Abstract
The pathogenesis of Ebola virus disease (EVD) is still incomplete, in spite of the availability of a nonhuman primate modelfor more than 4 decades. To further investigate EVD pathogenesis, a natural history study was conducted using 27 Chinese-origin rhesus macaques. Of these, 24 macaques were exposed intramuscularly to Kikwit Ebola virus and euthanized at predetermined time points or when end-stage clinical disease criteria were met, and 3 sham-exposed macaques were euthanized on study day 0. This study showed for the first time that Ebola virus causes uterine cervicitis, vaginitis, posthitis, and medullary adrenalitis. Not only was Ebola virus detected in the interstitial stromal cells of the genital tract, but it was also present in the epididymal and seminal vesicular tubular epithelial cells, ectocervical and vaginal squamous epithelial cells, and seminal fluid. Furthermore, as early as day 3 after exposure, Ebola virus replicative intermediate RNA was detected in Kupffer cells and hepatocytes. These findings in the nonhuman model provide additional insight into potential sexual transmission, possible disruption of sympathetic hormone production, and early virus replication sites in human EVD patients.
Keywords: EBOV, Ebola virus; EVD, Ebola virus disease; VP-40, Viral protein 40; GP, Glycoprotein; dpe, days of post-exposure; TC, Terminal control
Ebola virus (EBOV) is a large (19-kb) negative-sense, single-stranded RNA virus in the family Filoviridae.1 It causes a severe disease in humans, with case fatality rates of 40% to 50%.2 Although successful clinical trials have been conducted during EBOV outbreaks, conducting trials is limited by the frequency, size, and location of these outbreaks. Outbreaks of sufficient size to power a clinical trial are infrequent. As an alternative, countermeasures may still be evaluated and advanced under the Animal Efficacy Rule, codified 21 CFR 314.600 in 2002, which permits consideration of data obtained from animal efficacy studies in lieu of human clinical trials (US Food and Drug Administration, https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/subpart-I/section-314.600, last accessed November 11, 2021). This rule has facilitated the timely review of candidate Ebola virus disease (EVD) countermeasures to ensure availability of efficacious interventions to treat patients. Animal models are critical for furthering understanding of the pathogenic mechanisms of disease progression and development of medical countermeasures. Nonhuman primates (NHPs) have been used widely as animal models of EVD.3, 4, 5 Rhesus (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis) have been considered the gold standard animal models because they closely recapitulate the most pathogenic features of human EVD.6,7
Sexual transmission of EBOV in men and women is suspected.8, 9, 10, 11, 12 Remarkably, more than 2 years after recovery from EVD, EBOV RNA has been detected in seminal fluid samples.13 Sexual transmission is thought to be the origin of a new Ebola outbreak in Guinea this year (2021).11,14 Although EBOV infection has been confirmed in the interstitial mesenchymal tissues in the seminal vesicles, epididymis, testes, uterus, and ovaries in the acute NHP model,15,16 the origin of EBOV contamination of the genital fluid(s) in human survivors is not known. Adrenal cortical infection with degeneration and necrosis is observed commonly in the acute EVD NHP model.17,18 Whether EBOV can infect the chromaffin cells in adrenal medulla in the NHP model is unknown, although EBOV has been observed in some chromaffin cells in a guinea pig model.19 The liver is an important early target organ in acute EVD patients. Longitudinal studies in the NHP model have shown that, although rare, EBOV antigens and/or RNA can be detected in the liver as early as 3 days after exposure.17,20 It is unclear whether this is owing to viral replication in the liver or because of the presence of viremic blood within the tissue samples.21,22
The current study highlighted several previously unreported findings in reproductive organs, adrenal medulla, and liver, that may inform the pathogenesis of human EVD. Most other pathology and immunohistochemistry findings were similar to those described in previous EBOV natural history studies in the NHP model.3,4,18,23,24
Materials and Methods
Virus and Animals
The details regarding the viral variant and animals used have been published previously.25 Briefly, 27 (11 males and 16 females) 5- to 6-year-old Chinese-origin rhesus macaques (M. mulatta) were obtained from the NIH Animal Center (Dickerson, MD). Fifteen rhesus macaques were assigned randomly into five groups (three macaques/group) by day (D) of necropsy after EBOV exposure: D0 (uninfected control), D3 [3 days after exposure (dpe)], and D4 (4 dpe) to D6 (6 dpe). The additional 12 macaques were split equally into two terminal control (TC) groups: TC1 had no clinical manipulations after EBOV exposure and were euthanized humanely only after reaching experimental end points consistent with terminal EVD; and TC2 underwent routine blood sampling and other manipulations under anesthesia before being euthanized with terminal EVD. Kikwit EBOV (Ebola virus/Homo sapiens-terminal control-COD/1995/Kikwit-9510621) from BEI Resources, Manassas, VA was diluted to a target dose of 1000 plaque-forming units/mL before inoculation. Twenty-four macaques were inoculated intramuscularly with 1 mL of 1000 plaque-forming units/mL EBOV-Kikwit in the left lateral triceps muscle at study D0. Three uninfected control macaques in the D0 group were sham-exposed with 1 mL phosphate-buffered saline at the same anatomic location.
All macaques used in this research project were cared for and used humanely according to the following policies: the U.S. Public Health Service Policy on Humane Care and Use of Animals (2000); NIH’s Guide for the Care and Use of Laboratory Animals26; and the U.S. Government Principles for Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training (1985). All National Institute of Allergy and Infectious Diseases Integrated Research Facility animal facilities and programs are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. This study was performed in the Biosafety Level 4 Laboratory at the NIH/National Institute of Allergy and Infectious Diseases, Integrated Research Facility at Fort Detrick (Fredrick, MD).
Macroscopic Examination
All monkeys in the TC1 and TC2 groups reached EVD humane end point criteria and were euthanized and necropsied between 5 and 8 dpe, except for one rhesus in TC2 that succumbed shortly before euthanasia on 8 dpe. All other macaques were euthanized and necropsied at their predetermined time points. Complete postmortem examinations were performed on all macaques and gross findings were documented. Tissues were trimmed to less than 1-cm thick, placed in cassettes, and fixed for more than 72 hours in 10% neutral-buffered formalin for histopathology, immunohistochemical (IHC) assays for EBOV glycoprotein (GP) and viral protein 40 (VP40), and probed for EBOV RNA using RNAscope in situ hybridization (ISH) (catalog number 322360; Advanced Cell Diagnostics, Newark, CA).
Microscopic Examination
Microscopic examination was performed on routinely processed, paraffin-embedded, hematoxylin and eosin–stained tissue sections. The observations were scored by severity by a single American College of Veterinary Pathologists diplomate (DXL) using an ordinal category of severity of normal, minimal, mild, moderate, and severe.27
Immunohistochemistry
Immunohistochemical assays were performed on formalin-fixed, paraffin-embedded tissues, sectioned to 4-μm thick, using either primary mouse anti-EBOV matrix protein (VP40, catalog number 0201-016, 0.24 μg/mL; IBT Bioservices, Rockville, Maryland) or rabbit anti–surface GP (catalog number 0301-015, 0.36 μg/mL; IBT Bioservices) primary antibodies as previously described.28 For EBOV-VP40, the biotinylated target was detected by the VECTASTAIN ABC-AP kit (catalog number AK-5000; Vector Labs, Burlingame, California), and positive staining was visualized with ImmPACT Vector Red AP Substrate Kit chromogen (SK-5105; Vector Labs), while EBOV-GP–positive staining was visualized with 3,3′-diaminobenzidine (brown) chromogen, 3, 3' diaminobenzidine (DAB) (catalog number BDB2004L; Biocare Medical, Pacheco, California). Slides were counterstained with hematoxylin.
RNAScope In Situ Hybridization
Detection of EBOV genomic or antigenomic (replicative intermediate) RNA was performed on 4-μm sections of formalin-fixed, paraffin-embedded tissue collected at necropsy, using the manual ISH RNAscope 2.5 HD RED kit (Advanced Cell Diagnostics) according to the manufacturer's instructions and including modifications for protocol optimization validated by appropriate controls. Briefly, 20 ZZ probe pairs targeting the EBOV VP40 and VP35 genes were designed and synthesized (catalog numbers 507141 and 527491; ACD) for application to the ISH protocol. After deparaffinization with xylene, graded ethanol dehydration, and peroxidase blocking, the tissue sections were heated in Target Retrieval Solution (catalog number 322000; ACD) and enzymatically digested by Protease Plus (catalog number 323330; ACD, Newark, California). The sections then were exposed to the target probes and incubated in a humidified 40°C hybridization oven for 2 hours. After appropriate rinsing, the hybridized target signals were amplified with conjugated preamplifier and amplifier reagents, and a red substrate–chromogen solution was applied for 10 minutes at room temperature. The sections then were counterstained with hematoxylin (catalog number 7211; Richard-Allan Scientific, ThermoFisher, Waltham, MA), followed by graded ethanol dehydration, xylene clearing, and non–alcohol-based mounting medium coverslipping, as previously described.19
Results
EBOV Causes Cervicitis and Vaginitis in Females
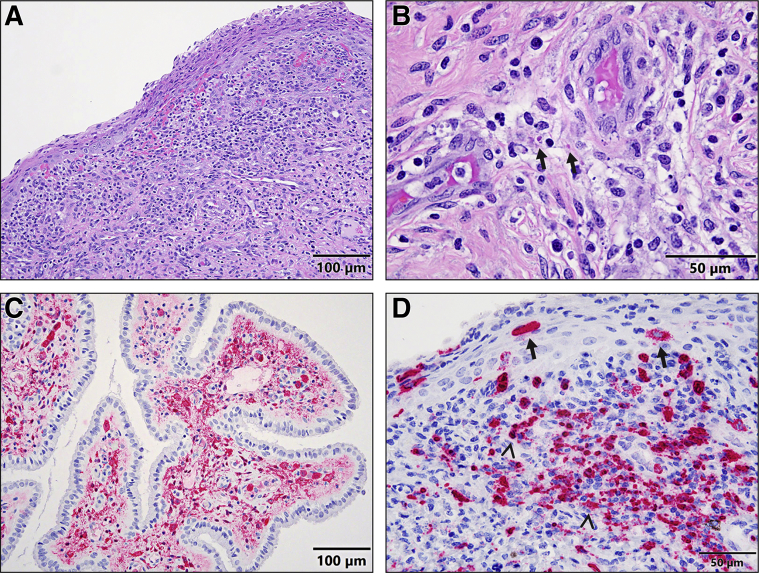
One of two D6 and three of six TC females showed multifocal acute cervicitis characterized by stromal hemorrhage, edema, and numerous degenerate neutrophils that expanded and infiltrated the lamina propria of both the ectocervix and endocervix with increased desquamation of epithelium (Figure 1A). Occasional EBOV inclusions were present in the cytoplasm of fibroblasts (Figure 1B). Many macrophages and fibroblasts in the lamina propria of both the ectocervix and endocervix (Figure 1C), as well as a few squamous epithelial cells in the ectocervix, were EBOV-VP40 and EBOV-GP antigen-positive in all D6 and TC females, as indicated by immunohistochemistry. To confirm the IHC results, EBOV genomic RNA was detected in these cell types, including squamous epithelial cells, in the cervices of three selected TC females by ISH (Figure 1D).
Figure 1.
A–D: Histopathology (hematoxylin and eosin) (A and B), immunohistochemistry (C), and RNAscope in situ hybridization (D) of cervix associated with EBOV infection. A: Acute ectocervicitis: the lamina propria is expanded and infiltrated by hemorrhage, edema, and numerous degenerate neutrophils with increased squamous epithelial desquamation in a female macaque 6 days after exposure. Scale bar: 100 μm. B: EBOV intracytoplasmic inclusion bodies (arrows) are observed in the fibroblast cells in the lamina propria from a female macaque 7 days after exposure. Scale bar: 50 μm C: Immunohistochemistry shows that many stromal cells and macrophages in the interstitium of an endocervical gland are strongly EBOV-VP40–antigen positive from a female macaque 6 days after exposure. Scale bar: 100 μm. D: RNAscope in situ hybridization confirms that many stromal cells and macrophages (arrowheads) of lamina propria and a few squamous epithelial cells (arrows) in an ectocervix are strongly EBOV–genomic RNA positive from a female macaque 6 days after exposure. Scale bar: 50 μm.
Acute hemorrhagic vaginitis was present in all D6 and TC females. Multifocally, the lamina propria was mildly to moderately expanded by perivascular edema, hemorrhage, and infiltrates of lymphocytes, plasma cells, and macrophages. Frequently, small veins were congested and/or contained many microthrombi (Figure 2A). Many macrophages and fibroblasts in the lamina propria were EBOV-VP-40- (Figure 2B) and EBOV-GP–antigen-positive (Figure 2C). In addition, vaginal squamous epithelial cells were EBOV-VP40- and EBOV-GP–antigen-positive (Figure 2C) in one of two D6 and four of six TC females. EBOV genomic RNA was detected in all of these cell types, including squamous cells, in the vaginas of three selected TC females by ISH, thereby confirming the IHC results (Figure 2D).
Figure 2.
A–D: Histopathology (hematoxylin and eosin) (A), immunohistochemistry (B and C), and RNAscope in situ hybridization (D) of vagina associated with EBOV infection. A: Acute hemorrhagic vaginitis and microthrombosis (arrows) are present in a female macaque 7 days after exposure. Scale bar: 100 μm. B and C: Immunohistochemistry shows that many submucosal macrophages and stromal cells (arrowheads) and squamous epithelial cells (arrows) are strongly EBOV-VP40–antigen (B) and EBOV-GP–antigen (C) positive from two female macaques, 6 and 8 days, respectively, after exposure. Scale bars: 100 μm and 50 μm, respectively. D: RNAscope in situ hybridization shows that many submucosal macrophages and stromal cells (arrowheads) and squamous epithelial cells (arrows) are strongly EBOV–genomic RNA positive from a female macaque 8 days after exposure. Scale bar: 50 μm.
All other findings in female reproductive organs were consistent with previous studies.15,16 Although significant histopathologic lesions associated with EBOV infection were not observed in the ovary, oviduct, and uterus, EBOV-VP40 and EBOV-GP antigens were detected in many macrophages and stromal cells in ovaries (one of one D4, and all D5, D6, and TC females), uteri (two of three D5, one of two D6, and all six TC females), and oviducts (four of six TC females). In addition, EBOV-VP40 and EBOV-GP antigens also were detected in many thecal cells and fewer granulosa cells in the ovaries (one of three D5 and all D6 and TC females) and oviductal endosalpingeal epithelial cells (two of six TC females).
EBOV Causes Posthitis, Infects Epididymal and Seminal Vesicular Tubular Epithelial Cells, and Contaminates Seminal Fluid in Males
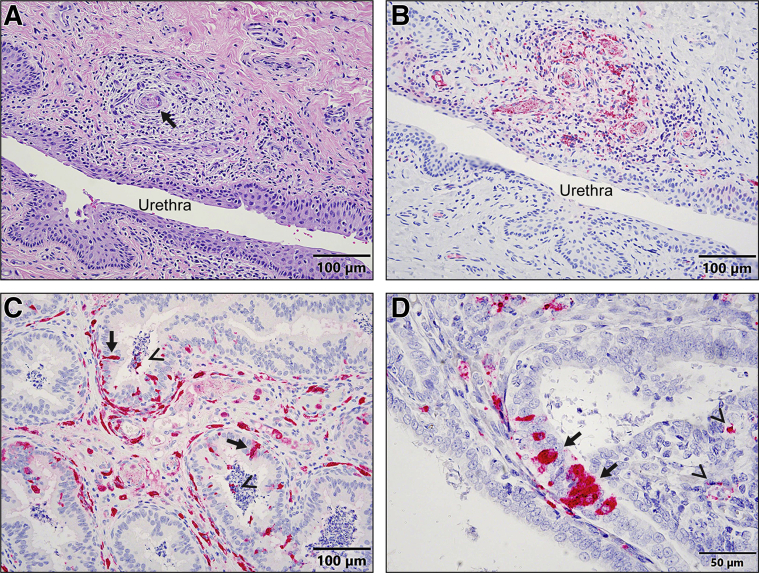
Acute, perivascular posthitis was observed in four of six TC males. Multifocally, the penile hypodermis and submucosa of the urethra were mildly to moderately expanded by perivascular edema, infiltrates of lymphocytes, plasma cells, and macrophages, with rare hemorrhages. Microthrombi were seen commonly in the lumina of the corpus cavernosum (Figure 3A). EBOV-VP40 (Figure 3B) and EBOV-GP antigen and genomic RNA were detected in the interstitial macrophages and fibroblasts in the penises in all D6 and TC males.
Figure 3.
A–D: Histopathology (hematoxylin and eosin) (A), immunohistochemistry (B and C), and RNAscope in situ hybridization (D) of male reproductive organs associated with EBOV infection. A: Acute perivascular posthitis with microthrombus (arrow) is observed in a male macaque 8 days after exposure. Scale bar: 100 μm. B: Immunohistochemistry shows that many macrophages, stromal cells, and/or circulating monocytes in the lamina propria of a penis (serial section from panel A) are strongly EBOV-VP40 positive. Scale bar: 100 μm. C: Immunohistochemistry shows that a few number (cell accounts)/amount (quanity of mixture) of epididymal tubular epithelial cells (arrows) and intraluminal mixture of secretory material and sperm (arrowheads) are EBOV-VP40–antigen positive from a male macaque 6 days after exposure. Scale bar: 100 μm. D: RNAscope in situ hybridization shows that a few number/amount of tubular epithelial cells (arrows) and intraluminal mixture of secretory material and cell debris (arrowheads) in a seminal versicle are EBOV–genomic RNA positive from a male macaque 6 days after exposure. Scale bar: 50 μm.
Acute hemorrhagic interstitial epididymitis was present in all D6 and TC males, and EBOV antigens were detected in the interstitial stromal cells and macrophages that were consistent with the previous findings.15,29 In addition, EBOV-VP40 (Figure 3C) and EBOV-GP antigen and genomic RNA (Figure 3D) were detected in some epididymal and seminal vesicular tubular epithelial cells, as well as intraluminal mixtures of sloughed cell debris and sperm or secreted material in the epididymis in two of six TC males and in the seminal vesicles in three of six TC males by IHC or ISH.
EBOV Infects Chromaffin Cells and Causes Medullary Adrenalitis
Acute medullary adrenalitis was present in two of three D5, two of three D6, and all TC macaques. Histopathologically, the adrenal medullae were infiltrated multifocally by variable numbers of lymphocytes, plasma cells, and macrophages admixed with congestion and hemorrhage. Chromaffin cells showed mild to moderate degeneration and necrosis characterized by cytoplasmic swelling with loss of granules and/or eosinophilic cytoplasm with pyknotic nuclei (Figure 4A). EBOV-VP40 (Figure 4B) and EBOV-GP (Figure 4C) antigens were detected in many chromaffin cells in the medulla in all D5, D6, and TC macaques, as well as interstitial stromal cells and macrophages. To confirm IHC results, adrenal glands from four TC monkeys were examined by ISH. EBOV genomic RNA was detected in the same types of cells, including chromaffin cells, in all examined adrenal glands (Figure 4D).
Figure 4.
A–D: Histopathology (hematoxylin and eosin) (A), immunohistochemistry (B and C), and RNAscope in situ hybridization (D) of the adrenal medulla from EBOV-infected macaques. A: A macaque 7 days after exposure shows acute medullary adrenalitis characterized by hemorrhage and many lymphoplasmacytic and histiocytic infiltrates in the adrenal medulla. Scale bar: 50 μm. B and C: Immunohistochemistry shows that many chromaffin cells (arrows) in the medulla are EBOV-VP40–antigen (B) and EBOV-GP–antigen (C) positive from two macaques, 6 and 7 days, respectively, after exposure. Scale bars: 50 μm. D: RNAscope in situ hybridization confirms that many chromaffin cells (arrows) in the adrenal medulla are EBOV–genomic RNA positive. Scale bar = 50 μm.
Other findings in adrenal glands were consistent with previous reports.16,17,30, 31, 32 Adrenal cortices showed multifocal, mild to moderate cortical cell degeneration, and necrosis admixed with hemorrhage and lymphoplasmacytic and histiocytic infiltration, especially in the zona fasciculata and reticularis, in all D5, D6, and two TC macaques. Occasionally, viral intracytoplasmic inclusions were observed in cortical cells. Immunohistochemistry and/or ISH showed that cortical endocrine cells and interstitial macrophages were EBOV antigens and genomic RNA-positive as early as 4 dpe (one of three D4 monkeys).
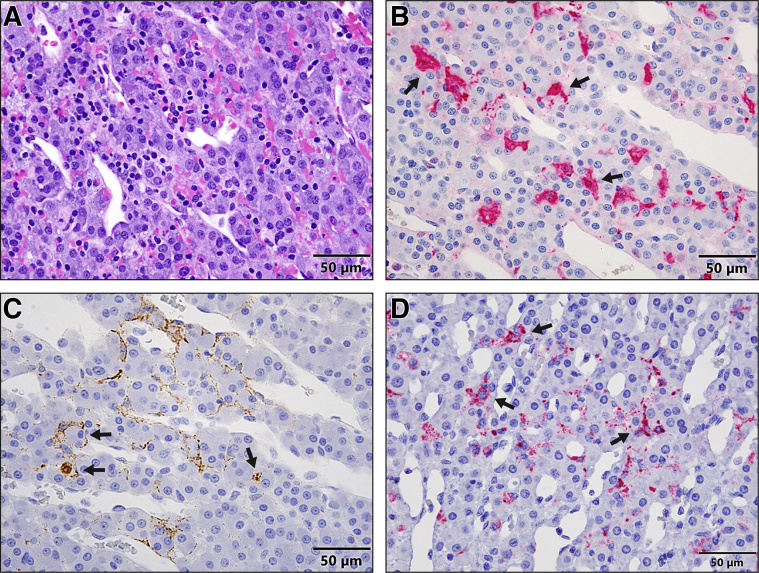
EBOV Infects and Replicates in Kupffer Cells and Hepatocytes in the Liver as Early as dpe 3
Hepatomegaly with rounded edges and friable texture was first noticed at necropsy in one of three D3 monkeys (Figure 5A). Histologically, minimal to mild Kupffer cell and hepatocyte degeneration and necrosis with intracytoplasmic inclusion bodies were present in all D3 monkeys (Figure 5B). Immunohistochemistry showed that many Kupffer cells and rare hepatocytes were EBOV-VP40– (Figure 5C) and EBOV-GP–antigen positive. Both genomic and antigenomic RNAs (Figure 5D) detected in both Kupffer cells and hepatocytes at all D3 monkeys by ISH confirmed the time of EBOV replication in the liver. Other findings in the liver at 4 dpe and thereafter were consistent with previous studies.3,18,24
Figure 5.
A–D: Gross pathology (A), histopathology (hematoxylin and eosin) (B), immunohistochemistry (C), and RNAscope in situ hybridization (D) in the liver of rhesus macaques 3 days after exposure to EBOV-Kikwit. A: Grossly, a liver from a macaque shows diffuse, moderate hepatomegaly with rounded edges and friable texture. B: Histopathologically, the liver from a macaque presents with early signs of hepatocellular degeneration (arrowheads). Many degenerate Kupffer cells contain intracytoplasmic, eosinophilic inclusion bodies (hematoxylin and eosin) (arrows). Scale bar: 50 μm. C: Immunohistochemistry shows that many Kupffer cells (arrows) and rare hepatocytes (arrowheads) are EBOV-VP40–antigen positive. Scale bar: 50 μm. D: RNAscope in situ hybridization shows that many Kupffer cells (arrows) and rare hepatocytes (arrowheads) are EBOV–anti–genomic RNA (replicative intermediate) positive. Scale bar = 50 μm.
Discussion
Nonhuman primates have proven to be excellent models for EBOV infection, closely mimicking the clinical manifestations of filovirus infections in humans.3,16,18,23 Generally, the pathology and immunohistochemistry findings in the current study were similar to those described in previous EBOV natural history studies in the rhesus monkey model.3,4,18,23,24 However, not only did this study reconfirm the most common pathology findings by time course and order of progression, herein, it also presented significant new pathology findings and virus tropism, as well as the earliest reported time of viral replication in the liver.
Sexual transmission from human EVD male survivors was reported recently, and is thought to be the initiating cause of the 2021 EBOV outbreak in Guinea and in the Democratic Republic of the Congo.11,14,33,34 EBOV genomic RNA has been detected in seminal fluid for more than 2 years after recovery from acute EVD in human patients,13 and in the testes at 43 dpe in the rhesus.35 Although seminal contamination by shedding of EBOV is hypothesized36 based on RT-PCR–positive seminal fluid, the mechanism of virus entry into seminal fluid is unknown. The detection of EBOV in submucosal macrophages; stromal cells; and epididymal, prostate glandular, and seminal vesicular epithelial cells in male reproductive organs has been reported in previous studies15,30 and in the current study. In addition, for the first time, this study provides direct evidence that EBOV can directly contaminate male genital fluid in acute EVD monkeys. These data support the shedding of infected epithelial cells being a potential source of EBOV contamination in male genital fluid in a NHP model. However, whether this is also the case in EVD human patients is unknown, owing to the scarcity of tissue samples from EVD patients/decedents.
EBOV can also be detected by PCR in vaginal fluid upto 3 weeks after the end of viremia,37,38 although sexual transmission from EVD female survivors has not been confirmed.12 Similar to the male reproductive organs of infected macaques, EBOV has been identified in the stromal cells and macrophages in female reproductive organs, such as the ovary and uterus, in NHPs.15,16 However, this study shows, for the first time, direct evidence that EBOV may contaminate female genital fluid through the shedding of infected cervical and vaginal squamous cells in the NHP model. Recently, EBOV was also detected in the vaginal stratified squamous epithelial cells in infected guinea pigs.19 Zika virus (a single strand RNA virus in the family Flaviviridae) was localized in the cervical epithelium in a Zika patient 11 days after the onset of symptoms, and in the vaginal epithelium in mice 18 days after Zika virus infection.39,40 These data suggest that EBOV potentially can be sexually transmitted through female EVD survivors.
It is well known that EBOV can infect adrenal cortical cells in NHPs.3,18,31 Recently, EBOV antigen was detected in the adrenal medulla of three cynomolgus monkeys, but the cell types were not specified.41 Herein, this study specified that EBOV can infect not only chromaffin cells, but it also can cause medullary adrenalitis. Although patients with acute EVD can develop neurologic symptoms, there is little information on endocrine disorders in EVD patients.42,43 Medullary adrenalitis associated with Marburg virus infection has been reported in rhesus monkeys and human patients.44,45 Recently, EBOV infection was observed in some chromaffin cells in a guinea pig model.19 These data suggest direct infection of chromaffin cells may cause neuroendocrine dysfunction in EVD patients.
The liver is one of the major and early target organs for EBOV and has been well studied.3,18,24,30,46 Previous NHP models with time point sacrifice studies show that there are no significant hepatic lesions upto 4 dpe and after,3,4,16,18 although rare viral antigen and genomic RNA are detectable in liver at 3 dpe.3,20 However, the presence of viral genomic RNA is not sufficient to show viral replication in the tissues because viruses may be present as a result of contamination from plasma and/or infected circulating monocytes.21,22 Herein, not only did this study present gross and histopathology lesions with typical intracytoplasmic EBOV inclusion bodies in liver as early as dpe 3, it also detected EBOV antigens, genomic RNA, and, most importantly, replicative intermediate (antigenome) RNA in both hepatocytes and Kupffer cells. These results strongly suggest that liver is one of earliest virus-replicating sites in EVD patients.
In summary, the pathology findings in the current study may shed light on the pathogenesis of sexual transmission in EVD survivors and warrants further study of the pathogenesis EVD. These data suggest that EBOV can cause neuroendocrine dysfunction and liver damage in acute EVD patients.
Acknowledgments
We thank Drs. Connie Schmaljohn and Travis Warren for supporting this study, Drs. Ian Crozier and Jens Kuhn for the comments on the manuscript, and Jiro Wada for preparation of the figures.
Footnotes
Supported in part by Battelle Memorial Institute's former contract with the National Institute of Allergy and Infectious Diseases (HHSN272200700016I) and Laulima Government Solutions, LLC, current contract with the National Institute of Allergy and Infectious Diseases (HHSN272201800013C).
Disclosures: D.X.L., T.K.C., D.L.P., L.M.H., A.M.W.H., R.J.H., N.I., R.B., D.R., K.C., R.R., J.L., M.R.H., and R.S.B. performed this work as former employees of Battelle Memorial Institute and current employees of Laulima Government Solutions, LLC, contract HHSN272201800013C.
Current address of J.L., Department of Microbiology and Immunology, University of Maryland School of Medicine, Baltimore, MD; of R.S.B., AbViro LLC, Bethesda, MD.
Contributor Information
David X. Liu, Email: xianhong.liu@nih.gov.
Lisa E. Hensley, Email: lisa.hensley@nih.gov.
References
- 1.Kuhn J.H., Amarasinghe G.K., Basler C.F., Bavari S., Bukreyev A., Chandran K., Crozier I., Dolnik O., Dye J.M., Formenty P.B.H., Griffiths A., Hewson R., Kobinger G.P., Leroy E.M., Muhlberger E., Netesov SV, Palacios G., Palyi B., Paweska J.T., Smither S.J., Takada A., Towner J.S., Wahl V., Ictv Report Consortium ICTV virus taxonomy profile: filoviridae. J Gen Virol. 2019;100:911–912. doi: 10.1099/jgv.0.001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob S.T., Crozier I., Fischer W.A., 2nd, Hewlett A., Kraft C.S., Vega M.A., Soka M.J., Wahl V., Griffiths A., Bollinger L., Kuhn J.H. Ebola virus disease. Nat Rev Dis Primers. 2020;6:13. doi: 10.1038/s41572-020-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisbert T.W., Hensley L.E., Larsen T., Young H.A., Reed D.S., Geisbert J.B., Scott D.P., Kagan E., Jahrling P.B., Davis K.J. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskerville A., Bowen E.T., Platt G.S., McArdell L.B., Simpson D.I. The pathology of experimental Ebola virus infection in monkeys. J Pathol. 1978;125:131–138. doi: 10.1002/path.1711250303. [DOI] [PubMed] [Google Scholar]
- 5.Carrion R., Jr., Ro Y., Hoosien K., Ticer A., Brasky K., de la Garza M., Mansfield K., Patterson J.L. A small nonhuman primate model for filovirus-induced disease. Virology. 2011;420:117–124. doi: 10.1016/j.virol.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baseler L., Chertow D.S., Johnson K.M., Feldmann H., Morens D.M. The pathogenesis of Ebola virus disease. Annu Rev Pathol. 2017;12:387–418. doi: 10.1146/annurev-pathol-052016-100506. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez A., Geisbert T.W., Feldmann H. Fields Virology. ed 5. Lippincott Williams and Wilkins; Philadelphia: 2007. Marburg and Ebola Viruses. [Google Scholar]
- 8.Christie A., Davies-Wayne G.J., Cordier-Lassalle T., Blackley D.J., Laney A.S., Williams D.E., Shinde S.A., Badio M., Lo T., Mate S.E., Ladner J.T., Wiley M.R., Kugelman J.R., Palacios G., Holbrook M.R., Janosko K.B., de Wit E., van Doremalen N., Munster V.J., Pettitt J., Schoepp R.J., Verhenne L., Evlampidou I., Kollie K.K., Sieh S.B., Gasasira A., Bolay F., Kateh F.N., Nyenswah T.G., De Cock K.M., Centers for Disease Control and Prevention Possible sexual transmission of Ebola virus - Liberia, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64:479–481. [PMC free article] [PubMed] [Google Scholar]
- 9.Diallo B., Sissoko D., Loman N.J., Bah H.A., Bah H., Worrell M.C., Conde L.S., Sacko R., Mesfin S., Loua A., Kalonda J.K., Erondu N.A., Dahl B.A., Handrick S., Goodfellow I., Meredith L.W., Cotten M., Jah U., Guetiya Wadoum R.E., Rollin P., Magassouba N., Malvy D., Anglaret X., Carroll M.W., Aylward R.B., Djingarey M.H., Diarra A., Formenty P., Keita S., Gunther S., Rambaut A., Duraffour S. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis. 2016;63:1353–1356. doi: 10.1093/cid/ciw601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mate S.E., Kugelman J.R., Nyenswah T.G., Ladner J.T., Wiley M.R., Cordier-Lassalle T., et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med. 2015;373:2448–2454. doi: 10.1056/NEJMoa1509773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupferschmidt K. Ebola virus may lurk in survivors for many years. Science. 2021;371:1188. doi: 10.1126/science.371.6535.1188. [DOI] [PubMed] [Google Scholar]
- 12.Dokubo E.K., Wendland A., Mate S.E., Ladner J.T., Hamblion E.L., Raftery P., et al. Persistence of Ebola virus after the end of widespread transmission in Liberia: an outbreak report. Lancet Infect Dis. 2018;18:1015–1024. doi: 10.1016/S1473-3099(18)30417-1. [DOI] [PubMed] [Google Scholar]
- 13.Fischer W.A., Brown J., Wohl D.A., Loftis A.J., Tozay S., Reeves E., Pewu K., Gorvego G., Quellie S., Cunningham C.K., Merenbloom C., Napravnik S., Dube K., Adjasoo D., Jones E., Bonarwolo K., Hoover D. Ebola virus ribonucleic acid detection in semen more than two years after resolution of acute Ebola virus infection. Open Forum Infect Dis. 2017;4:ofx155. doi: 10.1093/ofid/ofx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keita A.K., Koundouno F.R., Faye M., Düx A., Hinzmann J., Diallo H., et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature. 2021;597:539–543. doi: 10.1038/s41586-021-03901-9. [DOI] [PubMed] [Google Scholar]
- 15.Perry D.L., Huzella L.M., Bernbaum J.G., Holbrook M.R., Jahrling P.B., Hagen K.R., Schnell M.J., Johnson R.F. Ebola virus localization in the macaque reproductive tract during acute Ebola virus disease. Am J Pathol. 2018;188:550–558. doi: 10.1016/j.ajpath.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaax N.K., Davis K.J., Geisbert T.J., Vogel P., Jaax G.P., Topper M., Jahrling P.B. Lethal experimental infection of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival route of exposure. Arch Pathol Lab Med. 1996;120:140–155. [PubMed] [Google Scholar]
- 17.Geisbert T.W., Young H.A., Jahrling P.B., Davis K.J., Larsen T., Kagan E., Hensley L.E. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163:2371–2382. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twenhafel N.A., Mattix M.E., Johnson J.C., Robinson C.G., Pratt W.D., Cashman K.A., Wahl-Jensen V., Terry C., Olinger G.G., Hensley L.E., Honko A.N. Pathology of experimental aerosol Zaire ebolavirus infection in rhesus macaques. Vet Pathol. 2013;50:514–529. doi: 10.1177/0300985812469636. [DOI] [PubMed] [Google Scholar]
- 19.Cooper T.K., Huzella L., Johnson J.C., Rojas O., Yellayi S., Sun M.G., Bavari S., Bonilla A., Hart R., Jahrling P.B., Kuhn J.H., Zeng X. Histology, immunohistochemistry, and in situ hybridization reveal overlooked Ebola virus target tissues in the Ebola virus disease guinea pig model. Sci Rep. 2018;8:1250. doi: 10.1038/s41598-018-19638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg A., Huber B.R., Liu D.X., Logue J.P., Hischak A.M.W., Hart R.J., Abbott M., Isic N., Hisada Y.M., Mackman N., Bennett R.S., Hensley L.E., Connor J.H., Crossland N.A. Quantification of viral and host biomarkers in the liver of rhesus macaques: a longitudinal study of Zaire Ebolavirus strain Kikwit (EBOV/Kik) Am J Pathol. 2020;190:1449–1460. doi: 10.1016/j.ajpath.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanford R.E., Chavez D., Chisari F.V., Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G., Li K., Lea A.S., Li N.L., Abdulla N.E., Eltorky M.A., Ferguson M.R. In situ hybridization for the detection of hepatitis C virus RNA in human liver tissue. J Viral Hepat. 2013;20:183–192. doi: 10.1111/j.1365-2893.2012.01642.x. [DOI] [PubMed] [Google Scholar]
- 23.Jahrling P.B., Geisbert J.B., Swearengen J.R., Larsen T., Geisbert T.W. Ebola hemorrhagic fever: evaluation of passive immunotherapy in nonhuman primates. J Infect Dis. 2007;196(Suppl):S400–S403. doi: 10.1086/520587. [DOI] [PubMed] [Google Scholar]
- 24.Warren T., Zumbrun E., Weidner J.M., Gomba L., Rossi F., Bannister R., Tarrant J., Reed M., Lee E., Raymond J.L., Wells J., Shamblin J., Wetzel K., Donnelly G., Van Tongeren S., Lackemeyer N., Steffens J., Kimmel A., Garvey C., Bloomfield H., Blair C., Singh B., Bavari S., Cihlar T., Porter D. Characterization of Ebola virus disease (EVD) in rhesus monkeys for development of EVD therapeutics. Viruses. 2020;12:92. doi: 10.3390/v12010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett R.S., Logue J., Liu D.X., Reeder R.J., Janosko K.B., Perry D.L., Cooper T.K., Byrum R., Ragland D., St Claire M., Adams R., Burdette T.L., Brady T.M., Hadley K., Waters M.C., Shim R., Dowling W., Qin J., Crozier I., Jahrling P.B., Hensley L.E. Kikwit Ebola virus disease progression in the rhesus monkey animal model. Viruses. 2020;12:753. doi: 10.3390/v12070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council: Guide for the Care and Use of Laboratory Animals. Eighth Edition. National Academies Press; Washington, DC: 2011. [Google Scholar]
- 27.Gibson-Corley K.N., Olivier A.K., Meyerholz D.K. Principles for valid histopathologic scoring in research. Vet Pathol. 2013;50:1007–1015. doi: 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D.X., Perry D.L., Cooper T.K., Huzella L.M., Hart R.J., Hischak A.M.W., Bernbaum J.G., Hensley L.E., Bennett R.S. Peripheral neuronopathy associated with Ebola virus infection in rhesus macaques: a possible cause of neurological signs and symptoms in human Ebola patients. J Infect Dis. 2020;222:1745–1755. doi: 10.1093/infdis/jiaa304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskerville A., Fisher-Hoch S.P., Neild G.H., Dowsett A.B. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J Pathol. 1985;147:199–209. doi: 10.1002/path.1711470308. [DOI] [PubMed] [Google Scholar]
- 30.Davis K.J., Anderson A.O., Geisbert T.W., Steele K.E., Geisbert J.B., Vogel P., Connolly B.M., Huggins J.W., Jahrling P.B., Jaax N.K. Pathology of experimental Ebola virus infection in African green monkeys. Involvement of fibroblastic reticular cells. Arch Pathol Lab Med. 1997;121:805–819. [PubMed] [Google Scholar]
- 31.Johnson E., Jaax N., White J., Jahrling P. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int J Exp Pathol. 1995;76:227–236. [PMC free article] [PubMed] [Google Scholar]
- 32.Steele K., Crise B., Kuehne A., Kell W. Ebola virus glycoprotein demonstrates differential cellular localization in infected cell types of nonhuman primates and guinea pigs. Arch Pathol Lab Med. 2001;125:625–630. doi: 10.5858/2001-125-0625-EVGDDC. [DOI] [PubMed] [Google Scholar]
- 33.Thorson A.E., Deen G.F., Bernstein K.T., Liu W.J., Yamba F., Habib N., Sesay F.R., Gaillard P., Massaquoi T.A., McDonald S.L.R., Zhang Y., Durski K.N., Singaravelu S., Ervin E., Liu H., Coursier A., Marrinan J.E., Ariyarajah A., Carino M., Formenty P., Stroher U., Lamunu M., Wu G., Sahr F., Xu W., Knust B., Broutet N. Sierra Leone Ebola Virus Persistence Study Group: Persistence of Ebola virus in semen among Ebola virus disease survivors in Sierra Leone: a cohort study of frequency, duration, and risk factors. PLoS Med. 2021;18:e1003273. doi: 10.1371/journal.pmed.1003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tompkins K., Brown J., Tozay S., Reeves E., Pewu K., Johnson H., Williams G., Conneh T., Diggs J., DeMarco J., King K., McMillian D., Merenbloom C., Fischer W., Wohl D.A. The impact of semen testing for Ebola virus RNA on sexual behavior of male Ebola survivors in Liberia. PLoS Negl Trop Dis. 2020;14:e0008556. doi: 10.1371/journal.pntd.0008556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng X., Blancett C.D., Koistinen K.A., Schellhase C.W., Bearss J.J., Radoshitzky S.R., Honnold S.P., Chance T.B., Warren T.K., Froude J.W., Cashman K.A., Dye J.M., Bavari S., Palacios G., Kuhn J.H., Sun M.G. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat Microbiol. 2017;2:17113. doi: 10.1038/nmicrobiol.2017.113. [DOI] [PubMed] [Google Scholar]
- 36.Vetter P., Fischer W.A., 2nd, Schibler M., Jacobs M., Bausch D.G., Kaiser L. Ebola virus shedding and transmission: review of current evidence. J Infect Dis. 2016;214:S177–S184. doi: 10.1093/infdis/jiw254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez L.L., De Roo A., Guimard Y., Trappier S.G., Sanchez A., Bressler D., Williams A.J., Rowe A.K., Bertolli J., Khan A.S., Ksiazek T.G., Peters C.J., Nichol S.T. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl):S170–S176. doi: 10.1086/514291. [DOI] [PubMed] [Google Scholar]
- 38.Liu W.J., Sesay F.R., Coursier A., Knust B., Marrinan J.E., Whitmer S., McDonald S.L.R., Gaillard P., Liu Y., Su Q., Zhang Y., Crozier I., Ariyarajah A., Carino M., Massaquoi T., Broutet N., Xu W., Wu G., Stroher U., Gao G.F., Formenty P., Sahr F., Deen G.F. Sierra Leone Ebola Virus Persistence Study Group: Comprehensive clinical and laboratory follow-up of a female patient with Ebola virus disease: Sierra Leone Ebola Virus Persistence Study. Open Forum Infect Dis. 2019;6:ofz068. doi: 10.1093/ofid/ofz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prisant N., Bujan L., Benichou H., Hayot P.H., Pavili L., Lurel S., Herrmann C., Janky E., Joguet G. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000–1001. doi: 10.1016/S1473-3099(16)30193-1. [DOI] [PubMed] [Google Scholar]
- 40.Tang W.W., Young M.P., Mamidi A., Regla-Nava J.A., Kim K., Shresta S. A mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17:3091–3098. doi: 10.1016/j.celrep.2016.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cross R.W., Xu R., Matassov D., Hamm S., Latham T.E., Gerardi C.S., Nowak R.M., Geisbert J.B., Ota-Setlik A., Agans K.N., Luckay A., Witko S.E., Soukieh L., Deer D.J., Mire C.E., Feldmann H., Happi C., Fenton K.A., Eldridge J.H., Geisbert T.W. Quadrivalent VesiculoVax vaccine protects nonhuman primates from viral-induced hemorrhagic fever and death. J Clin Invest. 2020;130:539–551. doi: 10.1172/JCI131958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chertow D.S., Nath A., Suffredini A.F., Danner R.L., Reich D.S., Bishop R.J., Childs R.W., Arai A.E., Palmore T.N., Lane H.C., Fauci A.S., Davey R.T. Severe meningoencephalitis in a case of Ebola virus disease: a case report. Ann Intern Med. 2016;165:301–304. doi: 10.7326/M15-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasri S., Wiwanitkit V. Ebola infection and diabetes mellitus. Iran J Pathol. 2016;11:191–193. [PMC free article] [PubMed] [Google Scholar]
- 44.Geisbert T.W., Daddario-DiCaprio K.M., Geisbert J.B., Young H.A., Formenty P., Fritz E.A., Larsen T., Hensley L.E. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2. J Infect Dis. 2007;196(Suppl):S372–S381. doi: 10.1086/520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geisbert T.W., Jaax N.K. Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct Pathol. 1998;22:3–17. doi: 10.3109/01913129809032253. [DOI] [PubMed] [Google Scholar]
- 46.Jahrling P.B., Geisbert T.W., Jaax N.K., Hanes M.A., Ksiazek T.G., Peters C.J. Experimental infection of cynomolgus macaques with Ebola-Reston filoviruses from the 1989-1990 U.S. epizootic. Arch Virol Suppl. 1996;11:115–134. doi: 10.1007/978-3-7091-7482-1_11. [DOI] [PubMed] [Google Scholar]