the iPSCs into a high level of maturity and function without the need for serum, astrocytes, or conditioned medium is due to our long history of utilizing serum-free, defined systems to culture pure populations of cells, including hepatocytes (Oleaga et al., 2021), cardiac cells (Das et al., 2004; Stancescu et al., 2015), motoneurons (Guo et al., 2010), skeletal muscle (Badu-Mensah et al., 2020), as well as combination of the cells in multi-organ systems for up to 28 days (Oleaga et al., 2019).
LTP has been used in research as a quantitative measurement of cognitive functions related to learning and memory (Nicoll, 2017). It is defined as the ability of neurons to retain sustained, elevated firing rates following electrical or chemical stimulation (Caneus et al., 2020; Hata et al., 2020; Kumar, 2011). In vivo, LTP has been shown in mouse models as a measurement of cognition with respect to AD (Huh et al., 2016). Specifically, they have utilized LTP absence and deficits as the main readout following dosage with amyloid-β42, showing toxicity from plaque accumulation (Dragoi et al., 2003; Mango et al., 2019). The induction of LTP on MEAs has been shown previously in rat cortical neuron models (Narayanan and Johnston, 2007; Park et al., 2015) as well as hiPSC-derived cortical neurons with astrocytes and in serum-containing medium (Loscher, 2011; Odawara et al., 2016b). This study demonstrated the ability of iPSC-derived cortical neurons cultured on MEAs to exhibit LTP without glia in a serum-free medium. The ability of the culture to display LTP allows for modeling of CNS diseases related to learning and cognitive dysfunction, which are essential functional markers in clinical settings for neurodegenerative diseases such as AD (Koch et al., 2012), epilepsy (Zhou et al., 2007), and autism spectrum disorder (Chung et al., 2012).
The iPSC-cortical neuron-MEA system provides an ideal, non-invasive, and less labor-intensive approach than patch clamp electrophysiology to monitor neural activity, not only verifying the chronological maturation of the neuronal activity of the culture but also demonstrating controlled electrical and chemical modulation of this activity. Lidocaine, a Na+ channel blocker and local anesthetic (Onizuka et al., 2004), abolishes neuronal activity, while glutamate induced increased neuronal activity. Additionally, NBQX, an AMPA receptor antagonist, was used to investigate the neural circuit properties. The significant decrease in activity following dosage with NBQX (Figure 5B) suggests that approximately 50% of the recorded spontaneous activity was from synaptic inputs of the AMPA receptors. Furthermore, systems that were dosed with NBQX (Figure 5D) showed a significant decrease in activity following LTP induction both immediately and 24 h after HFS (Figure 5C). Therefore, it can be inferred that the persistent LTP observed in our cultures is based on the synaptic networks formed between the cortical neurons.
One important issue of in vitro cultures is controlling and defining the status of iPSC-cortical neurons as excitatory or inhibitory, which ultimately influences the ability of an individual neuron to fire action potentials and its capability of generating the cascade of neuronal firing seen in LTP models (Chapman et al., 1998; Reyes-Garcia et al., 2018). In alignment with the dual-transmitter phenomenon observed in multiple types of neurons during early stages of brain development (Vaaga et al., 2014), immunocytochemical analysis revealed that cortical neurons younger than day 40 co-stained for both the inhibitory and excitatory markers GABA and VGlut1, respectively, while those older than day 40 displayed almost a pure VGlut1 composition. As a result, it was expected that overall neuronal activity in the early cultures would be low because of the presence of GABAergic neurons in the culture, while the more functionally mature cultures would be excitatory. This hypothesis was further reinforced by weekly whole-cell patch clamp and spontaneous MEA data, indicating that neural activity did not reach its peak until day 40.
The GABAA receptor inhibitor picrotoxin was used to manipulate the immature cortical neurons for LTP induction and maintenance due to its ability to block inhibitory signal dispersion within the synapses (Lam et al., 2017; Xu et al., 2016). It was discovered that, while the exclusively glutamatergic culture at day 40 could retain LTP induction, the mixed population of immature neurons could not maintain the elevated firing rates after 1 h but could if the GABAergic inhibition was removed by picrotoxin. This study highlights that controlling the maturation level and inhibitory component of the culture influences its synaptic capabilities, which is important for in vitro LTP modeling.
Epilepsy is a chronic condition and occurs most commonly during childhood, and sometimes resolves of its own accord at the time of puberty. Our cortical neuron cultures younger than day 40 had a significant amount of GABAergic signaling, and blocking this inhibitory component was shown to dramatically increase neural activity. It is hard to conclude from this study whether the high occurrence of epilepsy in young populations is linked to a dysfunctional inhibitory signaling in the CNS, but the immature iPSC-cortical neuron culture could provide a valuable platform for simulating epilepsy by altering the inhibitory mechanism. Bicuculline has been previously observed in vitro to induce erratic firing deemed epileptiform activity, while valproic acid is used as a common anti-epileptic (Kumamaru et al., 2014; Nowak et al., 1982). Currently, the animal models of epilepsy are induced using the compounds kainic acid (Odawara et al., 2016b; Sharma et al., 2007), pilocarpine (Sharma et al., 2007), and bicuculline (Odawara et al., 2016b). While there has been a recent shift toward modeling epilepsy in vitro using human cells to compensate for the lack of species competency in these animal models, the optimization of the neuronal culture maturation and its capability in reproducing physiological and pathological properties is still a work in progress (Campos et al., 2018; Grainger et al., 2018). To develop an epilepsy model using these iPSC-derived cortical neurons, the effects of bicuculline were evaluated on immature cortical neuron cultures (Figure 7), because its working mechanism is to inhibit the GABAergic pathway by blocking GABAA receptors to regulate the balance of excitatory and inhibitory signals (Johnston, 2013). Subsequently, valproic acid enhances GABA transmission by inhibiting the degradation of GABA (Davies, 1995). It was observed that bicuculline was sufficient to induce an epileptic-like increase in activity in neuronal cultures and that the activity could subsequently be abolished following dosage with valproic acid.
The inherent co-expression of excitatory and inhibitory neurotransmission is potentially adaptable to other models that rely on this interplay, such as autism spectrum disorder (Lunden et al., 2019), alcoholism (Lieberman et al., 2012), depression, and anxiety. These models could be adapted to our system due to its ability to measure electrical activity and study both short- and long-term effects of compounds.
In summary, the iPSC-cortical neuron model exhibited the inhibitory to excitatory conversion during the maturation process. By integrating with MEA technology, it demonstrated functional neurocircuit formation without the need for added glial cells, especially the capacity to recapitulate LTP induction, which is an essential mechanism for neural plasticity and cognitive capability in neurodegenerative diseases such as dementia. It also reproduced the phenotype for epilepsy. These non-invasive functional human diseased systems would provide more clinically translatable platforms for drug testing. They are also potentially applicable to investigate drug interactions and off-target toxicity when adapted into a multi-organ-on-a-chip system, for either acute or chronic effects.
Experimental procedures
hiPSC-cortical neuron differentiation
The differentiation of cortical neurons from hiPSCs consisted of a timeline of three stages, starting out with the use of small molecules, and ending with an array of neuron-specific factors. hiPSCs (Coriell ND41865) were first grown to 90% confluence in mTESR1 culture medium on a surface coated with Corning Matrigel matrix. To induce the differentiation, cells received a full medium change and were then fed every other day with differentiation medium I, differentiation medium II, and differentiation medium III, according to the differentiation timeline featured in Figure 1A. Media formulations and methods for cortical neuron plating and maintenance, immunocytochemistry, flow cytometry, and patch clamp analysis are detailed in supplemental experimental procedures.
Multi-electrode array recordings and LTP induction
Cells were cultured on custom TiN electrode chips containing 10 electrodes, each 80 μm diameter, spaced at between 1,000 and 1,500 μm. The chips were coated with diethylenetriamine (DETA) (Varghese et al., 2010) followed by a poly-L-ornithine (PLO) and laminin protein adsorption coating. Cells were seeded at a density of 500 cells/mm2 and maintained for 28 days before neuronal activity could be recorded using a multi-channel systems (MCS) rig. Spontaneous activity was recorded for 5 min prior to the induction of LTP via electrical stimulation using an HFS protocol. All recordings were taken for a duration of 5 min unless otherwise specified. LTP induction protocol and MEA data analysis are detailed in supplemental experimental procedures.
Statistical analysis
Statistical analysis was performed on data collected over at least three independent experiments, with specific statistical methodology indicated in the manuscript. These include one-way ANOVA followed by Fisher's least significant difference (LSD) and Student's t test, depending on the experimental design for each dataset.
Author contributions
Conceptualization, K.A., X.G., and J.H.; data curation, K.A., X.G., J.R., C.L., N.A., and J.C.; formal analysis, K.A., X.G., J.R., C.L., M.J., and N.N.:; funding acquisition, J.H.; investigation and methodology, K.A., X.G., and J.R.; project administration, X.G. and J.H.; supervision, X.G. and J.H.; validation, X.G., D.M., and J.H.; visualization, K.A., X.G., D.M., and J.H.; roles/writing – original draft, K.A. and X.G.; writing – review & editing, K.A., X.G., D.M., and J.H.
Conflict of interests
The authors confirm that competing financial interests exist but there has been no financial support for this research that could have influenced its outcome. The only author with competing interest is J.H., who has ownership interest and is Chief Scientist and member of the Board of Directors in a company that may benefit financially as a result of the outcomes of the research or work reported in this publication.
Acknowledgments
Funding for this research was provided by the National Institutes of Health, National Center for Advancing Translational Sciences award number R44TR001326, and the National Institute on Aging award number R44AG058330.
Published: December 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.11.009.
Supporting citations
The following references appear in the Supplemental information: Edwards et al., 2013; Gonzalez et al., 2019; Obien et al., 2015; Patel et al., 2020.
Supplemental information
information
References
- Abraham W.C., Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. doi: 10.1002/(SICI)1098-1063(1997)7:2<137::AID-HIPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ahmed A.I., Shtaya A.B., Zaben M.J., Owens E.V., Kiecker C., Gray W.P. Endogenous GFAP-positive neural stem/progenitor cells in the postnatal mouse cortex are activated following traumatic brain injury. J. Neurotrauma. 2012;29:828–842. doi: 10.1089/neu.2011.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badu-Mensah A., Guo X., McAleer C., Rumsey J., Hickman J.J. Skeletal muscle model derived from familial ALS patient iPSCs recapitulates hallmarks of disease progression. Sci. Rep. 2020;10:14302. doi: 10.1038/s41598-020-70510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy C., van den Hurk M., Eames T., Marchand C., Hernandez R.V., Kellogg M., Gorris M., Galet B., Palomares V., Brown J., et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl. Acad. Sci. U S A. 2015;112:E2725. doi: 10.1073/pnas.1504393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafina A., Fontanet P.A., Paratcha G., Ledda F. GDNF/GFRα1 complex abrogates self-renewing activity of cortical neural precursors inducing their differentiation. Stem Cell Reports. 2018;10:1000–1015. doi: 10.1016/j.stemcr.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos G., Fortuna A., Falcão A., Alves G. In vitro and in vivo experimental models employed in the discovery and development of antiepileptic drugs for pharmacoresistant epilepsy. Epilepsy Res. 2018;146:63–86. doi: 10.1016/j.eplepsyres.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Caneus J., Akanda N., Rumsey J.W., Guo X., Jackson M., Long C.J., Sommerhage F., Georgieva S., Kanaan N., Morgan D., Hickman J.J. A human induced pluripotent stem cell-derived cortical neuron human-on-a chip system to study Aβ42 and tau-induced pathophysiological effects on long-term potentiation. Alzheimers Dement. (N Y) 2020;6:e12029. doi: 10.1002/trc2.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S.-Y., Hu Y., Chen C., Yuan F., Xu M., Li Q., Fang K.-H., Chen Y., Liu Y. Enhanced derivation of human pluripotent stem cell-derived cortical glutamatergic neurons by a small molecule. Sci. Rep. 2017;7:3282. doi: 10.1038/s41598-017-03519-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.L., Chan A.W.S. Pluripotent stem cells models for Huntington's disease: prospects and challenges. J. Genet. Genomics. 2012;39:253–259. doi: 10.1016/j.jgg.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C.A., Perez Y., Lacaille J.C. Effects of GABA(A) inhibition on the expression of long-term potentiation in CA1 pyramidal cells are dependent on tetanization parameters. Hippocampus. 1998;8:289–298. doi: 10.1002/(SICI)1098-1063(1998)8:3<289::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Chung L., Bey A.L., Jiang Y.-H. Synaptic plasticity in mouse models of autism spectrum disorders. Korean J. Physiol. Pharmacol. 2012;16:369–378. doi: 10.4196/kjpp.2012.16.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Molnar P., Gregory C., Riedel L., Hickman J.J. Long-term culture of embryonic rat cardiomyocytes on an organosilane surface in a serum free medium. Biomaterials. 2004;25:5643–5647. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Davies J.A. Mechanisms of action of antiepileptic drugs. Seizure. 1995;4:267–271. doi: 10.1016/s1059-1311(95)80003-4. [DOI] [PubMed] [Google Scholar]
- Dragoi G., Harris K.D., Buzsáki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Edwards D., Stancescu M., Molnar P., Hickman J.J. Two cell circuits of oriented adult hippocampal neurons on self-assembled monolayers for use in the study of neuronal communication in a defined system. ACS Chem. Neurosci. 2013;4:1174–1182. doi: 10.1021/cn300206k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle S.J., Blaha L., Kleiman R.J. Best practices for translational disease modeling using human iPSC-derived neurons. Neuron. 2018;100:783–797. doi: 10.1016/j.neuron.2018.10.033. [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I., Michelsen Kimmo A., Gall D., Linaro D., Hasche A., Bonnefont J., Bali C., Orduz D., Bilheu A., Herpoel A., et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Frega M., van Gestel S.H.C., Linda K., van der Raadt J., Keller J., Van Rhijn J.R., Schubert D., Albers C.A., Nadif Kasri N. Rapid neuronal differentiation of induced pluripotent stem cells for measuring network activity on micro-electrode arrays. JoVE. 2017;119:54900. doi: 10.3791/54900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak S., Dolatabadi N., Trudler D., Zhang X.T., Wu Y., Mohata M., Ambasudhan R., Talantova M., Lipton S.A. Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls. eLife. 2019;8:e50333. doi: 10.7554/eLife.50333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M., Guo X., Lin M., Stancescu M., Molnar P., Spradling S., Hickman J.J. Polarity induced in human stem cell derived motoneurons on patterned self-assembled monolayers. ACS Chem. Neurosci. 2019;10:2756–2764. doi: 10.1021/acschemneuro.8b00682. [DOI] [PubMed] [Google Scholar]
- Gorski J.A., Zeiler S.R., Tamowski S., Jones K.R. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J. Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger A.I., King M.C., Nagel D.A., Parri H.R., Coleman M.D., Hill E.J. In vitro models for seizure-liability testing using induced pluripotent stem cells. Front. Neurosci. 2018;12:590. doi: 10.3389/fnins.2018.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhanlar N., Shpak G., van der Kroeg M., Gouty-Colomer L.A., Munshi S.T., Lendemeijer B., Ghazvini M., Dupont C., Hoogendijk W.J.G., Gribnau J., et al. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol. Psychiatry. 2018;23:1336–1344. doi: 10.1038/mp.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.F., Johe K., Molnar P., Davis H., Hickman J.J. Characterization of a human fetal spinal cord stem cell line NSI-566RSC and its induction to functional motoneurons. J. Tissue Eng. Regen. Med. 2010;4:181–193. doi: 10.1002/term.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K., Araki O., Yokoi O., Kusakabe T., Yamamoto Y., Ito S., Nikuni T. Multicoding in neural information transfer suggested by mathematical analysis of the frequency-dependent synaptic plasticity in vivo. Sci. Rep. 2020;10:13974. doi: 10.1038/s41598-020-70876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh S., Baek S.-J., Lee K.-H., Whitcomb D.J., Jo J., Choi S.-M., Kim D.H., Park M.-S., Lee K.H., Kim B.C. The reemergence of long-term potentiation in aged Alzheimer's disease mouse model. Sci. Rep. 2016;6:29152. doi: 10.1038/srep29152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K.-D., Bak M.S., Kim S.J., Rhee S., Lee Y.-S. Restoring synaptic plasticity and memory in mouse models of Alzheimer’s disease by PKR inhibition. Mol. Brain. 2017;10:57. doi: 10.1186/s13041-017-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G.A.R. Advantages of an antagonist: bicuculline and other GABA antagonists. Br. J. Pharmacol. 2013;169:328–336. doi: 10.1111/bph.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Di Lorenzo F., Bonnì S., Ponzo V., Caltagirone C., Martorana A. Impaired LTP- but not LTD-like cortical plasticity in Alzheimer's disease patients. J. Alzheimer's Dis. 2012;31:593–599. doi: 10.3233/JAD-2012-120532. [DOI] [PubMed] [Google Scholar]
- Kumamaru E., Egashira Y., Takenaka R., Takamori S. Valproic acid selectively suppresses the formation of inhibitory synapses in cultured cortical neurons. Neurosci. Lett. 2014;569:142–147. doi: 10.1016/j.neulet.2014.03.066. [DOI] [PubMed] [Google Scholar]
- Kumar A. Long-term potentiation at CA3–CA1 hippocampal synapses with special emphasis on aging, disease, and stress. Front. Aging Neurosci. 2011;3:7. doi: 10.3389/fnagi.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam R.S., Töpfer F.M., Wood P.G., Busskamp V., Bamberg E. Functional maturation of human stem cell-derived neurons in long-term cultures. PLoS One. 2017;12:e0169506. doi: 10.1371/journal.pone.0169506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C., Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Libbey J.E., Hanak T.J., Doty D.J., Wilcox K.S., Fujinami R.S. NBQX, a highly selective competitive antagonist of AMPA and KA ionotropic glutamate receptors, increases seizures and mortality following picornavirus infection. Exp. Neurol. 2016;280:89–96. doi: 10.1016/j.expneurol.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R., Levine E.S., Kranzler H.R., Abreu C., Covault J. Pilot study of iPS-derived neural cells to examine biologic effects of alcohol on human neurons in vitro. Alcohol. Clin. Exp. Res. 2012;36:1678–1687. doi: 10.1111/j.1530-0277.2012.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Lunden J.W., Durens M., Phillips A.W., Nestor M.W. Cortical interneuron function in autism spectrum condition. Pediatr. Res. 2019;85:146–154. doi: 10.1038/s41390-018-0214-6. [DOI] [PubMed] [Google Scholar]
- Mango D., Saidi A., Cisale G.Y., Feligioni M., Corbo M., Nisticò R. Targeting synaptic plasticity in experimental models of Alzheimer's disease. Front. Pharmacol. 2019;10:778. doi: 10.3389/fphar.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D., Liu Q.Y., Maric I., Chaudry S., Chang Y.H., Smith S.V., Sieghart W., Fritschy J.M., Barker J.L. GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl- channels. J. Neurosci. 2001;21:2343–2360. doi: 10.1523/JNEUROSCI.21-07-02343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Morales P.L., Liste I. Stem cells as in vitro model of Parkinson's disease. Stem Cells Int. 2012;2012:980941. doi: 10.1155/2012/980941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadadhur A.G., Emperador Melero J., Meijer M., Schut D., Jacobs G., Li K.W., Hjorth J.J.J., Meredith R.M., Toonen R.F., Van Kesteren R.E., et al. Multi-level characterization of balanced inhibitory-excitatory cortical neuron network derived from human pluripotent stem cells. PLoS One. 2017;12:e0178533. doi: 10.1371/journal.pone.0178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R., Johnston D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron. 2007;56:1061–1075. doi: 10.1016/j.neuron.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M., Litt M., Tidball A., Li G., Aboud A., Hopkins C., Chamberlin R., Hong C., Ess K., Bowman A. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: comparison of PAX6 and SOX1 expression during neural induction. ACS Chem. Neurosci. 2012;3:482–491. doi: 10.1021/cn300029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoleau C., Varela C., Bonnefond C., Maury Y., Bugi A., Aubry L., Viegas P., Bourgois-Rocha F., Peschanski M., Perrier A.L. Embryonic stem cells neural differentiation qualifies the role of Wnt/β-catenin signals in human telencephalic specification and regionalization. Stem Cells. 2013;31:1763–1774. doi: 10.1002/stem.1462. [DOI] [PubMed] [Google Scholar]
- Nicoll R.A. A brief history of long-term potentiation. Neuron. 2017;93:281–290. doi: 10.1016/j.neuron.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Nowak L.M., Young A.B., Macdonald R.L. GABA and bicuculline actions on mouse spinal cord and cortical neurons in cell culture. Brain Res. 1982;244:155–164. doi: 10.1016/0006-8993(82)90913-1. [DOI] [PubMed] [Google Scholar]
- Obien M.E.J., Deligkaris K., Bullmann T., Bakkum D.J., Frey U. Revealing neuronal function through microelectrode array recordings. Front. Neurosci. 2015;8:1–30. doi: 10.3389/fnins.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odawara A., Katoh H., Matsuda N., Suzuki I. Induction of long-term potentiation and depression phenomena in human induced pluripotent stem cell-derived cortical neurons. Biochem. Biophys. Res. Commun. 2016;469:856–862. doi: 10.1016/j.bbrc.2015.12.087. [DOI] [PubMed] [Google Scholar]
- Odawara A., Katoh H., Matsuda N., Suzuki I. Physiological maturation and drug responses of human induced pluripotent stem cell-derived cortical neuronal networks in long-term culture. Sci. Rep. 2016;6:26181. doi: 10.1038/srep26181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odawara A., Matsuda N., Ishibashi Y., Yokoi R., Suzuki I. Toxicological evaluation of convulsant and anticonvulsant drugs in human induced pluripotent stem cell-derived cortical neuronal networks using an MEA system. Sci. Rep. 2018;8:10416. doi: 10.1038/s41598-018-28835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleaga C., Lavado A., Riu A., Rothemund S., Carmona-Moran C.A., Persaud K., Yurko A., Lear J., Narasimhan N.S., Long C.J., et al. Long-term electrical and mechanical function monitoring of a human-on-a-chip system. Adv. Funct. Mater. 2019;29:1805792. doi: 10.1002/adfm.201805792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleaga C., Platt V., Bridges L.R., Persaud K., McAleer C., Long C., Hickman J.J. A functional long-term serum-free human hepatic in vitro system for drug evaluation. Biotechnol. Prog. 2021;37:33069. doi: 10.1002/btpr.3069. [DOI] [PubMed] [Google Scholar]
- Onizuka S.M.D., Kasaba T.M.D., Hamakawa T.M.D., Ibusuki S.M.D., Takasaki M.M.D.P.D. Lidocaine increases intracellular sodium concentration through voltage-dependent sodium channels in an identified lymnaea neuron. Anesthesiology. 2004;101:110–120. doi: 10.1097/00000542-200407000-00018. [DOI] [PubMed] [Google Scholar]
- Park K., Heo H., Han M.E., Choi K., Yi J.H., Kang S.J., Kwon Y.K., Shin K.S. Learning-induced synaptic potentiation in implanted neural precursor cell-derived neurons. Sci. Rep. 2015;5:17796. doi: 10.1038/srep17796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Rumsey J.W., Lorance C., Long C.J., Lee B., Tetard L., Lambert S., Hickman J.J. Myelination and node of Ranvier formation in a human motoneuron–Schwann cell serum-free coculture. ACS Chem. Neurosci. 2020;11:2615–2623. doi: 10.1021/acschemneuro.0c00287. [DOI] [PubMed] [Google Scholar]
- Prè D., Nestor M.W., Sproul A.A., Jacob S., Koppensteiner P., Chinchalongporn V., Zimmer M., Yamamoto A., Noggle S.A., Arancio O. A time course analysis of the electrophysiological properties of neurons differentiated from human induced pluripotent stem cells (iPSCs) PLoS One. 2014;9:e103418. doi: 10.1371/journal.pone.0103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putrenko I., Schwarz S.K.W. Lidocaine blocks the hyperpolarization-activated mixed cation current, I h, in rat thalamocortical neurons. Anesthesiology. 2011;115:822–835. doi: 10.1097/ALN.0b013e31822ddf08. [DOI] [PubMed] [Google Scholar]
- Qi Y., Zhang X.-J., Renier N., Wu Z., Atkin T., Sun Z., Ozair M.Z., Tchieu J., Zimmer B., Fattahi F., et al. Combined small-molecule inhibition accelerates the derivation of functional, early-born, cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 2017;35:154–163. doi: 10.1038/nbt.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner S., Banos C., Bachay G., Li Y.N., Hunter D.D., Brunken W.J., Yee K.T. β2 and γ3 laminins are critical cortical basement membrane components: ablation of Lamb2 and Lamc3 genes disrupts cortical lamination and produces dysplasia. Dev. Neurobiol. 2013;73:209–229. doi: 10.1002/dneu.22057. [DOI] [PubMed] [Google Scholar]
- Reyes-Garcia S.Z., de Almeida A.-C.G., Ortiz-Villatoro N.N., Scorza F.A., Cavalheiro E.A., Scorza C.A. Robust network inhibition and decay of early-phase LTP in the hippocampal CA1 subfield of the Amazon rodent Proechimys. Front. Neural Circuits. 2018;12:81. doi: 10.3389/fncir.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F.B., Cugola F.R., Fernandes I.R., Pignatari G.C., Beltrão-Braga P.C.B. Induced pluripotent stem cells for modeling neurological disorders. World J. Transpl. 2015;5:209–221. doi: 10.5500/wjt.v5.i4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.K., Reams R.Y., Jordan W.H., Miller M.A., Thacker H.L., Snyder P.W. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol. Pathol. 2007;35:984–999. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- Stancescu M., Molnar P., McAleer C.W., McLamb W., Long C.J., Oleaga C., Prot J.-M., Hickman J.J. A phenotypic in vitro model for the main determinants of human whole heart function. Biomaterials. 2015;60:20–30. doi: 10.1016/j.biomaterials.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele P.M., Mauk M.D. Inhibitory control of LTP and LTD: stability of synapse strength. J. Neurophysiol. 1999;81:1559–1566. doi: 10.1152/jn.1999.81.4.1559. [DOI] [PubMed] [Google Scholar]
- Vaaga C.E., Borisovska M., Westbrook G.L. Dual-transmitter neurons: functional implications of co-release and co-transmission. Curr. Opin. Neurobiol. 2014;29:25–32. doi: 10.1016/j.conb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese K., Molnar P., Das M., Bhargava N., Lambert S., Kindy M.S., Hickman J.J. A new target for amyloid beta toxicity validated by standard and high-throughput electrophysiology. PLoS One. 2010;5:e8643. doi: 10.1371/journal.pone.0008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.D., Kriegstein A.R. Defining the role of GABA in cortical development. J. Physiol. 2009;587:1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.-C., Fan J., Wang X., Eacker S.M., Kam T.-I., Chen L., Yin X., Zhu J., Chi Z., Jiang H., et al. Cultured networks of excitatory projection neurons and inhibitory interneurons for studying human cortical neurotoxicity. Sci. Transl. Med. 2016;8:333ra348. doi: 10.1126/scitranslmed.aad0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.-L., Shatskikh T.N., Liu X., Holmes G.L. Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur. J. Neurosci. 2007;25:3667–3677. doi: 10.1111/j.1460-9568.2007.05598.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
information