Abstract
There are some concerns on the effect of infection with human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) on the outcome and mortality of COVID- 19. In this meta-analysis, we aimed to address this issue and assess the risk of mortality in COVID-19 patients who are co-infected with HIV. Two International electronic databases (PubMed, Scopus) were searched from the first time available to 12 August 2021. The targeted outcome was the pooled odds ratio to examine the effect of HIV infection on COVID-19 mortality. The crude odds ratio (OR) for all studies and the pooled OR were calculated with 95% confidence interval. The forest plot was used to graphically represent the result of conducted meta-analysis and calculated OR for individual studies. The I2 statistic was used to examine the Heterogeneity in the included studies. Eleven studies were included in our study consisting of 19,642,775 COVID-19 infected cases, 59,980 HIV-positive, and 4,373 deaths due to COVID-19 in HIV positive patients. The overall pooled odds ratio was 1.21 (CI: 1.02; 1.43) and P-value < 0.0277. The I^2 value was 89% (P-value < 0.0001), which shows that included studies are heterogeneous. In this study, the funnel plot analysis showed symmetry among the included studies. HIV-positive patients are 21% more likely to die because of COVID-19 infection than people without HIV. Special attention should be considered for the prevention and treatment of COVID-19 and consistent treatment for HIV infection, in HIV-positive patients.
Key Words: COVID-19, HIV, AIDS, SARS-CoV-2, mortality
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had infected more than 103 million people around the world in almost a year since its emergence in late 2019. The virus has been responsible for more than 2.2 million deaths all over the world as well1 The global pandemic of coronavirus disease 2019 (COVID-19) is having a major impact on health, economic and social aspects of life.2-5
Numerous studies have shown the effect of many conditions such as chronic diseases and co-infections with other viruses on the severity and outcome of the COVID-19 infections. Diabetes, hypertension, cardiovascular and hepatic diseases, obesity, and kidney diseases can increase the risk of COVID-19 mortality.6-11 However, there is a controversy on the effect of infection with human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) on the outcome and mortality of COVID-19.12
There are some concerns that HIV can suppress the immune system and make people who are infected with HIV more susceptible to SARS-CoV-2 infection and subsequent severe outcomes and death.13,14
On the other hand, some studies suggest that low CD4 count and immunosuppression in HIV-positive patients may protect these patients against cytokine storms observed in SARS-CoV-2 infected patients.15
There are almost 40 million people with HIV around the world; so it is essential to determine the outcome of COVID-19 infection in this group.16 It is especially the case in sub-Saharan Africa, where many countries are exposed to HIV and COVID-19 epidemics simultaneously, and the capacity to treat severe COVID- 19 is very limited.17
In this meta-analysis, we aimed to address this issue and assess the risk of mortality in COVID-19 patients who are co-infected with HIV.
Materials and Methods
The current meta-analysis is conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18 The targeted outcome was the pooled odds ratio to examine the effect of HIV infection on COVID-19 mortality, number of total HIV positive and SARS-CoV-2 co-infected patients, number of total HIV negative patients who are infected with SARS-CoV-2, number of COVID-19 deaths who are were infected with HIV, number of COVID-19 deaths who were HIV negative. Observational study - including cross-sectional, cohort, or case-control studies - was eligible for inclusion.
Search strategy
Two International electronic databases (PubMed, Scopus) were searched from the first time available to 12 August 2021. The following sets of key terms were used for searching international databases: 1) Human immunodeficiency virus, HIV, acquired immune deficiency syndrome, AIDS; 2) COVID-19, 2019- nCoV, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2 (Appendix I). No language limitation was applied in the search or the selection process.
Eligibility criteria
We included studies that provided data on the frequency of death due to COVID-19 and survival of COVID-19 patients, based on HIV status (positive or negative). We included studies where mortality due to COVID-19 in HIV positive and HIV negative patients is the main outcome, these methods could be PCR-based, antibodybased, molecular techniques or clinical/ hospital records, registries, or directly from patients. We excluded the studies that contain one or more of the following criteria: 1) Studies on any species of SARS other than SARS-CoV-2, 2) case reports, case series, systematic review, meta-analysis, grey literature, 3) Studies with less than three deaths due to COVID-19, 4) full text in any language other than English; 5) having infectious diseases other than HIV and COVID-19.
Screening, data extraction, and quality assessment
The titles and abstracts of all records retrieved from online databases were screened by two researchers independently. Eligible full texts were selected and were screened for data of interest and data were extracted. The quality of all included full texts were also evaluated. The extracted data were assessed by a third reviewer. Any disagreements were discussed with an expert as a fourth reviewer and were resolved. The following data was extracted from included studies: bibliometric information (name of the first author, year of publication), study implementation country, type of the study number of total HIV positive and SARS-CoV- 2 co-infected patients, number of total HIV negative patients who are infected with SARS-CoV-2, number of COVID-19 deaths who are were infected with HIV, number of COVID-19 deaths who were HIV negative, the mean or median age of patients, and the period in which mortality is accounted to be due to COVID-19. Based on previous studies, the quality of the eligible studies was assessed using the New-Castle Ottawa Scale (NOS) quality assessment scale. The quality score was presented for each study.19
Statistical Analyses
For data analysis, we used meta package in the R statistical software (version 4.0.5).20 The crude odds ratio (OR) was calculated with a 95% confidence interval (CI) for all studies. After that, the pooled odds ratio was estimated using Mantel-Haenszel Method. Because of heterogeneity greater than 75%, the randomeffects model was used.21 The pooled OR for the association between HIV infection and mortality due to COVID-19 was reported with 95% CI. The forest plot was used to graphically represent the result of conducted meta-analysis and calculated OR for individual studies. The I2 statistic was used to examine the Heterogeneity in the included studies. A value of more than 75% was considered as significant heterogeneity. The I2 statistic represents the percentage of total variation across studies due to heterogeneity.
The funnel plot was used to assess the small study effect. Observing any asymmetry visually in the funnel plot was considered as a Small study effect. As the small studies show different, treatment effects compared with the large studies, we used this method. The small study effect is the association between the size of the study and the effect in meta-analysis. Egger regression test of funnel plot asymmetry and Begg correlation method were conducted to test publication bias. A trimand- fill method was utilized to adjust the effect estimate for this bias.
Results
The flow diagram of the study selection is presented in Figure 1. After the removal of duplicate records, 3,262 studies were retrieved from online databases. These studies were screened based on title and/ or abstract. After that, 45 studies were fulfilled the inclusion criteria for full-text review. From these full-texts, 34 studies were excluded because 1) seventeen studies had insufficient data, 2) studies were not stratified by HIV status, 3) four studies did not have appropriate, 4) four studies have heterogeneous data; 5) studies had less than 10 deaths in HIV-positive and COVID-19 positive patients. Finally, the process led to a final inclusion of 11 studies. The characteristics of the selected studies are presented in Table1.
Eleven studies were included in our study consisting of 19.642.775 COVID-19 infected cases, 59.980 HIV positive, and 4,373 deaths due to COVID-19 in HIV positive patients and 167.800 deaths overall. Total HIV cases ranged from 111 to 27480, total COVID-19 cases ranged from 15.187 to 17.282.905. Total deaths in HIV-positive patients due to COVID-19 ranged from 20 to 3.407. Of the 11 studies, 9 were retrospective cohort and two were prospective cohort. Six studies were conducted in the United States; three and two studies were conducted in South Africa and the United Kingdom respectively. Five studies were conducted in 2021 and six ones were in 2021. The median age in the included studies ranged from 47 to 54 years. Five studies had a sample size greater than 100.000 people. The largest study in the United Kingdom was conducted by Bhaskaran et al. in 202. This study was a retrospective cohort study, in which 17.282.905 COVID-19 infected patients were recruited, in which 0.15% of the total study population was HIV positive. Twenty-five HIV-positive patients from 27.455 and 14.857 HIV-negative patients from 17.255.425 were died because of COVID-19 infection. The OR of COVID-19 mortality in HIV positive patients compared to HIV negative patients was 1.06 (CI: 0.71 to 1.56).22
Fig 1.
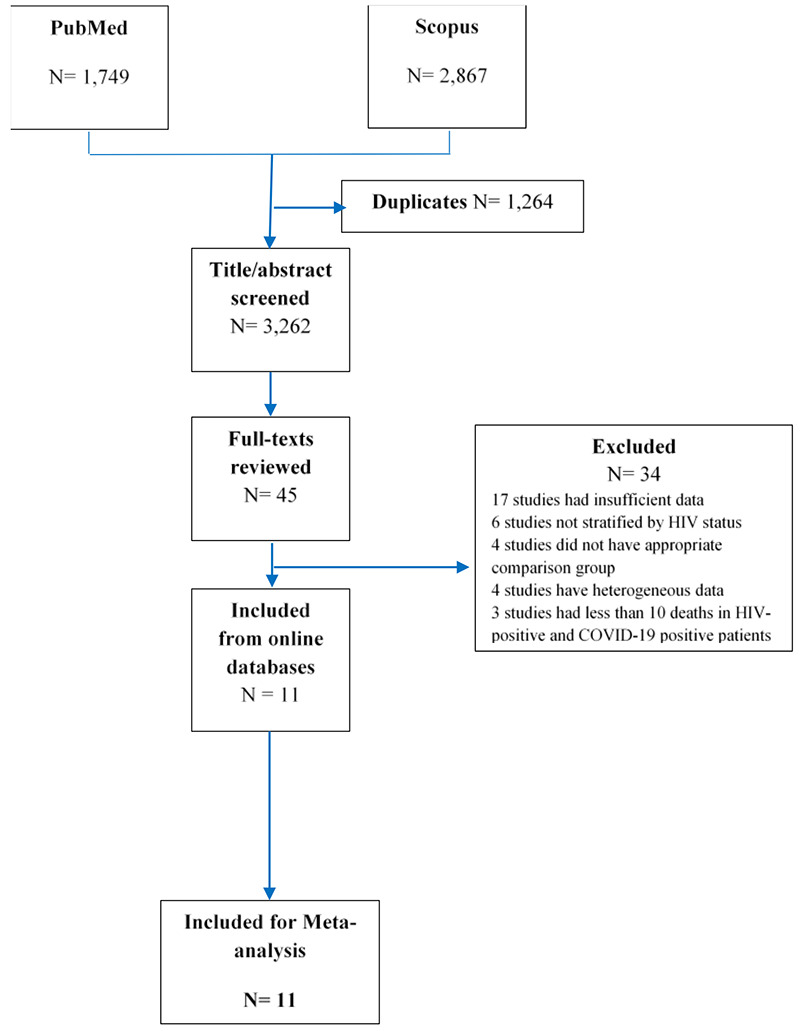
Flow diagram for Study selection
The largest retrospective cohort in the United States was conducted in 2021 by Sun et al. In this study 1.435.253 COVID-19 infected patients were recruited. In this study, 0.5% was HIV positive, were studied. Of 8.269 HIV-positive patients 195 and of 1.426.984 HIVnegative patients, 23.973 cases died because of COVID- 19 infection. The OR of COVID-19 mortality in HIV positive patients compared to HIV negative patients was 1.41 (CI: 1.23 to 1.63).23
The largest retrospective cohort in South Africa was conducted in 2021 by Jassat et al. In this study 151.779 COVID-19 infected patients were included. In this study, 9% of the population size was HIV positive. This study, with 3.407 deaths in HIV-positive patients, had the most mortality cases due to COVID-19. The OR of COVID-19 mortality in HIV positive patients compared to HIV negative patients was 1.15 (CI: 1.10 to 1.19).24
As it shows in Figure 2 the overall pooled odds ratio is 1.21 (CI: 1.02; 1.43), which suggests that the odds of mortality due to COVID-19 infection in HIV-positive patients was 21% higher than in HIV-negative patients (P-value < 0.0277). The I^2 value was 89% (P-value < 0.0001), which shows that included studies are heterogeneous. The evidence of the small study effect was tested by observation of funnel plot symmetry. In this study, the funnel plot analysis showed symmetry among the included studies (Figure 3). P-values for Egger regression test and Begg correlation method were 0.88 and 0.3 respectively, which indicate publication bias is non-significant. After moderating the small studies with the trimming method, the odds of mortality due to COVID-19 in HIV-positive patients was 1.21 (CI: 1.02; 1.43) times higher than HIV-negative patients, which did not show any change compared to the previous result.
Table 1.
Characteristic of the included studies
| ID | Author | Year | Country | Study design | Data source | Age (Range) | HIV (+) | HIV(-) | Mortality time | Quality of literature | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death | Total | Death | Total | |||||||||
| 1 | Bhaskaran (22) | 2021 | United Kingdom | Retrospective cohort | Data came from the OpenSAFELY platform on behalf of NHS England | median: 48(40-55) | 25 | 27480 | 14,857 | 17255425 | NA | H |
| 2 | Miyashita (25) | 2021 | United States | Retrospective cohort | Electronic medical records of the Mount Sinai Health System in NYC | NA | 23 | 161 | 1235 | 8751 | NA | H |
| 3 | Venturas (26) | 2021 | South Africa | Retrospective cohort | Medical wards and multi disciplinary intensive care unit (I CU) of Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) | Median: 50(39-60) | 16 | 108 | 54 | 276 | NA | H |
| 4 | Sun (27) | 2021 | United States | Retrospective cohort | A multicenter electronic medical record (EMR), data from across the United States | Median: 47 (32-61) | 195 | 8269 | 23,973 | 1426984 | Within 45 day | H |
| 5 | Jassat (24) | 2021 | South Africa | Retrospective cohort | Data submitted to DATCOV, a national active hospital surveillance system for COVID-19 hospital admissions | Median: 54(40-66) | 3407 | 13793 | 30,697 | 137986 | NA | H |
| 6 | Hadi (28) | 2020 | United States | Retrospective cohort | Multicenter research network TriNETX (Cambridge, Massachusetts, USA) | >10 | 20 | 404 | 1585 | 49763 | Within 30 days | H |
| 7 | Braunstein (29) | 2020 | United States | Retrospective cohort | Data from the New York City Department of Health and Mental Hygiene (DOHMH) HIV surveillance registry | NA | 312 | 2410 | 16,160 | 202012 | NA | H |
| 8 | Gudipati (30) | 2020 | United States | Prospective cohort | Patients living with HIV in Michigan | Median: 52 | 23 | 278 | 5919 | 64993 | NA | H |
| 9 | Tesoriero (31) | 2020 | United States | Retrospective cohort | Patients were diagnosed in New York State | NA | 207 | 2988 | 14,522 | 375260 | NA | H |
| 10 | Geretti (32) | 2020 | United Kingdom | Prospective cohort | Data from the International Severe Acute Respiratory and Emerging Infection Consortium | >18 years | 30 | 111 | 14,555 | 43015 | Within 28 days | H |
| 11 | Boulle (33) | 2020 | South Africa | Retrospective cohort | Data from adults attending public sector health facilities in the Western Cape, South Africa | >20 | 115 | 3978 | 510 | 18330 | NA | I |
Fig 2.
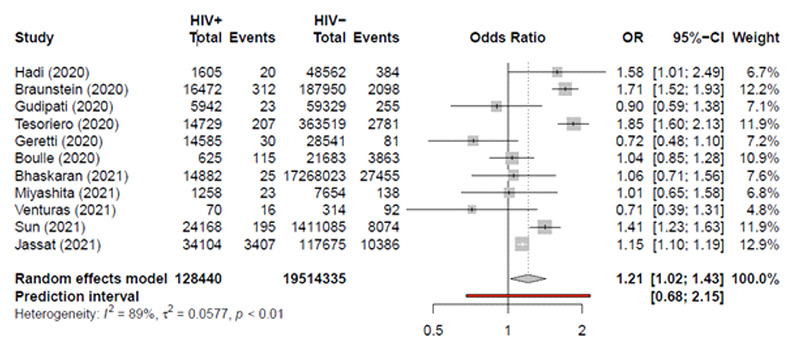
Forest plot exhibiting OR of each study and pooled OR of the association between HIV infection and mortality due to COVID-19
Discussion
In this meta-analysis, we assessed the mortality risk in HIV and COVID-19 co-infection compared to HIVnegative patients. It was revealed that persons living with HIV are 21% more likely to die because of COVID-19 infection than people without HIV. There are some explanations for the observed results. First, HIV-positive patients are more likely to have some preexisting chronic conditions that make them more susceptible to severe outcomes and mortality when infected with the COVID-19 virus.25-34 Some of these conditions are hypertension, diabetes, and cardiovascular diseases that numerous studies have shown their effectiveness in COVID-19 caused mortality and severity.35 Besides, HIV-positive patients have a compromised immune system, and subsequent attenuated immune response to COVID-19 infection and severity and mortality are followed13 The delayed SARS-CoV-2 specific antibody response caused by HIV infection may also increase the recovery time of lung lesions and provides the opportunity for the SARSCoV- 2 virus to spread and infect more areas of the lungs, but this hypothesis needs to be confirmed by future studies. In addition, HIV-positive patients are more susceptible to some conditions such as anemia, neutropenia, thrombocytopenia, and abnormal serum electrolytes.36 Co-infection with some other types of pneumonia and infectious diseases, which may worsen during the course of COVIS-19 infection, is also another possible explanation for the greater mortality rate of COVID-19 among HIV-positive patients.37 The mean age of patients with HIV and COVID-19 coinfection was 50 years, which is significantly younger than the mean age of hospitalized COVID-19 patients in the general population. This age difference results from premature aging of HIV-positive patients due to chronic inflammation or certain behavioral risk factors.38,39 With a greater chance of mortality in HIV-positive patients, they should follow all applicable recommendations for COVID-19 carefully to prevent SARS-CoV-2 acquisition. These recommendations include social or physical distancing, avoiding crowded areas, and wearing masks consistently.40 Besides, HIV-positive patients should be included in the category of high-risk medical conditions and should be of high priority to receive SARS-CoV-2 vaccines, and this should be done regardless of viral load or CD4 count. In addition to caution about SARS-CoV-2 infection control in HIVpositive patients, health care providers should ensure that HIV-positive patients maintain their HIV controlled and sustain antiretroviral therapy (ART) continuously with proper adherence to treatment.41 Addressing underlying medical conditions and achieving viral suppression could reduce COVID-19–associated mortality in HIV-positive patients, especially in highly affected regions with HIV including sub-Saharan Africa. Our study had some limitations. First, the studies included in our meta-analysis are from three regions, the United States, the United Kingdom and, South Africa, which may not be representative of the whole world. The latter limits the generalization of the results to other countries. Second, due to a limited number of studies, subgroup analysis, including age and sex groups, stages of HIV, HIV viral load, CD4 counts, ART regimen, and comorbidities were not possible. Therefore, future studies might take into account the association between HIV and COVID-19 outcomes by comorbidities, HIV viral load, CD4 count, and sex and age subgroups.
Fig 3.
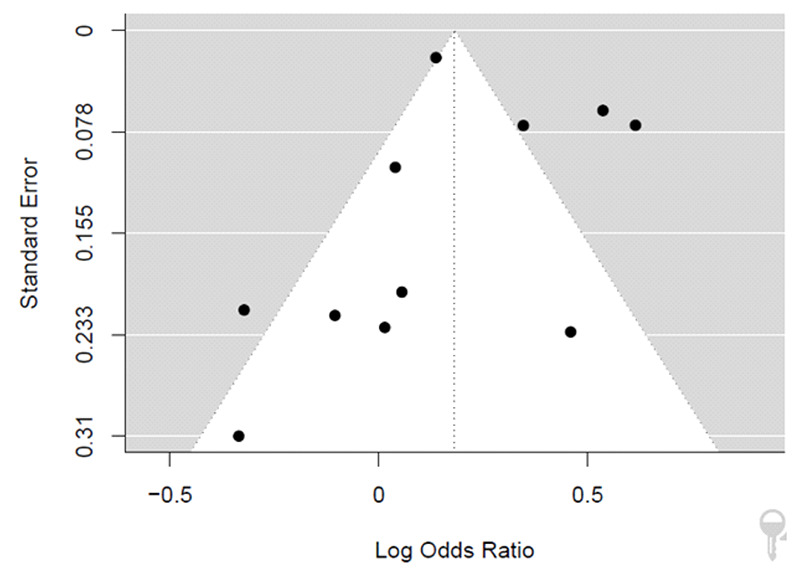
Funnel plot of the included studies in this meta-analysis. Each point represents a study; the y-axis represents standard error, and the x-axis displays the odds ratio of each study
In conclusion, HIV infection is associated with higher mortality due to COVID-19. HIV-positive patients are 21% more likely to die because of COVID-19 infection than people without HIV. Special attention should be considered for the prevention and treatment of COVID- 19 in HIV-positive patients.
Acknowledgments
None.
List of acronyms
- AIDS
acquired immunodeficiency syndrome
- CI
confidence interval
- OR
odds ratio
- HIV
human immunodeficiency virus
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Funding Statement
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
References
- 1.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/. Accessed on: January 31, 2021. [Google Scholar]
- 2.Jardine R, Wright J, Samad Z, Bhutta ZA. Analysis of COVID-19 burden, epidemiology and mitigation strategies in Muslim majority countries. East Mediterr Health J. 2020;26(10):1173-1183. doi: 10.26719/emhj.20.120. [DOI] [PubMed] [Google Scholar]
- 3.Jones DL, Rodriguez VJ, Salazar AS, Montgomerie E, Raccamarich PD, Uribe Starita C, Barreto Ojeda IT, Beauchamps L, Vazquez A, Martinez T, Alcaide ML. Sex Differences in the Association Between Stress, Loneliness, and COVID-19 Burden Among People with HIV in the United States. AIDS Res Hum Retroviruses. 2021;37(4):314-321. doi: 10.1089/AID.2020.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labgold K, Hamid S, Shah S, Gandhi NR, Chamberlain A, Khan F, Khan S, Smith S, Williams S, Lash TL, Collin LJ. Estimating the Unknown: Greater Racial and Ethnic Disparities in COVID-19 Burden After Accounting for Missing Race and Ethnicity Data. Epidemiology. 2021;32(2):157-161. doi: 10.1097/EDE.0000000000001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider M, Altersberger M, Binder C, Hengstenberg C, Binder T. The COVID-19 burden for health care professionals: Results of a global survey. Eur J Intern Med. 2021;83:96-98. doi: 10.1016/j.ejim.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eleni M, Evangelia M, Eleftheria K, Vasilios V, Vana S, Vissaria S, Evangelos B, Ioannis K. Clinical features and outcomes of hospitalized COVID-19 patients in a low burden region. Pathog Glob Health. 202;115(4):243-249. doi: 10.1080/20477724.2021.1893485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayinbuomwan SA, Mokogwu N, Akoria OA, Okwara BU, Omuemu CE, Obaseki DE. Arterial Oxygen Saturation and other Clinical Predictors of Survival in Patients with Covid-19: A Review of Cases in a Tertiary Care Hospital in Nigeria. West Afr J Med. 2021;38(2):109-113. PMID: 33641143. [PubMed] [Google Scholar]
- 8.Kumar N, Shahul Hameed SK, Babu GR, Venkataswamy MM, Dinesh P, Kumar Bg P, John DA, Desai A, Ravi V. Descriptive epidemiology of SARS-CoV-2 infection in Karnataka state, South India: Transmission dynamics of symptomatic vs. asymptomatic infections. EClinicalMedicine. 2021;32:100717. doi: 10.1016/j.eclinm.2020.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiabaud A, Iten A, Balmelli C, Senn L, Troillet N, Widmer A, Flury D, Schreiber PW, Vázquez M, Damonti L, Buettcher M, Vuichard-Gysin D, Kuhm C, Cusini A, Riedel T, Nussbaumer-Ochsner Y, Gaudenz R, Heininger U, Berger C, Zucol F, Bernhard-Stirnemann S, Corti N, Zimmermann P, Uka A, Niederer-Loher A, Gardiol C, Roelens M, Keiser O. Cohort profile: SARS-CoV-2/COVID-19 hospitalised patients in Switzerland. Swiss Med Wkly. 2021;151:w20475. doi: 10.4414/smw.2021.20475. [DOI] [PubMed] [Google Scholar]
- 10.Gacche RN, Gacche RA, Chen J, Li H, Li G. Predictors of morbidity and mortality in COVID- 19. Eur Rev Med Pharmacol Sci. 2021;25(3):1684-1707. doi: 10.26355/eurrev_202102_24880. [DOI] [PubMed] [Google Scholar]
- 11.Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, Kunze CA, Sinn BV, Gerhold M, Hauptmann K, Ingold-Heppner B, Miller F, Herbst H, Corman VM, Martin H, Radbruch H, Heppner FL, Horst D. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11(1):4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkar S, Khanna P, Singh AK. Impact of COVID- 19 in patients with concurrent co-infections: A systematic review and meta-analyses. J Med Virol. 2021;93(4):2385-2395. doi: 10.1002/jmv.26740. [DOI] [PubMed] [Google Scholar]
- 13.Tesoriero JM, Swain CE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, Gonzalez CJ, Udo T, Morne JE, Hart-Malloy R, Rajulu DT, Leung SJ, Rosenberg ES. Elevated COVID-19 outcomes among persons living with diagnosed HIV infection in New York State: Results from a population-level match of HIV, COVID-19, and hospitalization databases. medRxiv [Preprint]. 2020:2020.11.04.20226118. doi: 10.1101/2020.11.04.20226118. [Google Scholar]
- 14.Huang J, Xie N, Hu X, Yan H, Ding J, Liu P, Ma H, Ruan L, Li G, He N, Wei S, Wang X. Epidemiological, virological and serological features of COVID-19 cases in people living with HIV in Wuhan City: A population-based cohort study. Clin Infect Dis. 2020:ciaa1186. doi: 10.1093/cid/ciaa1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo W, Ming F, Dong Y, Zhang Q, Zhang X, Mo P. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China. AIDS Patients in Two Districts of Wuhan, China (3/4/2020) (2020). DOI: 10.2139/ssrn.3550029. [Google Scholar]
- 16.GBD 2017 HIV collaborators. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6(12):e831-e859. doi: 10.1016/S2352-3018(19)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.African COVID-19 Critical Care Outcomes Study (ACCCOS) Investigators. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study. Lancet. 2021;397(10288):1885-1894. doi: 10.1016/S0140-6736(21)00441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Well GA, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2011:1-12. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 20.Schwarzer G, Carpenter JR, Rücker G. Metaanalysis with R: Springer; 2015. DOI 10.1007/978-3-319-21416-0_1. [Google Scholar]
- 21.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105-14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, Eggo RM, Morton CE, Bacon SCJ, Inglesby P, Douglas IJ, Walker AJ, McDonald HI, Cockburn J, Williamson EJ, Evans D, Forbes HJ, Curtis HJ, Hulme WJ, Parry J, Hester F, Harper S, Evans SJW, Smeeth L, Goldacre B. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8(1):e24-e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S, Hou J, Chen Y, Lu Y, Brown L, Operario D. Challenges to HIV Care and Psychological Health During the COVID-19 Pandemic Among People Living with HIV in China. AIDS Behav. 2020. ;24(10):2764-2765. doi: 10.1007/s10461-020-02903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jassat W, Cohen C, Tempia S, Masha M, Goldstein S, Kufa T, Murangandi P, Savulescu D, Walaza S, Bam JL, Davies MA, Prozesky HW, Naude J, Mnguni AT, Lawrence CA, Mathema HT, Zamparini J, Black J, Mehta R, Parker A, Chikobvu P, Dawood H, Muvhango N, Strydom R, Adelekan T, Mdlovu B, Moodley N, Namavhandu EL, Rheeder P, Venturas J, Magula N, Blumberg L; DATCOV author group. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8(9):e554-e567. doi: 10.1016/S2352-3018(21)00151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyashita H, Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV Med. 2021;22(1):e1-e2. doi: 10.1111/hiv.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venturas J, Zamparini J, Shaddock E, Stacey S, Murray L, Richards GA, Kalla I, Mahomed A, Mohamed F, Mer M, Maposa I, Feldman C. Comparison of outcomes in HIV-positive and HIV-negative patients with COVID-19. J Infect. 2021;83(2):217-227. doi: 10.1016/j.jinf.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Patel RC, Zheng Q, Madhira V, Olex AL, Islam JY, French E, Chiang TP, Akselrod H, Moffitt R, Alexander GC, Andersen KM, Vinson AJ, Brown TT, Chute CG, Crandall KA, Franceschini N, Mannon RB, Kirk GD; National COVID Cohort Collaborative (N3C) Consortium. COVID-19 Disease Severity among People with HIV Infection or Solid Organ Transplant in the United States: A Nationally-representative, Multicenter, Observational Cohort Study. medRxiv [Preprint]. 2021:2021.07.26.21261028. doi: 10.1101/2021.07.26.21261028. [Google Scholar]
- 28.Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34(13):F3-F8. doi: 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braunstein SL, Lazar R, Wahnich A, Daskalakis DC, Blackstock OJ. Coronavirus Disease 2019 (COVID-19) Infection Among People With Human Immunodeficiency Virus in New York City: A Population-Level Analysis of Linked Surveillance Data. Clin Infect Dis. 2021;72(12):e1021-e1029. doi: 10.1093/cid/ciaa1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudipati S, Brar I, Murray S, McKinnon JE, Yared N, Markowitz N. Descriptive Analysis of Patients Living With HIV Affected by COVID-19. J Acquir Immune Defic Syndr. 2020;85(2):123-126. doi: 10.1097/QAI.0000000000002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesoriero JM, Swain CE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, Gonzalez CJ, Udo T, Morne JE, Hart-Malloy R, Rajulu DT, Leung SJ, Rosenberg ES. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw Open. 2021;4(2):e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, Villa G, Docherty A, Harrison EM, Turtle L, Openshaw PJM, Baillie JK, Sabin CA, Semple MG. Outcomes of COVID- 19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis. 2020:ciaa1605. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulle A, Davies MA, Hussey H, Ismail M, Morden E, Vundle Z, Zweigenthal V, Mahomed H, Paleker M, Pienaar D, Tembo Y, Lawrence C, Isaacs W, Mathema H, Allen D, Allie T, Bam JL, Buddiga K, Dane P, Heekes A, Matlapeng B, Mutemaringa T, Muzarabani L, Phelanyane F, Pienaar R, Rode C, Smith M, Tiffin N, Zinyakatira N, Cragg C, Marais F, Mudaly V, Voget J, Davids J, Roodt F, van Zyl Smit N, Vermeulen A, Adams K, Audley G, Bateman K, Beckwith P, Bernon M, Blom D, Boloko L, Botha J, Boutall A, Burmeister S, Cairncross L, Calligaro G, Coccia C, Corin C, Daroowala R, Dave JA, De Bruyn E, De Villiers M, Deetlefs M, Dlamini S, Du Toit T, Endres W, Europa T, Fieggan G, Figaji A, Frankenfeld P, Gatley E, Gina P, Govender E, Grobler R, Gule MV, Hanekom C, Held M, Heynes A, Hlatswayo S, Hodkinson B, Holtzhausen J, Hoosain S, Jacobs A, Kahn M, Kahn T, Khamajeet A, Khan J, Khan R, Khwitshana A, Knight L, Kooverjee S, Krogscheepers R, Jacque Kruger J, Kuhn S, Laubscher K, Lazarus J, Le Roux J, Lee Jones S, Levin D, Maartens G, Majola T, Manganyi R, Marais D, Marais S, Maritz F, Maughan D, Mazondwa S, Mbanga L, Mbatani N, Mbena B, Meintjes G, Mendelson M, Möller E, Moore A, Ndebele B, Nortje M, Ntusi N, Nyengane F, Ofoegbu C, Papavarnavas N, Peter J, Pickard H, Pluke K, Raubenheimer PJ, Robertson G, Rozmiarek J, Sayed A, Scriba M, Sekhukhune H, Singh P, Smith E, Soldati V, Stek C, van den Berg R, van der Merwe LR, Venter P, Vermooten B, Viljoen G, Viranna S, Vogel J, Vundla N, Wasserman S, Zitha E, Lomas-Marais V, Lombard A, Stuve K, Viljoen W, Basson V, Le Roux S, Linden-Mars E, Victor L, Wates M, Zwanepoel E, Ebrahim N, Lahri S, Mnguni A, Crede T, de Man M, Evans K, Hendrikse C, Naude J, Parak M, Szymanski P, Van Koningsbruggen C, Abrahams R, Allwood B, Botha C, Henndrik Botha M, Broadhurst A, Claasen D, Daniel C, Dawood R, du Preez M, Du Toit N, Erasmus K, Koegelenberg CFN, Gabriel S, Hugo S, Jardine T, Johannes C, Karamchand S, Lalla U, Langenegger E, Louw E, Mashigo B, Mhlana N, Mnqwazi C, Moodley A, Moodley D, Moolla S, Mowlana A, Nortje A, Olivier E, Parker A, Paulsen C, Prozesky H, Rood J, Sabela T, Schrueder N, Sithole N, Sithole S, Taljaard JJ, Titus G, Van Der Merwe T, van Schalkwyk M, Vazi L, Viljoen AJ, Yazied Chothia M, Naidoo V, Alan Wallis L, Abbass M, Arendse J, Armien R, Bailey R, Bello M, Carelse R, Forgus S, Kalawe N, Kariem S, Kotze M, Lucas J, McClaughlin J, Murie K, Najjaar L, Petersen L, Porter J, Shaw M, Stapar D, Williams M, Aldum L, Berkowitz N, Girran R, Lee K, Naidoo L, Neumuller C, Anderson K, Begg K, Boerlage L, Cornell M, de Waal R, Dudley L, English R, Euvrard J, Groenewald P, Jacob N, Jaspan H, Kalk E, Levitt N, Malaba T, Nyakato P, Patten G, Schneider H, Shung King M, Tsondai P, Van Duuren J, van Schaik N, Blumberg L, Cohen C, Govender N, Jassat W, Kufa T, McCarthy K, Morris L, Hsiao NY, Marais R, Ambler J, Ngwenya O, Osei-Yeboah R, Johnson L, Kassanjee R, Tamuhla T. Risk factors for COVID- 19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020:ciaa1198. doi: 10.1093/cid/ciaa1198. [Google Scholar]
- 34.Yang HY, Beymer MR, Suen SC. Chronic Disease Onset Among People Living with HIV and AIDS in a Large Private Insurance Claims Dataset. Sci Rep. 2019;9(1):18514. doi: 10.1038/s41598-019-54969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. 2020. Aug 26;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Santis GC, Brunetta DM, Vilar FC, Brandão RA, de Albernaz Muniz RZ, de Lima GM, Amorelli-Chacel ME, Covas DT, Machado AA. Hematological abnormalities in HIV-infected patients. Int J Infect Dis. 2011;15(12):e808-11. doi: 10.1016/j.ijid.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Abatenh E, Asmamaw A, Hiluf L, Mohammed N. Pneumonia Co-Infection in HIV AIDS Patients in Woldia. 2018. 2(3): 348-54. https://scientiaricerca.com/srcbmi/SRCBMI-02-00052.php [Google Scholar]
- 38.Nasi M, De Biasi S, Gibellini L, Bianchini E, Pecorini S, Bacca V, Guaraldi G, Mussini C, Pinti M, Cossarizza A. Ageing and inflammation in patients with HIV infection. Clin Exp Immunol. 2017;187(1):44-52. doi: 10.1111/cei.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med. 2012. Aug- Sep;20(3):101-5. PMID: 22954610; PMCID: PMC6148943. [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Centers for Disease Control and Prevention (CDC) to prevent acquisition of SARS-CoV-2. Reterived from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. [Google Scholar]
- 41.The Centers for Disease Control. People at increased risk and other people who need to take extra precautions. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extraprecautions/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fpeople-atincreased-risk.html. [Google Scholar]


