Abstract
Background:
Healthcare Workers (HCWs) are a key element in managing the COVID-19 pandemic, but they are also at high risk of infection.
Objective:
The aim of this study was to describe, in a large university hospital which provided healthcare services to patients with SARS-CoV-2 infection, the course of the epidemic among HCWs and effectiveness of COVID-19 vaccination in reducing SARS-CoV-2 infection and disease.
Methods:
Our case series included all “Fatebenefratelli Sacco” University Hospital workers. Data were collected until the 15th of May 2021 and analysed as part of the health surveillance program carried out by the Occupational Health Unit.
Results:
From March 2020 until May 2021, 14.4% of workers contracted COVID-19, with the highest incidence peak recorded during the second wave of the pandemic. The prevalence of infection was slightly higher in males than in females, and a greater number of cases was found in job categories characterized by direct patient care activities. We reported a higher prevalence of “serious/critical illness” in elder workers. A clear reduction of COVID-19 incidence was found in our population during the third pandemic wave, that coincided with the start of vaccination campaign.
Discussion:
HCWs have been at high risk of COVID-19 infection. Male sex and advanced age appear to be predisposing factor and negative prognostic factor respectively. An out-of-hospital setting appears to be the main source of COVID-19 confirming that the correct use of protective devices during work counters the risk of infection. Vaccination seems to reduce both documented cases of infection and severe illness.
Keywords: SARS-CoV-2, healthcare workforce, occupational medicine, health surveillance, BNT162b2 mRNA Vaccine
Introduction
On December 31st, 2019, Chinese health authorities reported an outbreak of pneumonia of unknown aetiology in the city of Wuhan (Hubei Province), China. On January 9th, 2020, the China CDC (China’s Centres for Disease Control and Prevention) identified a new coronavirus (provisionally called 2019-nCoV and then called SARS-CoV-2) as an etiological agent of this disease, called COVID-19 (1). On the 22nd of January 2020, the WHO reported in its communications that there was evidence of human to human transmission of the new virus. This led to the start of the alert phase and a national task force to counter SARS-CoV-2 infection was set up also in Italy by the Minister of Health. On March 11, 2020 the WHO declared COVID-19 a pandemic (2). As of May 2021, about 160 million cases of COVID-19 and more than 3 million deaths have been reported worldwide (3). In Italy, after the first two Italian cases of COVID-19 infection reported on January 30, 2020, starting from February 21, 2020, several outbreaks of infection have been detected (4). The evolution of the COVID-19 epidemic in Italy, until today, recognizes three main waves. The first wave of infection, from February 2020 to July 2020, was followed by a second increase in infections and deaths (second wave) from August 2020 to December 2020, and finally a third wave which from January 2021 is still ongoing. Overall, in Italy, from February 2020 to May 2021, COVID-19 disease was diagnosed in more than 4 million people and caused about 124,000 deaths (5). Healthcare workers (HCWs) are a key element in managing this COVID-19 pandemic but they are also at high risk of infection and a source of transmission for patients and other staff (6,7).
On December 27, 2020 the “Vaccine day” was held throughout Italy as well as throughout Europe, marking the “symbolic” start of the COVID-19 vaccination campaign. The nationwide vaccination campaign was therefore launched, initially aimed at the higher risk categories, including HCWs (8). BNT162b2 mRNA COVID-19 vaccine was chosen for higher risk categories vaccination by the Italian Minister of Health. Recent population studies suggested that BNT162b2 mRNA COVID-19 vaccine is effective for a wide range of COVID-19–related outcomes like documented infection, symptomatic COVID-19, hospitalization and COVID-19 severe disease (9).
The aim of this study was to describe, in a large University Hospital which provided healthcare services to patients with SARS-CoV-2 infection, the course of the epidemic of SARS-CoV-2 among HCWs, the sources of their infection, the severity of clinical symptoms, and the effectiveness of COVID-19 vaccination in reducing SARS-CoV-2 infection and disease.
Methods
Fatebenefratelli-Sacco University Hospital is part of the Italian public healthcare system and is composed of four Hospital Centers (Sacco, Fatebenefratelli, Macedonio Melloni, Buzzi) and several Territorial Outpatient Units in Milan. The University Hospital employs 5605 workers. It also has an agreement with the University of Milan, and its departments and clinics are attended by numerous students and resident doctors. During the COVID-19 pandemic there was a progressive change in clinical activities within hospital wards: in relation to the local epidemiological trend of COVID-19 cases, an increasing number of beds were made available by converting various departments (internal medicine, surgery, etc.) into COVID-19 units which provided three levels of care: intensive care, subintensive care and regular care. These changes initially involved the Sacco Hospital, that is one of the reference hospitals for the treatment of infectious diseases in Italy, and then the other hospital centers. All HCWs involved in COVID Units were first properly informed, educated and trained about the correct use of personal protective equipment (PPEs) and compliance with the anti-contagion rules. Adequate information and training on the rules to combat the spread of SARS-CoV-2 infection was also given both to HCWs operating in COVID-free areas and administrative and other non-sanitary workers.
Since the beginning of the pandemic, the Occupational Health Unit has carried out an intense activity in contact tracing and management of SARS-CoV-2 positive HCWs, following the national and regional legislative directives. After the recognition of a positive molecular nasopharyngeal swab (NPS; in HCWs as well as in patients) the Occupational Unit, in collaboration with Epidemiological Office, performed a survey to identify close contacts of this confirmed SARS-CoV-2 case. A “close contact” was defined as a person who had a face-to-face contact or who spent at least 15 min in an indoor environment with a COVID- 19 patient, without wearing a personal protective device (PPE, surgical mask, etc.) and at a distance of less than two meters. According to national and regional legislative directives, all close contacts underwent an initial NPS. Symptomatic close contacts were quarantined at home pending the NPS result, while asymptomatic ones remained at work. The close contacts with negative NPS result continued to work monitoring their symptoms for at least 2 weeks and underwent other NPS in the middle and at the end of the clinical monitoring period. HCWs with SARS-CoV-2 infection were isolated at home or hospitalized in relation to the severity of the illness. SARS-CoV-2-positive workers were considered virus-free after the resolution of respiratory infection symptoms and negative control NPS for SARS-CoV-2: according to directives of the Italian Minister of Health, until October 2020 negative result of two consecutive NPS were required to define the subject’s recovery, subsequently only a single negative NPS was needed.
From December 28, 2020 all physicians of the Occupational Unit have also been actively involved in the anti-COVID-19 vaccination of all workers of the Fatebenefratelli-Sacco University Hospital. The vaccine used was BNT162b2 mRNA COVID-19 vaccine (Pfizer) and it was administered according to a two-dose schedule (21-day interval).
Our case series included all workers of the Fatebenefratelli-Sacco University Hospital. Data were collected from the beginning of the pandemic until the 15th of May 2021 and analyzed as part of the health surveillance program carried out by the Occupational Health Unit. Data were also compared with COVID-19 infection incidence data in the Italian and Milan populations, available at: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati (10) and at: https://statistichecoronavirus.it/coronavirus-italia/coronavirus-lombardia/coronavirus-milano/ (11), respectively. These data collected during the third wave of the pandemic - which coincided with the start of the vaccination campaign – were also used as an indirect and approximate measure of COVID-19 vaccine effectiveness. Furthermore, to estimate vaccine effectiveness, we calculated COVID-19 cumulative incidence and odds ratio of infection during a period of 90 days comparing two groups of workers: vaccinated group (n. 2037 who received vaccine second dose by January 31, 2021) and unvaccinated group (n. 441, who were still unvaccinated at the end of the 90-days monitoring period). Workers previously affected by COVID-19 were excluded. The beginning of the 90-days monitoring period coincided with the eighth day after administration of the second dose of vaccine for subjects included in the vaccine group; for unvaccinated subjects the beginning of the monitoring period was considered coinciding with the end of recruitment period of vaccinated ones (January 31, 2021). The choice of a 90-days monitoring period was driven by the need to acquire data for a reasonable period of time while maintaining a sufficient number of subjects in the unvaccinated group. The monitoring periods for the two groups, although chosen according to different criteria, mostly overlap.
All data showed in our study were expressed as absolute number, percentage and/or mean ± SD. We have tested associations between categorical variables using Pearson’s Chi-square test and between continuous variables with the student’s t-test. A p-value < 0.05 was considered significant. Descriptive analysis was performed for vaccinated vs unvaccinated subjects, in terms of age, female sex and job category. Between-group differences for the continuous variables were analysed by the student’s t-test while categorical variables were analysed by Pearson’s Chi-square test. Tests were two tailed, with significance set at a p-value of 0.05. The crude odds ratio (OR) and the adjusted odds ratio (aOR) were calculated using univariate and multivariate logistic regression analysis adjusted for potential confounding factors (age, gender, job category). Given the high potential for confounding by monitoring time, we stratified analysis to mitigate confounding by this covariate. All analyses were performed using counts of unique cases. Statistical analysis was performed with Microsoft Excel (Microsoft Office vers. 2003) and R-Studio (R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/).
Results
Fatebenefratelli-Sacco University Hospital employed a total of 5605 workers. As shown in Table 1, out of all workers, 72.6% were female and the mean age was 44.6 ± 11.9 years old, with a higher prevalence of workers over the age of 50 (30.2%) compared to other age groups. 31.3% of workers were nurses (n. 1754), 20.6% were physicians (n. 1154), and 9% were nursing assistants (n. 507). Job category “other health professional” included radiology technicians, laboratory technicians, biologists and other health professionals not included in the main groups reported: they represented 16.4% of workers (n. 918). Non sanitary workers (including technical and administrative staff) count about 16.6 % of all workers (n. 932). Lastly, in connection with the agreement with the University of Milan, hospital departments and clinics were attended by about 340 resident doctors (6.1% of total workers).
Table 1.
Characteristics of the study population, COVID-19 prevalence and clinical characteristics of infection: data stratified by job category
| All workers | All NPS + cases | Physicians (n. 1154) NPS+ cases | Nurses (n. 1754) NPS+ cases | Nursing Assistant (n. 507) NPS+ cases | Other health professionals (n. 918) NPS+ cases | Non Sanitary workers (n. 932) NPS+ cases | Resident doctors (n. 340) NPS+ cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | |
| Total | 5605 | 100 | 805 | 14.4 | 169 | 14.6 (§) | 315 | 17.9 (†) | 96 | 18.9 (‡) | 102 | 11.1 | 79 | 8.5 | 44 | 12.9 |
| Gender | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % |
| Females | 4072 | 72.6 | 548 | 68.1 | 92 | 54.4 | 235 | 74.6 | 67 | 69.8 | 80 | 78.4 | 45 | 57 | 29 | 65.9 |
| Males | 1533 | 27.4 | 257 | 31.9 | 77 | 45.6 | 80 | 25.4 | 29 | 30.2 | 22 | 21.6 | 34 | 43 | 15 | 34.1 |
| Age Group | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % |
| 18-30 31-40 41-50 51-60 >60 |
1023 1144 1303 1692 443 |
18.2 20.4 23.2 30.2 8 |
123 174 202 255 51 |
12 15.2 15.5 15.1 11.5 |
1 41 48 54 25 |
0.6 24.3 28.4 31.9 14.8 |
54 74 81 99 7 |
17.1 23.5 25.7 31.4 2.3 |
10 19 28 33 6 |
10.4 19.8 29.2 34.4 6.2 |
20 19 28 28 7 |
19.6 18.6 27.4 27.4 7 |
6 9 17 41 6 |
7.6 11.4 21.5 51.9 7.6 |
32 12 0 0 0 |
72.7 27.3 0 0 0 |
| Source of infection | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % |
| In-hospital | - | - | 175 | 21.7 | 35 | 20.7 (*) | 86 | 27.3 (*) | 18 | 18.7 (*) | 19 | 18.6 (*) | 6 | 7.6 | 11 | 25 (*) |
| Out-of-hospital | - | - | 270 | 33.5 | 53 | 31.4 | 99 | 31.4 | 25 | 26 | 43 | 42.1 | 37 | 46.8 | 13 | 29.5 |
| Unknown | - | - | 360 | 44.8 | 81 | 47.9 | 130 | 41.3 | 53 | 55.3 | 40 | 39.3 | 36 | 45.6 | 20 | 45.5 |
| Clinical course | - | - | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % | n. | % |
| Asymptomatics | - | - | 235 | 29.2 | 62 | 36.7 | 80 | 25.4 | 20 | 20.8 | 35 | 34.3 | 20 | 25.3 | 18 | 40.9 |
| Mild conditions | - | - | 514 | 63.4 | 90 | 53.2 | 212 | 67.3 | 66 | 68.7 | 65 | 63.7 | 57 | 72.1 | 24 | 54.5 |
| Serious/critical illness (hospitalized ones) | - | - | 56 | 7.4 | 17 | 10.1 | 23 | 7.3 | 10 | 10.5 | 2 | 2 | 2 | 2.6 | 2 | 4.6 |
§ p = 0.001 vs non sanitary workers; † p < 0.001 vs non sanitary workers; ‡ p < 0.001 vs non sanitary workers; * p = 0.02 vs non sanitary workers
In the period from March 2020 until May 2021, 805 workers (14.4% of all workers) contracted COVID-19 as demonstrated by the positive NPS result. There was no clear difference in the prevalence of SARS-CoV-2 infection by most representative age group. The prevalence of infection was slightly higher in males (16.8%) than in females (13.5%) (p = 0.09). A higher prevalence of COVID-19 infection was found in job categories characterized by direct patient care activities (17.9% in nurses group, 18.9% in nursing assistant group, 14.6% in physicians group) than in non sanitary workers (8.5%).
Contact tracing has allowed to recognize the source of the infection in 55.2% of cases. In particular, 21.7% of infected workers reported an in-hospital exposure (related to risky contact with a COVID-19 positive patient and/or collegue) while 33.5% of infected workers reported an out-of-hospital exposure (related to risky contact with infected relatives or friends). Unknown exposure (44.8% of cases) was correlated to the absence of evident risky situations both in the workplace and outside the workplace. When it was possible to recognize the source of the infection, out-of-hospital exposure was reported more frequently by workers of all job categories. A higher prevalence of in-hospital exposure was found in job categories characterized by direct patient care activities (27.3% in nurses group, 18.7% in nursing assistant group, 20.7% in physicians group) than in non sanitary workers (7.6%) (p = 0.02).
Symptoms associated with COVID-19 and their severity were different, from absent to severe; 29.2% of infected workers (n. 235) were totally asymptomatics while 514 COVID-19 cases had mild conditions (63.4%) and needed home care. 7.4% of infected workers (n. 56) had more serious/critical conditions that required hospitalization. During the whole monitoring period, no case of death was recorded among our workers. Elder workers affected by COVID-19 needed hospitalization most frequently than younger ones as show in figure 1. In particular, we reported an increase of “serious/critical illness” from 1.6% in 18-30 years old workers to 19.6% in infected workers over the age of 60 (p<0.001).
Figure 1.
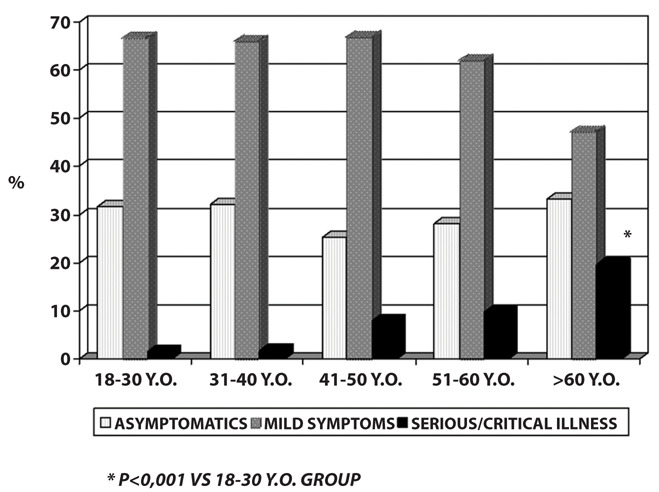
COVID-19 clinical characteristics: data stratified by age groups
Table 2 shows the main characteristics of COVID-19 infection in the study population stratified by pandemic waves. In the first wave of COVID-19 pandemic (from February 2020 to July 2020) we recorded 176 cases of COVID-19 (21.9% of all of infected workers). In-hospital exposure was most frequently reported by infected workers in that pandemic wave as cause of infection (39.7%) and the course of COVID-19 was characterized by mild symptoms or no symptoms in most cases (69.9% and 21.6%, respectively). The largest number of COVID-19 cases (n. 559; 69.4% of infected workers) was diagnosed during the second wave of the pandemic (from August 2020 to December 2020). The source of infection most frequently reported in that phase was the out-of-hospital one (36.5% of cases) and the clinical characteristics of the infection was similar to the previous phase: in particular, 28.3% of cases had no symptoms and 64.8% had a mild course of the illness. 6.9% of infected workers during the second wave needed hospitalization.
Table 2.
COVID-19 prevalence and clinical characteristics: data stratified by pandemic waves
| COVID-19 cases (all) | COVID-19 cases (first wave) | COVID-19 cases (second wave) | COVID-19 cases (third wave) | |||||
|---|---|---|---|---|---|---|---|---|
| n. | % | n. | % | n. | % | n. | % | |
| Total | 805 | 100 | 176 | 21,9 | 559 | 69,4 | 70 | 8,7 |
| Source of infection | ||||||||
| In-hospital | 175 | 21,7 | 70 | 39,7 | 89 | 15,9 | 16 | 22,8 |
| Out-of-hospital | 270 | 33,5 | 36 | 20,6 | 204 | 36,5 | 30 | 42,9 |
| Unknown | 360 | 44,8 | 70 | 39,7 | 266 | 47,6 | 24 | 34,3 |
| Clinical course | ||||||||
| Asymptomatics | 235 | 29,2 | 38 | 21,6 | 158 | 28,3 | 39 | 55,7 (*) |
| Mild conditions | 514 | 63,4 | 123 | 69,9 | 362 | 64,8 | 29 | 41,4 |
| Serious/critical conditions (hospitalized ones) | 56 | 7,4 | 15 | 8,5 | 39 | 6,9 | 2 | 2,9 |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
* p < 0.001 compared to the number of asymptomatic cases diagnosed during the second wave of the COVID-19 pandemic.
The beginning of the third wave (January 2021) coincided with the start of the national vaccination campaign which involved primarily HCWs. In the Fatebenefratelli-Sacco University Hospital, the vaccination campaign against COVID-19 started on December 28, 2020 and 2215 HCWs received a second vaccine dose within the following month. As of May 2021, 90.4% of all workers were fully vaccinated. Seventy COVID-19 cases (8.7% of all infected workers) were diagnosed during the third wave: 35% of them were not vaccinated, while 8% were partially vaccinated and 57% were fully vaccinated. More than 55% of COVID-19 cases diagnosed during this phase had no symptom of infection (p<0.001 vs. second wave cases), about 41% of them had a mild course and only two cases had a “serious/critical illness” Sars-CoV-2-related and needed hospitalization. These two hospitalized cases were not vaccinated. The out-of-hospital setting was the most frequent source of infection identified by infected workers during the third wave.
In the period from March 2020 to May 2021, 805 workers of our University Hospital (14.4%) contracted COVID-19. Epidemiological data released by the Italian Superior Institute of Health (10, 11) showed a prevalence of COVID-19 of about 7% in the Italian population and of about 8.8% in the population of Milan. In figure 2 we compared COVID-19 incidence data in our population with those of the Italian and Milan populations. Data showed a higher COVID-19 incidence during the second wave in our population when compared both to Milan population and Italian national data. Conversely, a clear reduction of the COVID-19 new cases in our population was found during the third wave when compared both to Italian and Milan population: in particular, while during this pandemic wave the incidence of new COVID-19 cases reached a peak in March 2021 in Italian and in Milan population, a progressive decrease in the number of monthly diagnosed COVID-19 cases was observed from January 2021 to May 2021 in our population, also as a result of the beginning of vaccination campaign.
Figure 2.
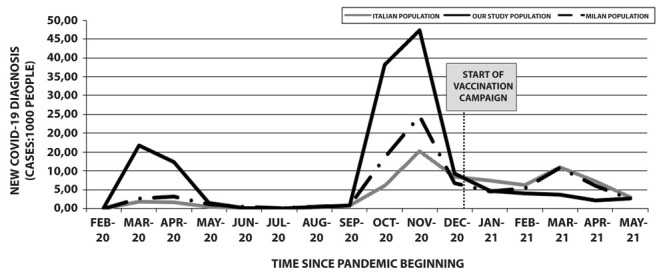
COVID-19 incidence among our population study and comparison with Italian and Milan population data
To evaluate COVID-19 vaccine effectiveness, we also compared SARS-CoV-2 infection incidence in vaccinated (n. 2037 who received vaccine second dose by January 31, 2021) and unvaccinated subjects (n. 441, who were still unvaccinated at the end of the 90-days monitoring period). As shown in table 3, the two groups had similar characteristics in age but there was a greater prevalence of females in unvaccinated than in vaccinated group. After a monitoring period of 90 days, 22 COVID-19 cases were detected in the unvaccinated group (5%), 13 in the vaccinated group (0.6%). Multivariate logistic regression analysis adjusted for potential confounding factors (age, gender, job category) showed an adjusted Odds Ratio (aOR) of infection of 0.11 (0.05-0.22; p: 0.001) in the vaccinated group after 90 days since vaccination. In the vaccinated group we found 53.8% of asymptomatic cases and 46.2% of cases with mild symptoms. Conversely, in the unvaccinated group an asymptomatic course of infection was described in 27.3%, while 63.6% had mild symptoms and 2 cases (9.1%) had severe illness requiring hospitalization.
Table 3.
Descriptive and univariate/multivariate analysis of COVID occurrence in vaccinated vs unvaccinated subjects during a 90-days monitoring program
| Unvaccinated group (n. 441) | Vaccinated group (n. 2037) | p | ||||
|---|---|---|---|---|---|---|
| Age, yrs # | ||||||
| Mean (SD) | 44.9 (11.5) | 44.8 (11.7) | 0.9 | |||
| Gender, n (%) * | ||||||
| Female | 351 (79.6) | 1418 (69.9) | <0.001 | |||
| Job category, n (%) * | n. (%) | n. (%) | ||||
| Sanitary workers | 337 (76.4) | 1932 (94.8) | < 0.001 | |||
| COVID-19 occurrence | n. (%) | n. (%) | OR (95% CI) | p | aOR § (95% CI) | p |
| Stratified analysis: | ||||||
| 8 to 30 days later | 12 (2.7) | 2 (0.1) | 0.04 (0.01;0.13) |
< 0.001 | 0.03 (0.01;0.11) |
< 0.001 |
| after 60 days | 19 (4.3) | 8 (0.4) | 0.09 (0.04;0.20) |
< 0.001 | 0.07 (0.03;0.16) |
< 0.001 |
| after 90 days | 22 (5.0) | 13 (0.6) | 0.12 (0.06;0.24) |
< 0.001 | 0.11 (0.05;0.23) |
< 0.001 |
| Subgroup analysis on clinical characteristics: | ||||||
| Asymptomatics | 6 (27.3) | 7 (53.8) | Ref. group | - | Ref. group | - |
| Symptomatics | 16 (72.7) | 6 (46.2) | 0.32 (0.07;1.33) |
> 0.05 | 0.28 (0.04;1.63) |
> 0.05 |
SD: standard deviation; OR: Odds Ratio; 95% CI: confidence interval 95%
# t-test
* chi-squared test
§ Adjustment for gender, age, job categories (sanitary workers, non-sanitary workers)
Discussion
The aim of this study was to describe, in a large University Hospital which provided healthcare services to patients with SARS-CoV-2 infection, the course of the COVID-19 epidemic among HCWs, the sources of their infection, the severity of clinical symptoms and the effectiveness of COVID-19 vaccination in reducing SARS-CoV-2 infection and disease.
The evolution of the COVID-19 epidemic in Italy, until today, recognizes three main waves. The first wave of infection, from February 2020 to July 2020, was followed by a second largest increase in infections and deaths (second wave) from August 2020 to December 2020, and finally a third wave which started in January 2021 and is still ongoing (10). From March 9, 2020 to May 4, 2020 the Italian government imposed a national total lockdown, restricting the movement of the population except for necessity, work (only for few job categories), and health circumstances, in response to the growing COVID-19 pandemic in the Country. A large percentage of workers worked from home (“smart working” mode) in order to limit interpersonal contacts and contrast the spread of the virus. From May 4, 2020 there has been a partial reopening of the work activities in Italy maintaining the restrictions on not-essential movements for the general population. Since the beginning of the pandemic, HCWs, together with a few other job categories, continued to carry out their activity in the workplace as considered of primary necessity (12). The Occupational Health Unit of our University Hospital is part of the group of the “Occupational Medicine Hospital Units”: they are a peculiarity of the Lombardy Health System and, in the context of the COVID pandemic, have played a central role in homogenizing procedures for managing cases of infection and contact tracing, providing recommendations as a result of national and regional directives. In the period from March 2020 to May 2021, 805 workers of our university hospital (14.4%) contracted COVID-19. Epidemiological data released by the Italian Superior Institute of Health (10) showed a prevalence of COVID-19 in the Italian population of about 7% while in the Milan population 8.8% contracted COVID-19 from March 2020 to May 2021 (11). The higher prevalence of SARS-CoV-2 infection observed among HCWs when compared with data from the general population seems to suggest that HCWs are at high risk of infection and other studies appear to confirm this data (6, 7). However, the national legislative directives, indicated above, may have influenced COVID-19 prevalence data in our HCWs compared to general population. In particular, the start of the lockdown and other control measures ordered by the Italian government, reducing interpersonal contacts and gathering situations, seem to have allowed a reduction in the spread of the infection within the general population. Conversely, HCWs continued to work tirelessly throughout this period, exposing themselves to a greater risk of contagion despite an adequate training and information about the correct use of PPEs and respect of hygiene rules, both in hospital and in out-of-hospital setting. Furthermore, if we analyze the prevalence of infection stratified by pandemic wave, we can see that the largest number of new cases in our study population was recorded during the second pandemic phase. In this period, both more rigorous regional directives in contact tracing programs among healthcare workers and a greater availability of NPS in our university hospital have certainly favoured the diagnosis of infection even in asymptomatic or paucisymptomatic subjects. Specific directives ordered by the Government for COVID-19 sorveillance in HCWs (for example: periodic NPS for HCWs that work with weak patients, more frequent NPS for close contacts of COVID-19 cases that continue to work, performing NPS even in case of very mild symptoms) and the higher NPS availability in our university hospital compared to Italian and Milan general populations could partly explain the important gap highlighted in our study. An association between healthcare activities and increased risk of infection is, however, suggested by our finding of a higher prevalence of infection in job categories characterized by direct patient care activities (nurses, nursing assistants and physicians) than in non-sanitary workers. Literature studies disagree on this aspect: some previous studies showed similar results with a greater risk of infection in HCWs compared to non-sanitary ones (13, 14); differently, a higher risk in non sanitary workers have been reported in other investigations (15). These dissimilar results can be explained by possible different information, education and training programs about the correct use of personal protective equipment (PPE) and compliance with the anti-contagion rules among the study populations involved. In our university hospital all HCWs involved in COVID units were first properly informed, educated and trained about the correct use of PPEs and compliance with the anti-contagion rules. Adequate information and training on the rules to combat the spread of SARS-CoV-2 infection was also given both to HCWs operating in COVID-free areas and administrative and other non-sanitary workers. In a recent study, Al Maskari et al (16) evaluated the principal sources of COVID-19 among HCWs: the most common setting of infection acquiring was the community (61.3%, n = 125), followed by hospital setting (25.5%, n = 52). They also showed a significant association between acquiring COVID-19 in the hospital and carrying out activities characterized by direct contact to patients (doctors and nurses). Concerning this data, our study highlighted that, when it was possible to recognize the source of the infection, out-of-hospital exposure was reported more frequently by workers of all job categories. However we found a higher prevalence of in-hospital exposure in job categories characterized by direct patient care activities than in non sanitary workers. These data suggest that, within a population in which all individuals have the same basal degree of information and education on the respect of hygiene rules against Sars-CoV-2 and the correct use of protective devices and PPEs (with specific differences job category-related), occupational exposure is an addictional risk factor for contagion. Therefore, the correct use of PPEs and compliance with the anti-contagion rules, both during care and non-care working moments and during social life, are essential to prevent COVID-19 infection.
In addition to a greater risk of infection, meta-analysis studies reported a prevalence of hospitalization among HCWs affected by COVID-19 of 15.1% and a mortality rate of 1.5% (17). Conversely, our data showed a lower hospitalization rate (7.4%) compared to literature data; no case of death was registered among our HCWs during the entire monitoring period. Our study showed a prevalence of infection slightly higher in males (16.8%) than in females (13.5%). This finding is consistent with literature data (14, 18). In particular, Nanshan Chen et al, in one of the first published studies about COVID-19, suggested that 2019-nCoV is more likely to infect adult males than females. The same Authors highlighted a higher prevalence of infection in the older age groups (67% of COVID-19 cases registered in subjects over 50 years of age) (18). In our study, however, there was no clear difference in the prevalence of SARS-CoV-2 infection by most representative age groups (31-40; 41-50; 51-60 years). Nevertheless, we found a correlation between age and severity of clinical manifestations: in particular, an increase of “serious/critical illness” from 1.6% in 18-30 years old workers to 19.6% in infected workers over the age of 60 was found in our study population. Previous studies confirmed this finding: CDC data showed that in the United States 62% of hospitalized patients with COVID-19 were older than 55. Conversely, less than 1% of hospitalized patients were 19 years old or younger (19). In a retrospective cohort study of 1591 patients in Italy (20), the Authors showed a median age of 63 years old in COVID-19 infected subjects requiring hospitalization (only 13% of hospitalized patients were younger than 51 years old).
To evaluate the effect of BNT162b2 mRNA COVID-19 vaccine, at first we compared the prevalence of COVID-19 infection in our population during the three pandemic waves and we found a clear reduction in the number of cases of infection during the third wave, which coincided with the start of the vaccination campaign in our University Hospital. During the same period, Italian and Milan population data described a third wave characterized by an increase in COVID-19 cases that reached a peak incidence in March 2021 (10, 11). These data seem to indicate, althought indirectly, the effectiveness of BNT162b2 mRNA COVID-19 vaccine in preventing COVID-19 infection. Furthermore, in our study, we also found a significant reduction of severe/critical illness and an increase of asymptomatic COVID-19 cases in the third wave compared with previous periods. All these data appear to be consistent with those reported by a recent Israelian population study (9): in this study Noa Dagan et al estimated BNT162b2 mRNA vaccine effectiveness for both documented infection and symptomatic COVID-19. Comparing two groups, vaccinated vs. unvaccinated, they also estimated that vaccine effectiveness during the follow-up period starting 7 days after the second dose was 92% for documented infection, 94% for symptomatic COVID-19, 87% for hospitalization, and 92% for severe COVID-19. The Authors concluded suggesting that effectiveness is high for the most serious outcomes (such as hospitalization, severe illness, and death). In our study, we also compared vaccinated and unvaccinated groups and found a significant reduction of SARS-CoV-2 infection in vaccinated HCWs during a 90-days monitoring period (aOR of infection in vaccinated vs. unvaccinated subjects was 0.11). As reported by Dagan et al., when comparing the two groups we also found a significant difference in infection outcome: in particular, we found a lower severity of symptoms and a consequent lower risk of hospitalization in vaccinated HCWs. Our data about COVID-19 incidence and severity of symptoms in HCWs after vaccination were consistent with data of a recent Italian study (21): Sansone et al. monitored the SARS-CoV-2 infection and COVID-19 symptoms among HCWs of a large Hospital in Northern Italy and showed that vaccination campaign effectively reduced the appearance of symptoms and the incidence of infections among vaccinated HCWs.
Although the vaccine has led to a clear reduction in cases of infection, the risk of infection is not eliminated in vaccinated subjects, as shown by our results and previous studies (9, 21). This aspect has already been considered by several international companies who have reiterated the absolute need for all HCWs to use appropriate PPEs and adopt correct hygiene habits until the end of the COVID-19 pandemic (22).
Conclusions
HCWs have been a high risk group for COVID-19 infection. Male sex and older age are confirmed to be predisposing factor and negative prognostic factor, respectively. The use of protective devices (PPEs, surgical mask, etc.) during work seems to reduce the risk of infection: indeed, the main source of COVID-19 appears to be in an out-of-hospital setting, as suggested by previous studies. Vaccination appears to be able to reduce both documented cases of infection and severe illness. However, COVID-19 vaccine does not prevent all cases of infection and HCWs should continue to wear personal protective equipment, observe physical distancing and other measures against the spread of SARS-CoV-2 both in “in-hospital” and “out-of-hospital” settings until the end of COVID-19 pandemic.
Acknowledgments:
The Authors would like to thank all staff of the Occupational Health Unit of the Fatebenefratelli-Sacco University Hospital of Milan and Luca Tosti and Francesca Metruccio MD of the International Centre for Pesticides and Health Risk Prevention, for the kind help and support.
Declaration of Interest:
The Authors declare no conflict of interest.
References
- 1.Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133(9):1015–1024. doi: 10.1097/CM9.0000000000000722. doi:10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus Disease (COVID-19) Situation Reports. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports . (last accessed: May 2021)
- 3.World Health Organization (WHO) WHO Coronavirus Disease (COVID-19) Weekly Epidemiological Update-11 May 2021 [Google Scholar]
- 4.La Maestra S, Abbondandolo A, De Flora S. Epidemiological trends of COVID-19 epidemic in Italy over March 2020: From 1000 to 100 000 cases. J Med Virol. 2020;92(10):1956–1961. doi: 10.1002/jmv.25908. doi:10.1002/jmv.25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novel coronavirus: daily report. Italian Minister of Health. Avaiable from: https://www.salute.gov.it/portale/nuovocoronavirus/homeNuovoCoronavirus.jsp?lingua=english . (last accessed: May 2021)
- 6.Eurosurveillance Editorial Team. Updated rapid risk assessment from ECDC on coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill. 2020;25(12):2003121. doi: 10.2807/1560-7917.ES.2020.25.12.2003261. doi:10.2807/1560-7917.ES.2020.25.12.2003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China [published correction appears in JAMA. 2021 Mar 16;325(11):1113] JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. doi:10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novel Coronavirus – daily vaccine report. Italian Minister of Health. Available at: https://www.governo.it/it/cscovid19/report-vaccini/ . (last accessed: May 2021)
- 9.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. doi:10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Superior Health Institute. COVID-19 integrated surveillance: the main national data. Avaiable at: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati . (last accessed: May 2021)
- 11.Torresi M. Statistiche Coronavirus nel Mondo. Fonti: Worldometer, CSSE, Protezione civile. Avaiable at: https://statistichecoronavirus.it/coronavirus-italia/coronavirus-lombardia/coronavirus-milano/ (last accessed: September 2021)
- 12.Coronavirus: la normativa vigente. Italian Government. Avaiable at: https://www.governo.it/it/coronavirus-normativa . (last accessed: May 2021)
- 13.Mandić-Rajčević S, Masci F, Crespi E, et al. Source and symptoms of COVID-19 among hospital workers in Milan. Occup Med (Lond) 2020;70(9):672–679. doi: 10.1093/occmed/kqaa201. doi:10.1093/occmed/kqaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consonni D, Bordini L, Nava C, et al. COVID-19: What happened to the healthcare workers of a research and teaching hospital in Milan, Italy. Acta Biomed. 2020;91(3):e2020016. doi: 10.23750/abm.v91i3.10361. Published 2020 Sep 7. doi:10.23750/abm.v91i3.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garzaro G, Clari M, Ciocan C, et al. COVID-19 infection and diffusion among the healthcare workforce in a large university-hospital in northwest Italy. Med Lav. 2020;111(3):184–194. doi: 10.23749/mdl.v111i3.9767. Published 2020 Jun 26. doi:10.23749/mdl.v111i3.9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Maskari Z, Al Blushi A, Khamis F, et al. Characteristics of healthcare workers infected with COVID-19: A cross-sectional observational study. Int J Infect Dis. 2021;102:32–36. doi: 10.1016/j.ijid.2020.10.009. doi:10.1016/j.ijid.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gholami M, Fawad I, Shadan S, et al. COVID-19 and healthcare workers: A systematic review and meta-analysis. Int J Infect Dis. 2021;104:335–346. doi: 10.1016/j.ijid.2021.01.013. doi:10.1016/j.ijid.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. doi:10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. Published 2020 Mar 27. doi:10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy [published correction appears in JAMA. 2021 May 25;325(20):2120] JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. doi:10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sansone E, Tiraboschi M, Sala E, et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 among healthcare workers. Med Lav. 2021;112(3):250–255. doi: 10.23749/mdl.v112i3.11747. doi: 10.23749/mdl.v112i3.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Centre for Disease Prevention and Control. ECDC: Stockholm; 2021. Interim guidance on the benefits of full vaccination against COVID-19 for transmission risks and implications for non-pharmaceutical interventions – 21 April 2021. [Google Scholar]


