Abstract
Background:
In Italy, healthcare workers (HCWs) were among the first to receive COVID-19 vaccination. Aim of the present study is to evaluate frequency and severity of adverse events (AEs) following the second dose of BNT162b2 vaccine among HCWs of a large university hospital in Milan, Italy.
Methods:
One month after having received the second dose of vaccine, HCWs filled-in a form about type, severity, and duration of post-vaccination local and systemic symptoms. We calculated the overall frequency of AEs and used multivariable Poisson regression models (adjusted for sex, age, BMI, smoking, allergy history, previous SARS-CoV-2 infection, anti-hypertensive therapy, and occupation) to calculate risk ratios (RR) and 95% confidence intervals (CI) of AEs according to selected variables.
Results:
We included 3659 HCWs. Overall, 2801 (76.6%) experienced at least one local event, with pain at injection site being the most frequent (2788, 76.2%). Systemic events were reported by 2080 (56.8%) HCWs, with fatigue (52.3%), muscle pain (42.2%), headache (37.7%), joint pain (31.9%), and fever (26.2%) being the most frequent. Risks of systemic events were associated with female gender (RR=1.14, CI: 1.06-1.24), age (strong decrease with increasing age, p-trend<0.001), allergy history (RR=1.13, CI: 1.05-1.20), SARS-CoV-2 infection more than 180 days before second dose (RR=1.16, CI:1.01-1.32), and current smoking (RR=0.90, CI: 0.84-0.97).
Conclusions:
Both local and systemic acute effects after second dose of BNT162b2 vaccine were frequently reported. However, symptoms were mostly light/mild and of short duration. Thus, our findings support the safety of COVID-19 vaccination in adults in relatively good health.
Keywords: COVID-19 Vaccination, healthcare workers, adverse events
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to spread, having already affected more than two hundred million people and caused more than four million deaths (1). Given the lack of a specific therapy, with the aim of reducing the occurrence of severe cases, industry and academic institutions have made huge efforts to develop an effective vaccine (2, 3). To do that, they have employed a wide range of technologies that include live attenuated, viral vectored, mRNA-based, protein-based, and inactivated vaccines (4). To date, 194 vaccines are in pre-clinical development, 126 vaccines are in clinical development, and 7 vaccines have already been approved for use (5).
BNT162b2 was the first vaccine authorized for emergency use by the European Medicines Agency (EMA), on December 21, 2020 (6), following the Food and Drug Administration (FDA) approval on December 11, 2020 (7). In Italy, on December 22nd, 2020, the Italian Medicines Agency also authorized the use of BNT162b2 vaccine, and mass vaccination started on December 27th, 2020 (8). The first phase of the immunization program was primarily focused on people with an increased risk of getting infected, such as healthcare workers (HCWs), or with an enhanced risk of severe disease, like elderly people in long-term care facilities or individuals older than 80 years (8).
BNT162b2 is a lipid nanoparticle–formulated, nucleoside-modified RNA vaccine, developed by Pfizer–BioNTech, that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full length spike protein (9). BNT162b2 efficacy was evaluated in a phase-3 clinical trial showing that two injections, performed 21 days apart, gave a 95% protection against COVID-19 in people 16 years of age or older. That trial also evaluated the safety profile, showing that vaccinated people, especially the young, reported more local reactions, such as mild-to-moderate pain at the injection site, and systemic reactions, such as fatigue, headache, and fever, as compared to people in the placebo group. Moreover, the rate of reported adverse events (AEs) was slightly higher after the second dose, as compared to the first dose (10). However, phase-3 clinical trials have limitations in assessing vaccine safety, as they include a small number of participants and a healthier-than-average sample population. As a consequence, they are not adequate to identify less common AEs, and post-marketing surveillance is needed to assess the safety of vaccines in real-world settings (11).
In Italy, at the time of writing (October 19th, 2021), 62,512,723 total doses of BNT162b2 vaccine have been administered (12), and the administration of the third vaccine dose is currently underway. Despite the huge number of doses administered, knowledge on AEs is still limited and primarily based on pharmacovigilance surveillance reports by Italian Medicines Agency database (13) and few other studies. Continuous documentation of local and systemic reactions to vaccination outside clinical trials is necessary. In a previous publication, we focused on the role of anti-hypertensive treatment in developing cutaneous reactions after vaccination and we found that people who took ACE inhibitors had a greater risk of developing urticaria/angioedema (14). The aim of the present study was the comprehensive evaluation of the BNT162b2 safety profile after second vaccination dose in HCWs of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan (Italy), who received the recommended two doses (30 mg, 0.3 mL each), administered intramuscularly, 3 weeks apart, between December 27th, 2020 and June 9th, 2021. Information on AEs was collected through a self-administered questionnaire at the time individuals came to perform a serology test to verify antibody response to vaccination, about one month after the second dose.
Methods
HCWs were recruited from Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan (Italy), a large university hospital with about 800 beds, and with the capability of providing assistance to more than 34,000 hospitalized subjects (about 24,500 after the spread of the pandemic, during 2020). The whole personnel (about 8,200 workers) performing healthcare activities was eligible, including: a) hospital and University physicians, nurses, midwifes, healthcare assistants, and clerical workers and technicians (about 5,700 workers including those with temporary contracts); and b) University residents and students (about 2,500). In the present work, we loosely refer to all these occupations as HCWs.
HCWs who agreed to get vaccinated, one month after the second dose, were invited to fill-in a form at the time of blood collection to test for seroconversion by assessment of anti-Spike-1 antibodies. The form contained questions about type and duration of several selected local and systemic symptoms occurred after the second vaccine dose (Appendix A). Use of medication, consultation with a physician, admission to an emergency room, and admission to hospital because of those symptoms were also asked.
Vaccination data were obtained by local databases and completed after linkage with vaccination files of Lombardy Region. Information on occupation, BMI, and smoking habit was taken from a separate database containing data collected at the time of first vaccine dose administration. Information on previous SARS-CoV-2 infection (documented by positive RT-PCR test on nasopharyngeal swab) was derived from routinely collected laboratory files. We first calculated the overall number (%) of adverse events (AEs). Then, we examined the influence of selected variables, such as gender, age, occupation, BMI, smoking, previous SARS-CoV-2 infection (stratified by recent infection, i.e. occurred ≤180 days before the second dose, and remote infection, i.e. >180 days before, because we previously noted different anti-S antibody responses to vaccination between these two groups) (15), history of allergy, and use of anti-hypertensive drugs (angiotensin-converting enzyme inhibitors, ACEI, and angiotensin II receptor blocker, ARBs) on AEs frequency, by fitting univariate and multivariable Poisson regression models (adjusted for the above-mentioned variables) with robust standard error to calculate adjusted risk ratios (RR) and 95% confidence intervals (CI) (16). To visualize frequency of symptoms by age (age and squared age) we fitted two logistic regression models to males and females separately, and then we obtained predicted probabilities. Analyses were performed with Stata 17 (StataCorp. 2021). The study was approved by the hospital’s ethics committee (Milano Area 2, Prot. No. 828_2021bis).
Results
We included in analysis 3659 HCWs, 2610 females (71.3%) and 1049 males (28.7%), who filled-in the AEs form. On average, females were about two years younger, with only 20.2% aged 55 or more, against 27.6% of males (Table 1). Most females were nurses/midwifes, while the majority of males were physicians. Males had higher BMI and higher proportions of former/current smokers (45.8%), and ARBs use (3.4%), as compared to females. History of allergy was more frequent in females (23.6%), while frequency of previous documented SARS-CoV-2 infection was similar between the two genders.
Table 1.
Characteristics of healthcare workers of a large university hospital included in the analysis of adverse events after the second dose of COVID-19 vaccination with BNT162b2, Milan, Italy
| Variable | Women | Men | |||
|---|---|---|---|---|---|
| N | % | N | % | P-value | |
| All | 2610 | 100 | 1049 | 100 | |
| Age (years), mean (SD) | 42.4 | (12.8) | 44.5 | (13.2) | <0.001 |
| Age category (years) | |||||
| <35 | 934 | 35.8 | 345 | 32.9 | <0.001 |
| 35-44 | 464 | 17.8 | 190 | 18.1 | |
| 45+54 | 685 | 26.2 | 225 | 21.4 | |
| 55+ | 527 | 20.2 | 289 | 27.6 | |
| Occupation | |||||
| Physicians | 423 | 16.2 | 314 | 29.9 | <0.001 |
| Residents | 203 | 7.8 | 124 | 11.8 | |
| Nurses, midwives | 786 | 30.1 | 219 | 20.9 | |
| Health-care assistants | 156 | 6.0 | 67 | 6.4 | |
| Health technicians | 482 | 18.5 | 109 | 10.4 | |
| Clerical workers, technicians | 391 | 15.0 | 194 | 18.5 | |
| Students | 169 | 6.5 | 22 | 2.1 | |
| BMI (kg/m2), mean (SD) | 23.1 | (4.4) | 25.0 | (3.5) | <0.001 |
| BMI category (kg/m2) | |||||
| <20 | 613 | 23.5 | 41 | 3.9 | <0.001 |
| 20-24 | 1207 | 46.2 | 522 | 49.8 | |
| 25-29 | 441 | 16.9 | 352 | 33.6 | |
| 30+ | 193 | 7.4 | 83 | 7.9 | |
| Missing | 156 | 6.0 | 51 | 4.9 | |
| Cigarette smoking | |||||
| Never | 1519 | 58.2 | 525 | 50.0 | <0.001 |
| Former | 377 | 14.4 | 223 | 21.3 | |
| Current | 583 | 22.3 | 257 | 24.5 | |
| Missing | 131 | 5.0 | 44 | 4.2 | |
| History of allergy | 617 | 23.6 | 127 | 12.1 | <0.001 |
| Previous SARS-CoV-2 infection | 294 | 11.3 | 138 | 13.2 | 0.11 |
| ACEI therapy | 42 | 1.6 | 21 | 2.0 | 0.41 |
| ARB therapy | 42 | 1.6 | 36 | 3.4 | 0.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
At least an adverse event was reported in 3205 (87.6%) subjects (Table 2). In total, 2801 (76.6%) HCWs experienced at least one local event, with pain at injection site being the most frequent (average duration 1.8 days; 7-31 days in 20 subjects). Redness (average duration 3.1 days; 7-11 days in 9 subjects) and swelling (average duration 3.2 days; 7-48 days in 10 subjects) were also reported by 151 (4.1%) and 198 (5.4%) subjects, respectively.
Table 2.
Type and degree of adverse events after the second dose of COVID-19 vaccination with BNT162b2 among healthcare workers in a large university hospital, Milan, Italy
| Adverse events and consequences | Degree | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | Light | Mild | Severe | |||||
| N | % | N | % | N | % | N | % | |
| Any event | 3205 | 87.6 | ||||||
| Local events | 2801 | 76.6 | ||||||
| Pain at injection site | 2788 | 76.2 | 1581 | 43.2 | 1056 | 28.9 | 151 | 4.1 |
| Redness | 151 | 4.1 | ||||||
| Swelling | 198 | 5.4 | ||||||
| Systemic events | 2080 | 56.8 | ||||||
| Fatigue | 1914 | 52.3 | 869 | 23.8 | 773 | 21.1 | 272 | 7.4 |
| Muscle pain | 1543 | 42.2 | 574 | 15.7 | 711 | 19.4 | 258 | 7.0 |
| Headache | 1380 | 37.7 | 675 | 18.4 | 535 | 14.6 | 170 | 4.6 |
| Joint pain | 1166 | 31.9 | 434 | 11.9 | 527 | 14.4 | 205 | 5.6 |
| Fever | 960 | 26.2 | ||||||
| Lymph-node swelling | 348 | 9.5 | 164 | 4.5 | 155 | 4.2 | 29 | 0.8 |
| Diarrhea | 137 | 3.7 | 82 | 2.2 | 43 | 1.2 | 12 | 0.3 |
| Nausea/vomiting | 119 | 3.2 | 81 | 2.2 | 26 | 0.7 | 12 | 0.3 |
| Skin rash | 54 | 1.5 | 28 | 0.8 | 16 | 0.4 | 10 | 0.3 |
| Shortness of breath | 38 | 1.0 | 25 | 0.7 | 10 | 0.3 | 3 | 0.1 |
| Chills | 37 | 1.0 | ||||||
| Face/tongue/throat swelling | 18 | 0.5 | 13 | 0.4 | 3 | 0.1 | 2 | 0.05 |
| Use of medications | 1276 | 34.9 | ||||||
| Consultation of a physician | 63 | 1.7 | ||||||
| Admission to an emergency department | 10 | 0.3 | ||||||
Systemic events were reported by 2080 (56.8%) subjects, with the most frequent being fatigue (52.3%), muscle pain (42.2%), headache (37.7%), and joint pain (31.9%). Among these events, sizable proportions of mild (14-21%) or severe (5-7%) symptoms were observed. Average duration of fatigue was 2.0 days (7-34 days in 37 subjects), of muscle pain 2.0 days (7-34 days in 24 subjects), of headache 1.8 days (7-21 days in 21 subjects), and of joint pain 2.1 days (7-34 days in 19 subjects).
Fever was reported by one fourth of subjects, with median/average temperature of 38.0°C and a maximum of 42.1°C; lasting 1 day in 492 (74.4%), 2 days in 167 (24.1%), and 3-4 days in 33 subjects (4.8%), respectively. The remainder of symptoms listed in Table 2 concerned less than 10% of HCWs. In total, 1276 (34.9%) individuals took medications (mostly analgesic/antipyretic drugs) consequently to these events, and 63 (1.7%) consulted a physician, including 10 subjects (0.3%) who were admitted to an emergency department, because of esophageal/gastric pain (n=3), suspected severe allergic reactions (n=2), flu-like symptoms (n=2), hemifacial paresthesia (n=1), cochleo-vestibular neuritis (n=1), and hemi-thoracic pain (n=1). None of them required hospitalization.
At univariate analyses, risk of reported acute systemic reactions was associated with gender (higher in females), age (strong decrease with increasing age, P-trend<0.001), BMI (decrease with increasing BMI, P-trend=0.001), smoking (lower risks in former and current smokers, P-trend=0.002), occupation (higher in resident doctors, nurses, technicians and students) and allergy history (Table 3). The decrease by age was marked and gradual in both genders (Figure 1). Previous SARS-CoV-2 infection occurred more than 180 days before the second dose was positively associated with risk of systemic events, while recent SARS-CoV-2 infection and ACEI/ARB therapy were associated with lower risks.
Table 3.
Risks of systemic events (N=2080) after the second dose of COVID-19 vaccination with BNT162b2 according to selected variables among healthcare workers in a large university hospital, Milan, Italy
| Variable | Systemic events | RR crude | RR adjusted* | |||
|---|---|---|---|---|---|---|
| N | % | RR | 95% CI | RR | 95% CI | |
| Gender | ||||||
| Male | 524 | 50.0 | 1.00 | Reference | 1.00 | Reference |
| Female | 1556 | 59.6 | 1.19 | 1.11-1.28 | 1.14 | 1.06-1.24 |
| Age category (years) | ||||||
| <35 | 816 | 63.8 | 1.00 | Reference | 1.00 | Reference |
| 35-44 | 395 | 60.4 | 0.95 | 0.88-1.02 | 0.93 | 0.85-1.01 |
| 45-54 | 508 | 55.8 | 0.87 | 0.81-0.94 | 0.86 | 0.79-0.93 |
| 55+ | 361 | 44.2 | 0.69 | 0.64-0.76 | 0.69 | 0.62-0.76 |
| P-trend | <0.001 | <0.001 | ||||
| Occupation | ||||||
| Physicians | 376 | 51.0 | 1.00 | Reference | 1.00 | Reference |
| Residents | 198 | 60.6 | 1.19 | 1.06-1.33 | 1.01 | 0.87-1.14 |
| Nurses | 580 | 57.7 | 1.13 | 1.04-1.24 | 1.08 | 0.98-1.18 |
| Healthcare assistants | 120 | 53.8 | 1.05 | 0.92-1.21 | 1.08 | 0.92-1.25 |
| Health technitians | 364 | 61.6 | 1.21 | 1.10-1.33 | 1.12 | 1.02-1.24 |
| Clerical workers, technicians | 324 | 55.4 | 1.09 | 0.98-1.20 | 1.14 | 1.03-1.27 |
| Students | 118 | 61.8 | 1.21 | 1.06-1.38 | 1.00 | 0.86-1.15 |
| BMI category (kg/m2) | ||||||
| <20 | 405 | 61.9 | 1.00 | Reference | 1.00 | Reference |
| 20-24.99 | 992 | 57.4 | 0.93 | 0.86-1.00 | 0.99 | 0.92-1.07 |
| 25-29.99 | 423 | 53.3 | 0.86 | 0.79-0.94 | 0.99 | 0.90-1.08 |
| 30+ | 144 | 52.2 | 0.84 | 0.74-0.96 | 0.94 | 0.82-1.07 |
| P-trend | <0.001 | 0.61 | ||||
| Cigarette smoking | ||||||
| Never | 1200 | 58.7 | 1.00 | Reference | 1.00 | Reference |
| Former | 332 | 55.3 | 0.94 | 0.87-1.02 | 1.01 | 0.93-1.09 |
| Current | 443 | 52.7 | 0.90 | 0.83-0.97 | 0.90 | 0.84-0.97 |
| P-trend | 0.003 | 0.02 | ||||
| History of allergy | ||||||
| No | 1569 | 55.1 | 1.00 | Reference | 1.00 | Reference |
| Yes | 465 | 62.5 | 1.13 | 1.06-1.21 | 1.13 | 1.05-1.20 |
| Previous SARS-CoV-2 infection | ||||||
| No | 1841 | 57.0 | 1.00 | Reference | 1.00 | Reference |
| ≤180 days before | 145 | 50.5 | 0.89 | 0.79-1.00 | 0.86 | 0.76-0.98 |
| >180 days before | 94 | 64.8 | 1.14 | 1.00-1.29 | 1.16 | 1.01-1.32 |
| P-trend | 0.71 | 0.63 | ||||
| ACEI therapy | ||||||
| No | 2002 | 56.8 | 1.00 | Reference | 1.00 | Reference |
| Yes | 32 | 50.8 | 0.89 | 0.70-1.14 | 1.02 | 0.80-1.30 |
| ARB therapy | ||||||
| No | 1999 | 56.9 | 1.00 | Reference | 1.00 | Reference |
| Yes | 35 | 44.9 | 0.79 | 0.62-1.01 | 0.97 | 0.75-1.25 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Figure 1.
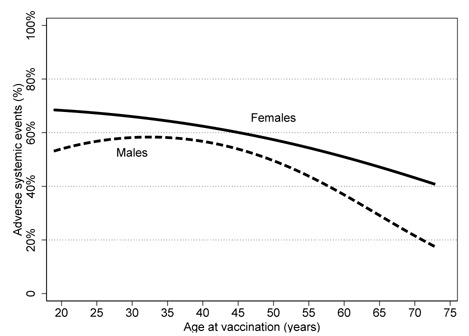
Frequency of adverse systemic events after the second dose of COVID-19 vaccination with BNT162b2, by age among healthcare workers of a large university hospital, Milan, Italy
At multivariate analysis (Table 3), the strong association of systemic AEs with gender, age, and allergy history was confirmed, while the association with BMI became negligible. A 10% decreased risk was found for current smokers only. Healthcare technicians and workers not involved in healthcare activities (clerical workers and technicians) were at greater risk of reporting AEs. Subjects previously infected with SARS-CoV-2 were at lower risk if they had a recent infection history (≤180 days), while workers who had a previous infection occurred more than 180 days before the second dose were at increased risk. Finally, no association was found with ACEI/ARB therapy.
Discussion
In this study, we found that BNT162b2 vaccine caused frequent (88%) transient events, either local (77%) or systemic (57%). However, most symptoms were of light-mild severity. Although one third of participants declared to have taken medications, only few individuals needed access to emergency department and none was hospitalized. Risk of systemic effects was higher in women and individuals with previous allergy history, and lower with increasing age and in current smokers. Finally, HCWs previously infected by SARS-CoV-2 180 days or more before the second dose were at increased risk. We verified that the apparent lower risk by increasing BMI can be explained by confounding by age.
Our results are similar to those observed in clinical trial settings, where fatigue and headache were the most common systemic AEs (10). Moreover, similar AEs, following BNT162b2 vaccination, were also found in recent post-marketing reports. A study conducted among HCWs in the USA, reported that localized soreness, generalized weakness, myalgia, headache, chills, fever, joint pain and nausea were the most common symptoms after vaccination (17). The Report of the First Month of COVID-19 Vaccine Safety Monitoring, conducted in the USA, which analyzed the data from the AEs observed during the first month of mass vaccination, found that the largest majority of AEs were non-serious, including headache, fatigue and dizziness. However, this study also reported rare cases of anaphylaxis after receipt of BNT162b2 vaccine (18). A similar study conducted in the UK, which analyzed data reported by people who used the COVID Symptom Study app, observed that headache, fatigue, pain and tenderness at injection site were the most common side-effects (19).
Systemic and local non-serious side-effects after vaccination were expected because of reactogenicity, the physical manifestation of the inflammatory response to vaccination. In general, vaccines contain antigens and adjuvants that stimulate the immune system, which, consequently, produces a complex series of innate immune events, including phagocytosis, and release of inflammatory mediators in the injection site. These phenomena are crucial for activating the immune response, in a process of antigen recognition and subsequent development of adaptive immune responses necessary to achieve the protection against disease. At the same time, they may also lead to the development of signs and symptoms of injection-site inflammation (pain, redness and swelling) in vaccinated individuals. Moreover, mediators and products of inflammation in the circulation can affect the entire body, causing systemic side effects (such as fever, fatigue, and headache) (20). Continued monitoring of reactogenicity of COVID-19 vaccines outside of clinical trial settings may provide additional information for healthcare practitioners and the public about transient local and systemic reactions. This will allow patients to alleviate some of the potential anxiety concerns due to postvaccination reactogenicity (21).
Our findings are in agreement with previous investigations showing that females and younger individuals were more likely to report adverse events after BNT162b2 vaccination (18, 19, 22, 23). Older individuals normally report less adverse events, probably because the intensity of innate immune responses reduces during lifetime. This hypothesis is supported by the fact that older people display lower systemic levels of IL-6, IL-10 and CRP after vaccination (24), which could contribute to their tendency to report fewer systemic adverse events, in particular fever. In addition, women are more likely to experience more adverse events, as compared to men, possibly because of genetic and hormonal differences (25). Indeed, sex hormones have been shown to influence immune responses and cytokine levels, with androgens and high doses of estrogens being immunosuppressive (26, 27). The role of smoking status and BMI levels in predicting adverse events have not been investigated in previous publications. However, people with higher BMI levels may experience less adverse events, because of the low-level chronic inflammation which characterizes them (28). Moreover, also current smokers may be at lower risk of developing AEs, probably because of the immunosuppressive effect of smoking (29, 30).
Finally, our study shows that HCWs recently infected with SARS-CoV-2 were at lower risk, while workers who had resulted positive to SARS-CoV-2 more than six months before vaccination were at greater risk of experiencing AEs. Discordant results were also found in the literature. In some previous studies, history of SARS-CoV-2 infection was associated with an increased risk of AEs after both BNT162b2 doses, either in the general population (19) or among HCWs (31). However, in another study from Milan, higher rates of AEs in HCWs with a history of SARS-CoV-2 infection were observed after the first dose of vaccine, while the contrary was observed after the second dose (23). In addition, in another small Italian study among HCWs a positive association with previous SARS-CoV-2 infection after the first BNT162b2 dose, but not after the second was found (22). On one hand, subjects with a prior SARS-CoV-2 infection are expected to report more AEs, due to the increased immunogenicity (32, 33). On the other hand, it is also well known that high recall and anamnestic responses to vaccination are associated with intervals of at least 3–4 months between stimuli, with longer intervals associated with generally greater responses (15).
The present study has some strengths. This is one of the first studies investigating the side-effects following BNT162b2 vaccination in a real-world setting. Moreover, the large sample size allowed us to provide a comprehensive picture of different AEs. At the same time, we recognize several limitations. First, due to time constraints, we were not able to collect data on AEs after the first vaccine dose. Therefore, we could not evaluate the full picture of BNT162b2 vaccine safety nor evaluate how many HCWs did not complete the vaccination due to severe AEs after the first dose. However, either the BNT162b2 RCT (10) or other studies among HCWs reported no serious AEs after the first dose (19, 22, 23). Also a study in Milan reported only 5 HCWs out of 3078 who refused the second dose because of “moderate symptoms” (23). Therefore, we have no reason to expect a different behavior in our hospital. In fact, the main reason for not doing the second dose was a recommendation of the Italian Ministry of Health, who in March 2021 (when 70% of HCWs had already been vaccinated) declared that subjects infected 3-6 months prior to vaccination can be considered fully vaccinated with only one dose.
A second limitation is that not all HCWs responded to the invitation to perform a serology test. Although this fact reduced the sample size, we believe that selection bias in reporting AEs after vaccination is implausible, because participation depended on willingness to check one’s anti-S antibody level, not on the development of AEs. Notably, the frequency of systematic AE after the second BNT162b2 dose in our study (56.8%) was similar to that found in the first BNT162b2 clinical trial (10) and in a study in Milan (52.0%) (23), higher than in a study in Italy (37.1% moderate and 4.7% severe) (22), and much higher than in a large UK study (22.0%) (19). In summary, we feel our study gave a fair picture of AEs occurrence.
A third limitation is that our analysis was based on subjects’ self-reports and not on clinical diagnosis, which may introduce information bias. However, this limitation is common in pharmacoepidemiologic studies.
Conclusions
In conclusion, our study shows that the second dose of BNT162b2 vaccine causes frequent local and systemic AEs, with females, younger ages, people with allergy history, who had a history of SARS-CoV-2 180 days or more before second dose, and not currently smoking being at a greater risk. However, AEs were mostly light/mild and of short duration, therefore our findings, in agreement with other studies, support the safety of the vaccine. In interpreting these results, one should consider that our study was limited to a working population in relatively good health. AEs in specific population subgroups, such as pregnant women, younger and older subjects, and people with severe diseases should be assessed in different settings.
Acknowledgements:
We would like to thank the following people that helped in data collection, database preparation, and data input: Silvia Adamoli, Francesco De Palo, Vittoria Di Donna, Nicola Ernesto Di Laurenzio, Sara Franchetti, Giulia Gallo, Michele Gatti, Gabriele Ghigliazza, Lidia Guerrieri, Maurizio Lecce, Marco Leoni, Francesco Mandara, Patrizia Marazzi, Barbara Marinelli, Andrea Midi, Francesco Oliani, Alkis Piliafas, Marco Polonioli, Antonella Savoia, Enrico Radice, Daniele Serra, Francesco Tafuri, Navpreet Tiwana, Adriana Torri, the personnel of SPIO (Servizio Prevenzione e Igiene Ospedaliera).
Funding:
This research was partially funded by the “Fondazione Romeo ed Enrica Invernizzi”.
Institutional review board statement:
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the hospital’s ethics committee (Milano Area 2, Prot. No. 828_2021bis).
Informed consent statement:
Informed consent was obtained from all subjects involved in the study.
Conflict of interests:
The authors declare no conflict of interest.
References
- 1.Johns Hopkins University and Medicine. Coronavirus Resource Center. Published 2021. Accessed October 14, 2021. https://coronavirus.jhu.edu/
- 2.Haque A, Pant AB. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines. 2020;8(4) doi: 10.3390/vaccines8040739. doi:10.3390/vaccines8040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KD, Hwang I, Ku KB, Lee S, Kim SJ, Kim C. Progress and Challenges in the Development of COVID-19 Vaccines and Current Understanding of SARS-CoV-2-Specific Immune Responses. J Microbiol Biotechnol. 2020;30(8):1109–1115. doi: 10.4014/jmb.2006.06006. doi:10.4014/jmb.2006.06006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng WH, Liu X, Mahalingam S. Development of vaccines for SARS-CoV-2 [version 1; peer review: 1 approved] F1000 Res. 2020;9:1–15. doi: 10.12688/f1000research.25998.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. COVID-19 vaccine tracker and landscape. Published 2021. Accessed October 20, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
- 6.European Medicines Agency. EMA recommends first COVID-19 vaccine for authorisation in the EU. Published 2020. Accessed October 21, 2021. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu .
- 7.U.S. Food and Drug Administration. FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. Published 2020. Accessed October 21, 2021. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 .
- 8.Italian Ministry of Health. Covid-19 vaccine plan. Published 2021. Accessed October 14, 2021. https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5452&area=nuovoCoronavirus&menu=vuoto .
- 9.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. doi:10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. doi:10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. doi:10.1056/nejmoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Il Sole 24 Ore. Vaccini in tempo reale. Published 2021. Accessed October 21, 2021. https://lab24.ilsole24ore.com/numeri-vaccini-italia-mondo/
- 13.Italian Medicines Agency. Rapporto sulla Sorveglianza dei vaccini. Published online 2021. https://www.aifa.gov.it/farmacovigilanza-vaccini-covid-19 .
- 14.Cugno M, Consonni D, Lombardi A, et al. Increased risk of urticaria/angioedema after BNT162b2 mRNA COVID-19 vaccine in health care workers taking ACE inhibitors. Vaccines. 2021;9(9):1–7. doi: 10.3390/vaccines9091011. doi:10.3390/vaccines9091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lombardi A, Consonni D, Oggioni M, et al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J Infect Public Health. 2021;14(8):1120–1122. doi: 10.1016/j.jiph.2021.07.005. doi:10.1016/j.jiph.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. doi:10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 17.Kadali RAK, Janagama R, Peruru S, Malayala S V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. doi:10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gee J, Paige Marquez, Su J, et al. MMWR, First Month of COVID-19 Vaccine Safety Monitoring — United States, December 14, 2020–January 13, 2021. 2020. https://www.meddra. [DOI] [PMC free article] [PubMed]
- 19.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. doi:10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Da Silva FT. The how’s and what’s of vaccine reactogenicity. npj Vaccines. 2019;4(1) doi: 10.1038/s41541-019-0132-6. doi:10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mrna-based covid-19 vaccines. JAMA - J Am Med Assoc. 2021;325(21):2201–2202. doi: 10.1001/jama.2021.5374. doi:10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 22.Ripabelli G, Tamburro M, Buccieri N, et al. Active Surveillance of Adverse Events in Healthcare Workers Recipients After Vaccination with COVID-19 BNT162b2 Vaccine (Pfizer-BioNTech, Comirnaty): A Cross-Sectional Study. J Community Health. 2021:0123456789. doi: 10.1007/s10900-021-01039-3. doi:10.1007/s10900-021-01039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.d’Arminio Monforte A, Tavelli A, Perrone PM, et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: Data from 3,078 health care workers. EClinicalMedicine. 2021:36. doi: 10.1016/j.eclinm.2021.100914. doi:10.1016/j.eclinm.2021.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Yousfi M, Mercier S, Breuillé D, et al. The inflammatory response to vaccination is altered in the elderly. Mech Ageing Dev. 2005;126(8):874–881. doi: 10.1016/j.mad.2005.03.008. doi:10.1016/j.mad.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9. doi:10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trigunaite A, Dimo J, J⊘rgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294(2):87–94. doi: 10.1016/j.cellimm.2015.02.004. doi:10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. doi: 10.1016/j.cellimm.2015.01.018. doi:10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. 2017;127(1):65–73. doi: 10.1172/JCI88882. doi:10.1172/JCI88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.M. S. Effects of cigarette smoke on the immune system.Nat Rev Immunol 2002;2:372–377. Nat Rev Immunol. 2002;2(May):372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 30.Qiu F, Liang CL, Liu H, et al. Oncotarget 268 www.impactjournals.com/oncotarget Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget. 2017;8(1):268–284. doi: 10.18632/oncotarget.13613. www.impactjournals.com/oncotarget/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raw RK, Kelly CA, Rees J, Wroe C, Chadwick DR. Previous COVID-19 infection, but not Long-COVID, is associated with increased adverse events following BNT162b2/Pfizer vaccination. J Infect. 2021;83(3):381–412. doi: 10.1016/j.jinf.2021.05.035. doi:10.1016/j.jinf.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. doi:10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise J. Covid-19: People who have had infection might only need one dose of mRNA vaccine. BMJ. 2021;372:n308. doi: 10.1136/bmj.n308. doi:10.1136/bmj.n308. [DOI] [PubMed] [Google Scholar]


